Preparation, Characterization and Magneto-Optical Properties of Sm-Doped Y2O3 Polycrystalline Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Sample Preparation
3. Results and Discussion
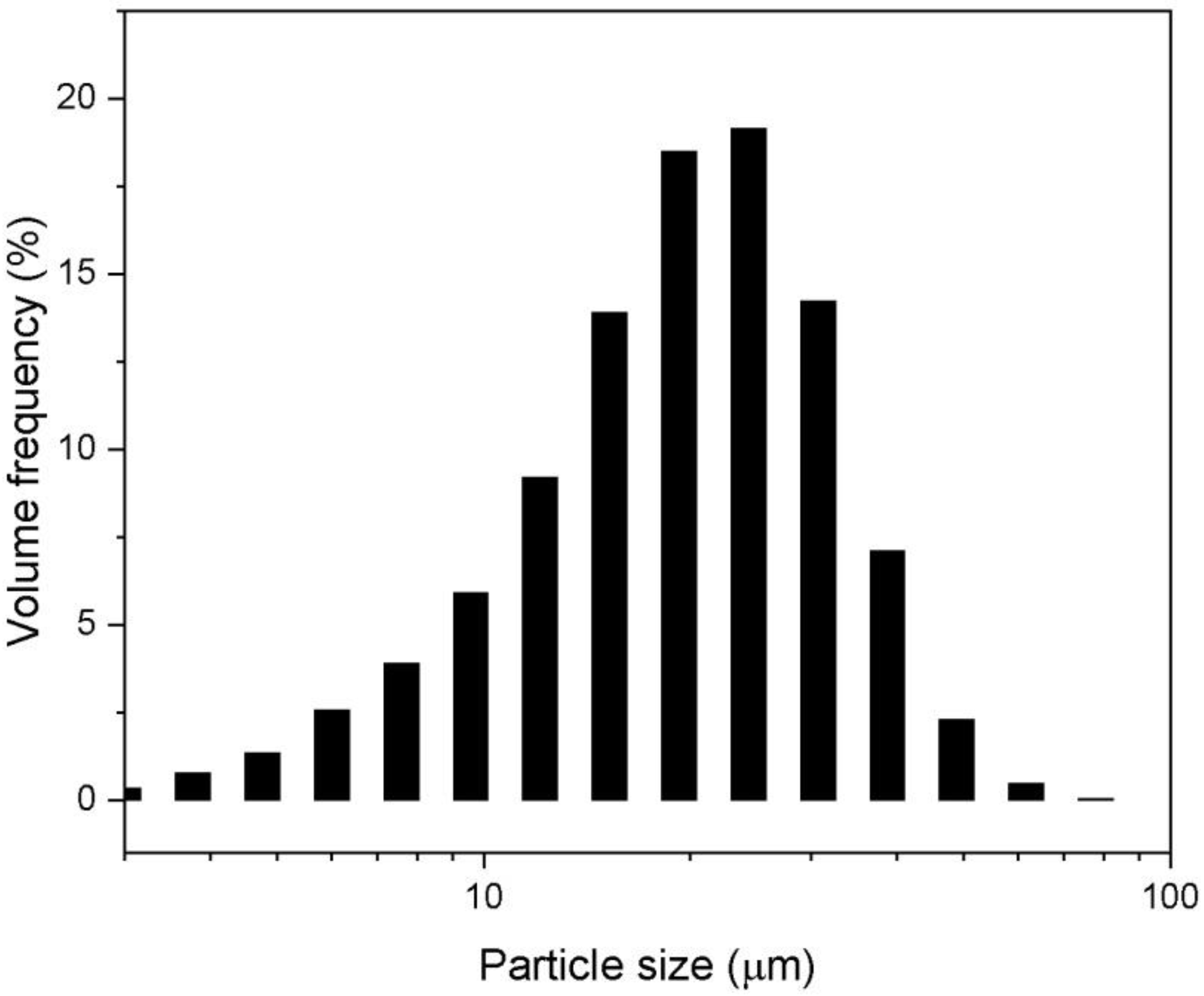
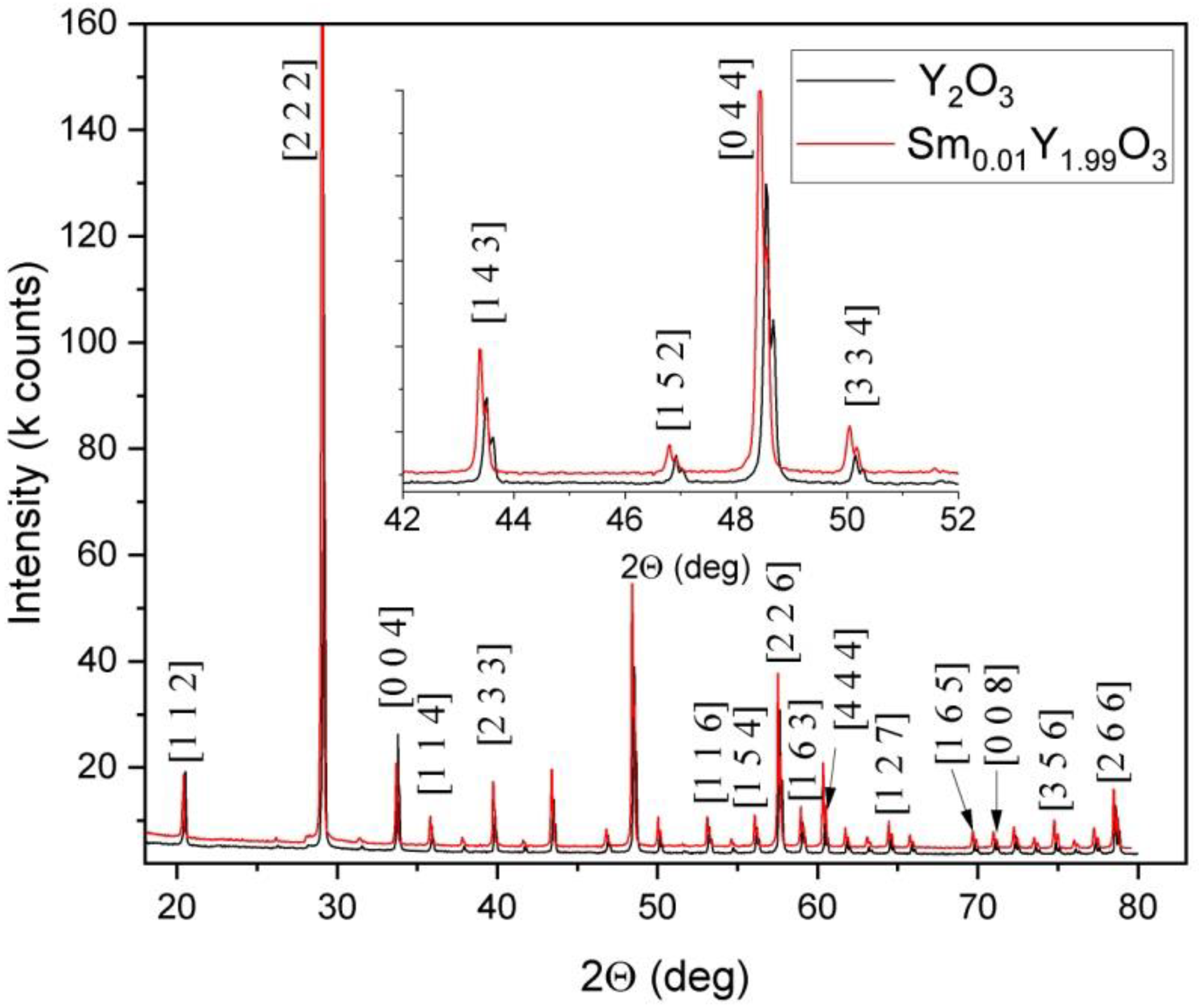
