Stainless Steel Foil-Based Label-Free Modular Thin-Film Electrochemical Detector for Solvent Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation
2.3. Thin-Film-Coated Electrode Preparation
2.4. Thin-Film-Coated Glass Substrate Preparation
2.5. Sensor Device Assembly
2.6. Electrochemical Measurements
2.7. Conductometry
2.8. UV-Vis Transmittance Spectroscopy
3. Results
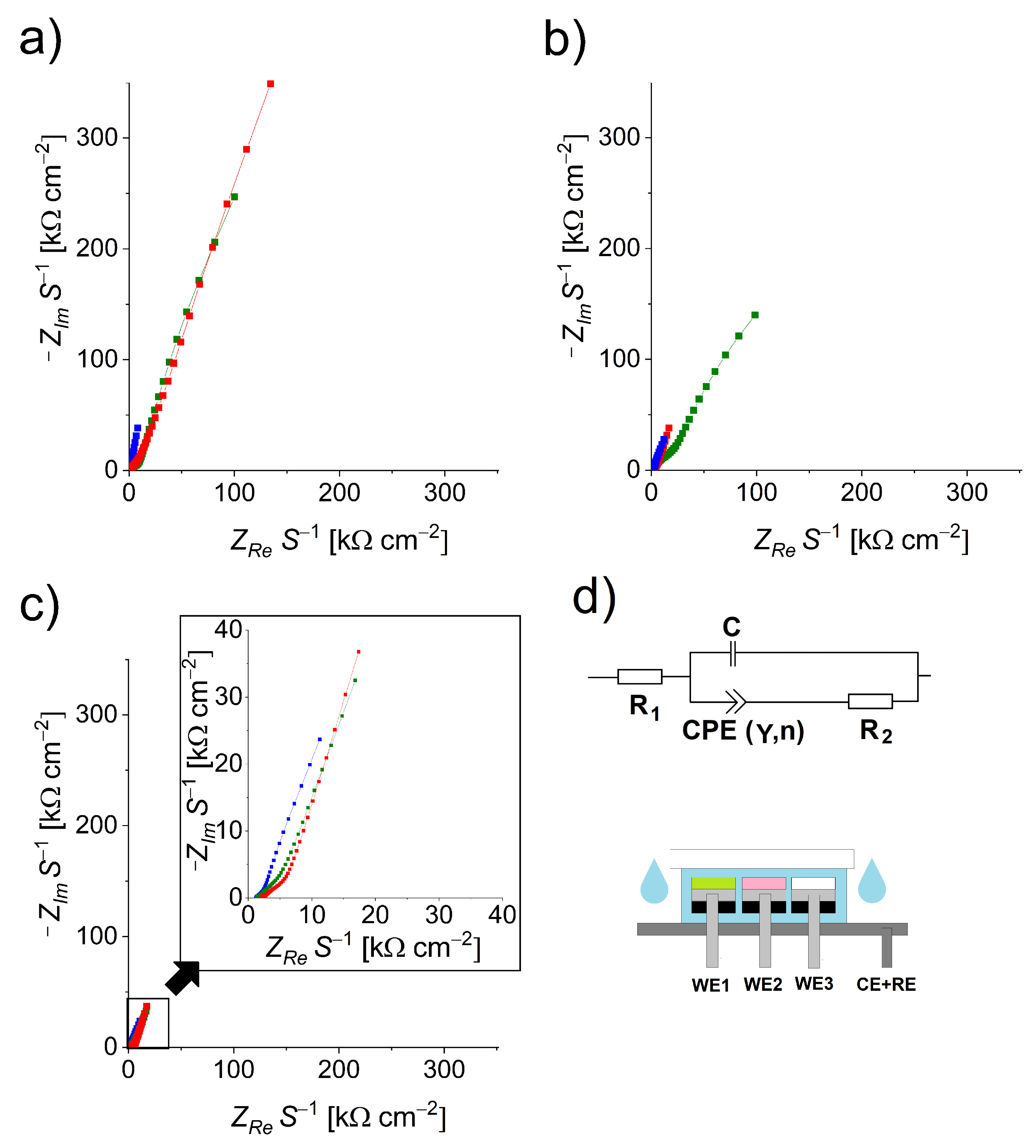
3.1. Electrochemical Impedance Spectroscopy (EIS)
3.2. Cell Resistance Measurement (CRM)
3.3. Conductometry Measurements
3.4. Spectrophotometric Analysis of Thin Films
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EtOH | ethanol |
| THF | tetrahydrofuran |
| EtOH-STF | ethanol-susceptible thin film |
| THF-STF | tetrahydrofuran-susceptible thin film |
| EIS | electrochemical impedance spectroscopy |
| CRM | cell resistance measurement |
| WE | working electrode |
Appendix A
Appendix A.1

Appendix A.2
| Sensor System/Treatment | [] | [] | C [F] | CPE (Y,n) [; A.U.] |
|---|---|---|---|---|
| uncoated WE exposed to water | 185 | 880 | ; 0.699 | |
| EtOH-STF-coated WE exposed to water | 187 | 4406 | ; 0.765 | |
| THF-STF-coated WE exposed to water | 186 | 3470 | ; 0.779 | |
| THF-STF-coated WE exposed to ethanol | 186 | 6270 | ; 0.6528 |

References
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Li, W.; Luo, W.; Li, M.; Chen, L.; Chen, L.; Guan, H.; Yu, M. The impact of recent developments in electrochemical POC sensor for blood sugar care. Front. Chem. 2021, 9, 723186. [Google Scholar] [CrossRef]
- Cole, L.A.; Sutton-Riley, J.M.; Khanlian, S.A.; Borkovskaya, M.; Rayburn, B.B.; Rayburn, W.F. Sensitivity of over-the-counter pregnancy tests: Comparison of utility and marketing messages. J. Am. Pharm. Assoc. 2005, 45, 608–615. [Google Scholar] [CrossRef]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-Cov-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef]
- Masson, J.-F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Killner, M.H.M.; Danieli, E.; Casanova, F.; Rohwedder, J.J.R.; Blümich, B. Mobile compact 1H NMR spectrometer promises fast quality control of diesel fuel. Fuel 2017, 203, 171–178. [Google Scholar] [CrossRef]
- van Kollenburg, G.H.; van Manen, H.-J.; Admiraal, N.; Gerretzen, J.; Jansen, J.J. Low-cost handheld NIR spectroscopy for identification of organic solvents and low-level quantification of water contamination. Talanta 2021, 223, 121865. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Vempatapu, B.P.; Kanaujia, P.K. Monitoring petroleum fuel adulteration: A review of analytical methods. Trends Anal. Chem. 2017, 92, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C.; Reniers, G.; Huang, L.; Kang, L.; Zhang, L. The future of hazardous chemical safety in china: Opportunities, problems, challenges and tasks. Sci. Total Environ. 2018, 643, 1–11. [Google Scholar] [CrossRef]
- Athar, M.; Mohd Shariff, A.; Buang, A.; Shuaib Shaikh, M.; Ishaq Khan, M. Review of process industry accidents analysis towards safety system improvement and sustainable process design. Chem. Eng. Technol. 2019, 42, 524–538. [Google Scholar] [CrossRef]
- Henretig, F.M.; Kirk, M.A.; McKay, C.A. Hazardous chemical emergencies and poisonings. N. Engl. J. Med. 2019, 380, 1638–1655. [Google Scholar] [CrossRef]
- Kriaa, S.; Pietre-Cambacedes, L.; Bouissou, M.; Halgand, Y. A survey of approaches combining safety and security for industrial control systems. Reliab. Eng. Syst. Saf. 2015, 139, 156–178. [Google Scholar] [CrossRef]
- Şennik, E.; Çolak, Z.; Kılınç, N.; Öztürk, Z.Z. Synthesis of highly-ordered TiO2 nanotubes for a hydrogen sensor. Int. J. Hydrog. Energy 2010, 35, 4420–4427. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Jin, Y.M.; Ouyang, H.; Zou, Y.; Wang, X.X.; Xie, L.X.; Li, Z. Flexible piezoelectric nanogenerator in wearable self-powered active sensor for respiration and healthcare monitoring. Semicond. Sci. Technol. 2017, 32, 064004. [Google Scholar] [CrossRef]
- Kiew, L.-V.; Chang, C.-Y.; Huang, S.-Y.; Wang, P.-W.; Heh, C.-H.; Liu, C.-T.; Cheng, C.-H.; Lu, Y.-X.; Chen, Y.-C.; Huang, Y.-X.; et al. Development of flexible electrochemical impedance spectroscopy-based biosensing platform for rapid screening of SARS-Cov-2 inhibitors. Biosens. Bioelectron. 2021, 183, 113213. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose nanowhisker modulated 3D hierarchical conductive structure of carbon black/natural rubber nanocomposites for liquid and strain sensing application. Compos. Sci. Technol. 2016, 124, 44. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Lengalova, A.; Svoboda, P.; Saha, P. Multi-wall carbon nanotube networks as potential resistive gas sensors for organic vapor detection. Carbon 2011, 49, 2499. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Hammock, M.L.; Appleton, A.L.; Schwartz, G.; Mei, J.; Lei, T.; Pei, J.; Bao, Z. Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 2014, 5, 2954. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, H.J.; Jung, G.Y.; Han, A.-R.; Kwak, S.K.; Kim, B.J.; Oh, J.H. Highly sensitive and selective liquid-phase sensors based on a solvent-resistant organic-transistor platform. Adv. Mater. 2015, 27, 1540. [Google Scholar] [CrossRef]