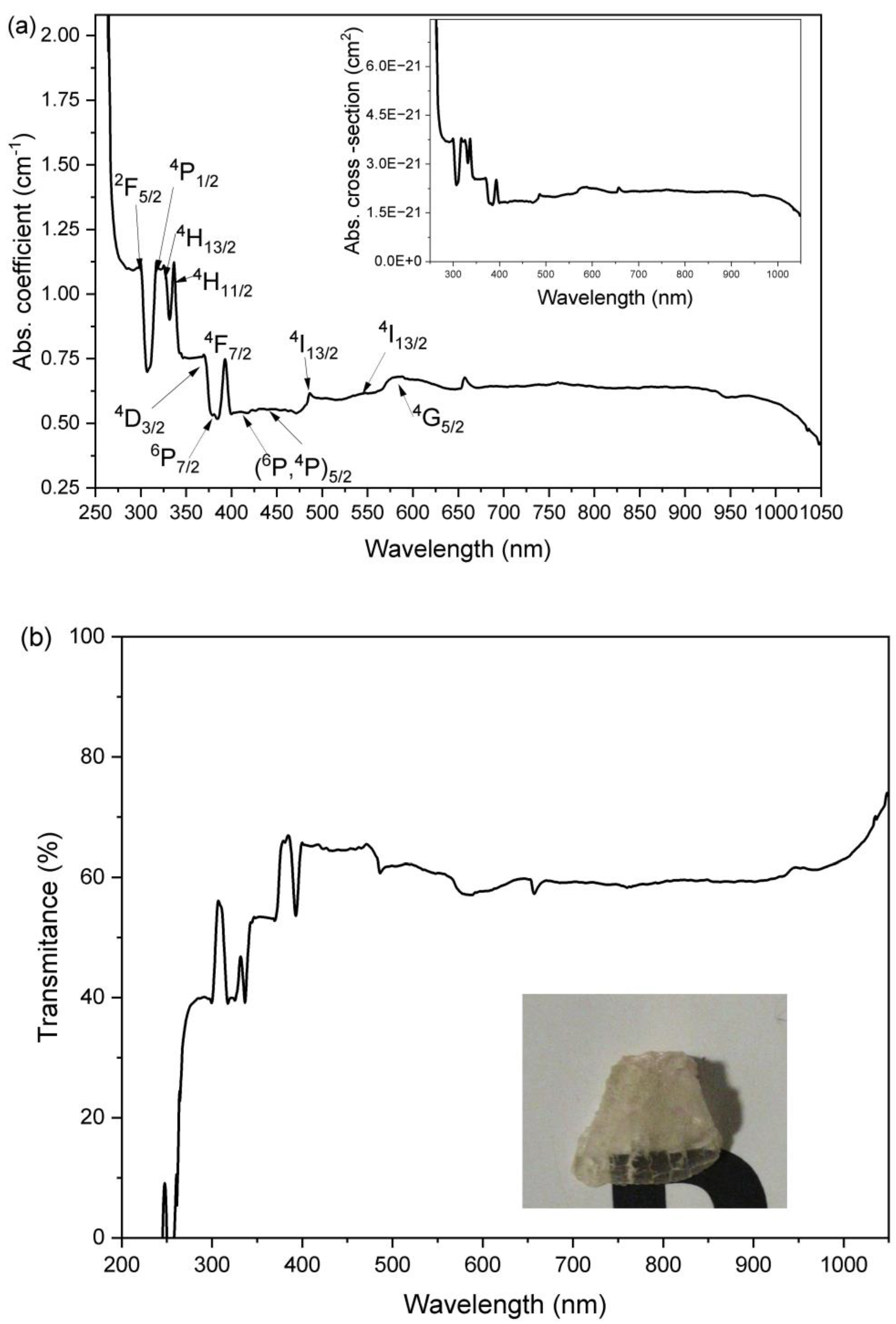
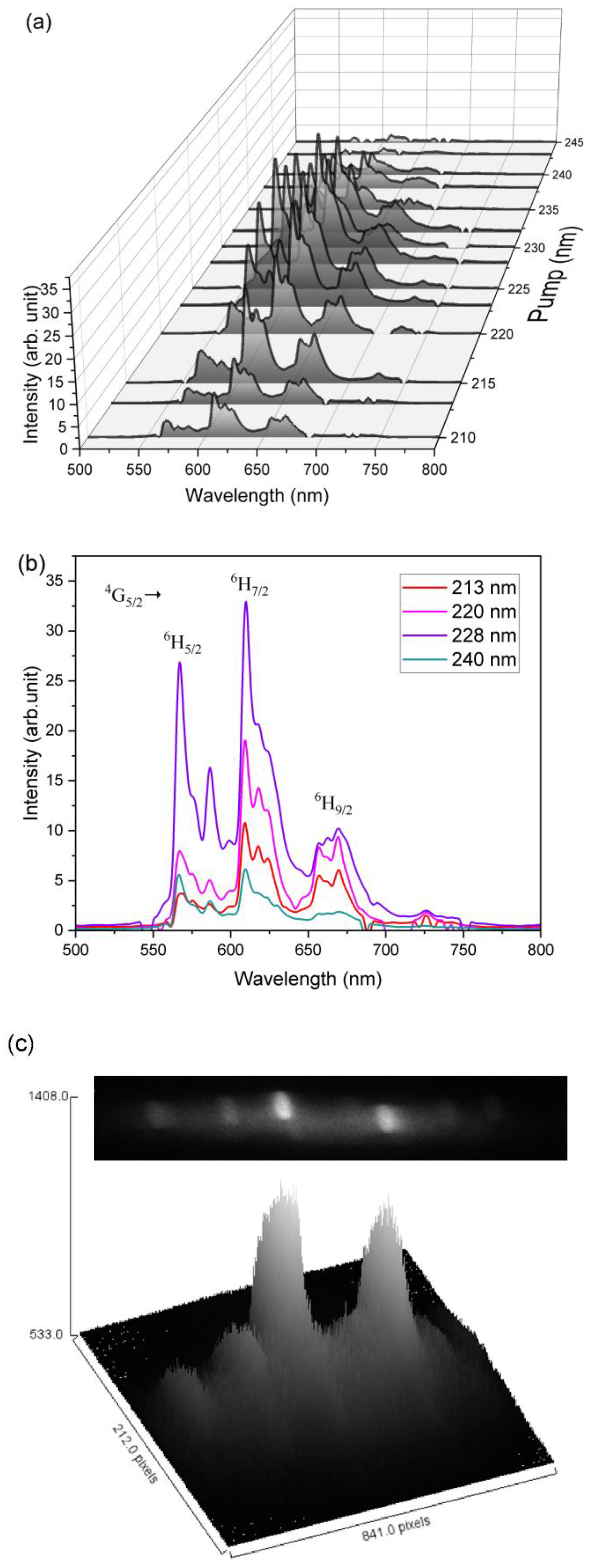
3.1. Powder Characteristics