- Rozman, M.; Štukovnik, Z.; Sušnik, A.; Pakseresht, A.; Hočevar, M.; Drobne, D.; Bren, U. A HepG2 cell-based biosensor that uses stainless steel electrodes for hepatotoxin detection. Biosensors 2022, 12, 160. [Google Scholar] [CrossRef]
- Štukovnik, Z.; Bren, U.; Rozman, M. Model electrochemical biosensor for the detection of methanol in aqueous solutions with yeast cells. Acta Chim. Slov. 2021, 68, 773. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. Progress in Chemometrics Research; Pomerantsev, A.L., Ed.; Nova Science Publishers: New York, NY, USA, 2005; pp. 89–102. Available online: http://www.abc.chemistry.bsu.by/vi/analyser/ (accessed on 25 November 2022).
- Dielectric Conestant of Common Solvents. Available online: https://depts.washington.edu/eooptic/linkfiles/dielectric_chart%5B1%5D.pdf (accessed on 25 November 2022).
| Solvent | Working Electrode | [k/cm] |
|---|---|---|
| No exposure | uncoated | |
| EtOH-STF | 12,760 | |
| THF-STF | 10,680 | |
| Ethanol | uncoated | |
| EtOH-STF | ||
| THF-STF | ||
| THF | uncoated | |
| EtOH-STF | ||
| THF-STF |
| Solvent | Specific Conductivity [mS/cm] |
|---|---|
| stock electrolyte | 11.66 |
| electrolyte-water | 10.74 |
| electrolyte-EtOH | 7.84 |
| electrolyte-THF | 7.68 |
| Solvent | Working Electrode (WE) | Transmittance [%] |
|---|---|---|
| No exposure | EtOH-STF | |
| THF-STF | ||
| Ethanol | EtOH-STF | |
| THF-STF | ||
| THF | EtOH-STF | |
| THF-STF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman, M.; Lukšič, M. Stainless Steel Foil-Based Label-Free Modular Thin-Film Electrochemical Detector for Solvent Identification. Micromachines 2022, 13, 2256. https://doi.org/10.3390/mi13122256
Rozman M, Lukšič M. Stainless Steel Foil-Based Label-Free Modular Thin-Film Electrochemical Detector for Solvent Identification. Micromachines. 2022; 13(12):2256. https://doi.org/10.3390/mi13122256
Chicago/Turabian StyleRozman, Martin, and Miha Lukšič. 2022. "Stainless Steel Foil-Based Label-Free Modular Thin-Film Electrochemical Detector for Solvent Identification" Micromachines 13, no. 12: 2256. https://doi.org/10.3390/mi13122256
APA StyleRozman, M., & Lukšič, M. (2022). Stainless Steel Foil-Based Label-Free Modular Thin-Film Electrochemical Detector for Solvent Identification. Micromachines, 13(12), 2256. https://doi.org/10.3390/mi13122256