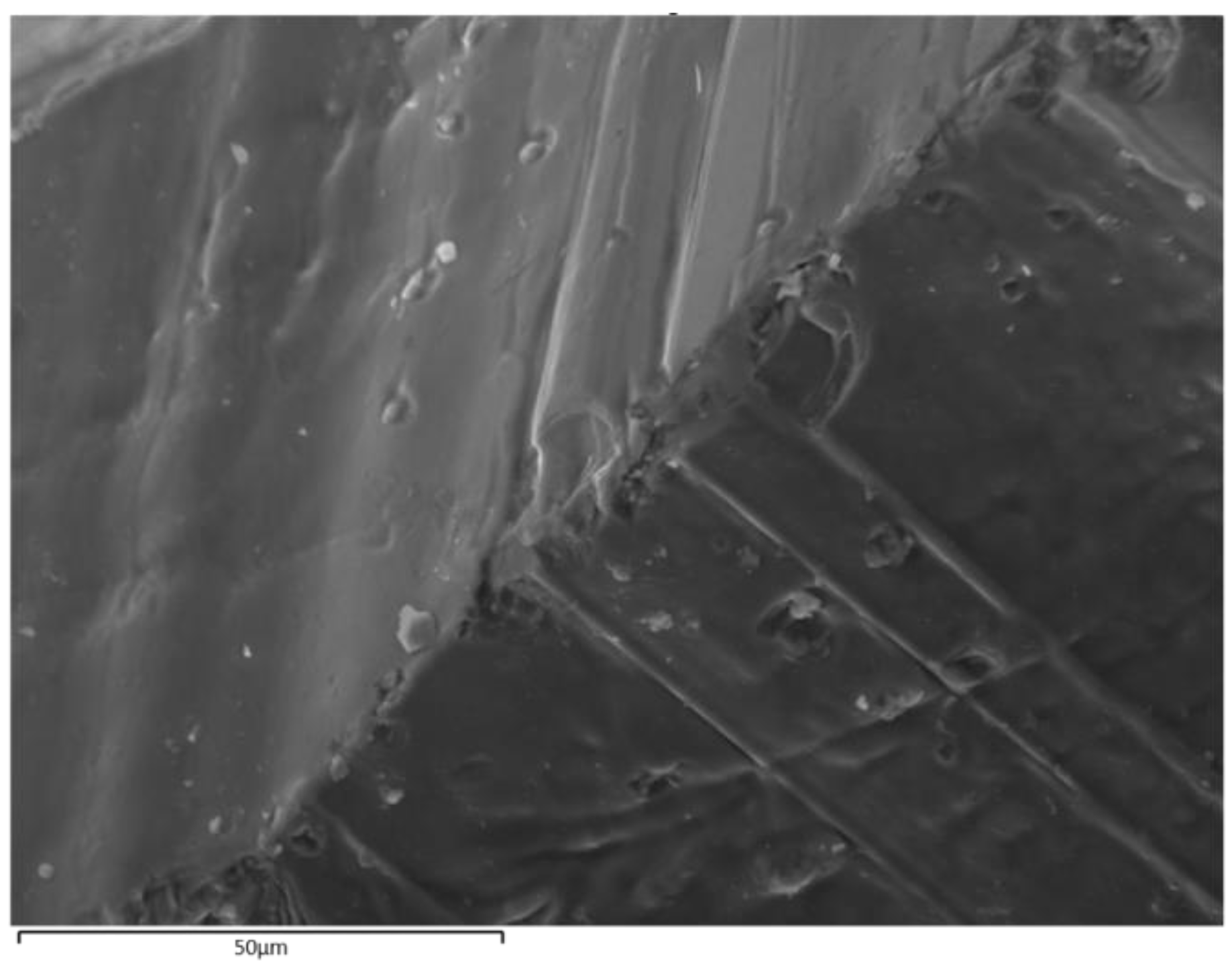
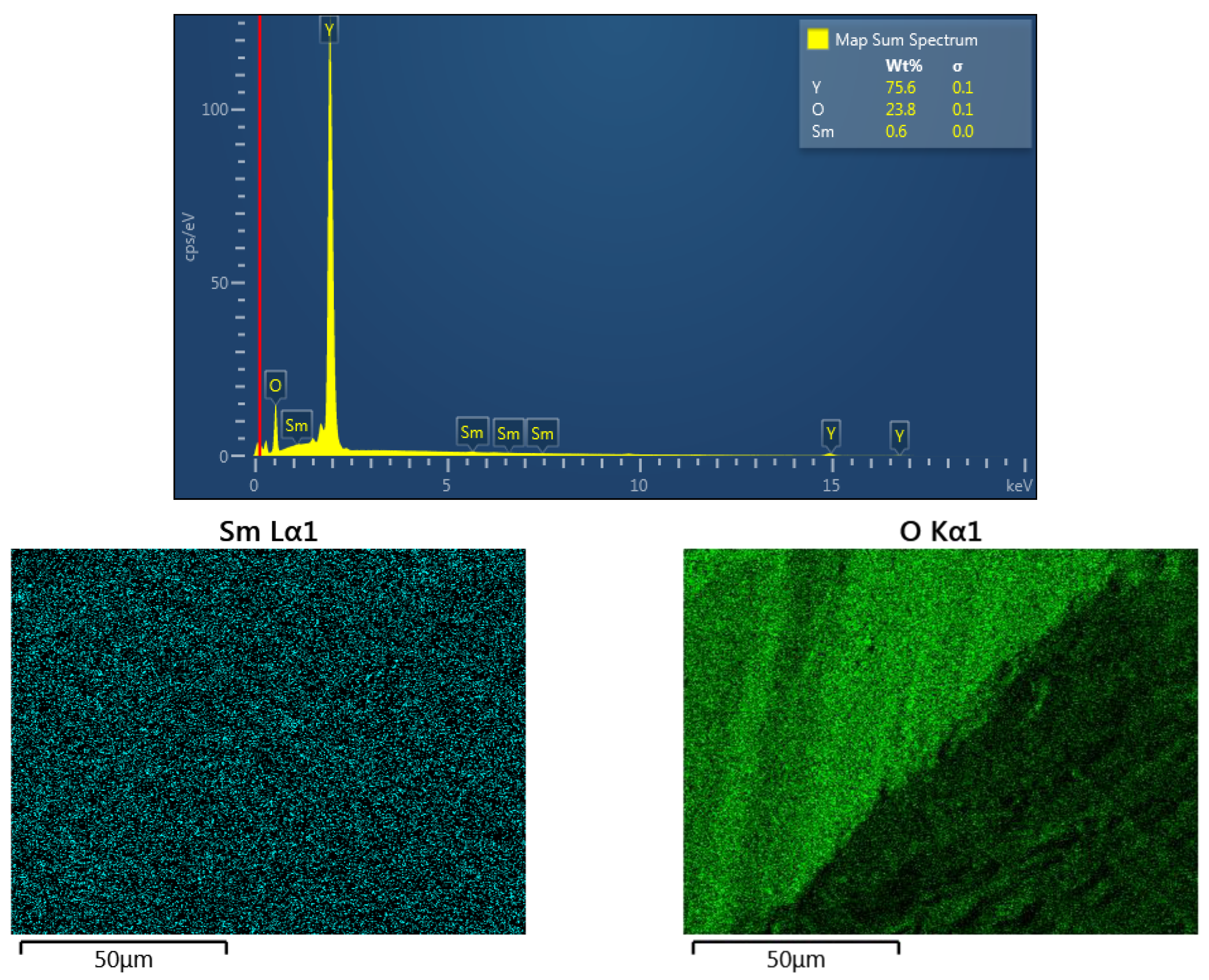
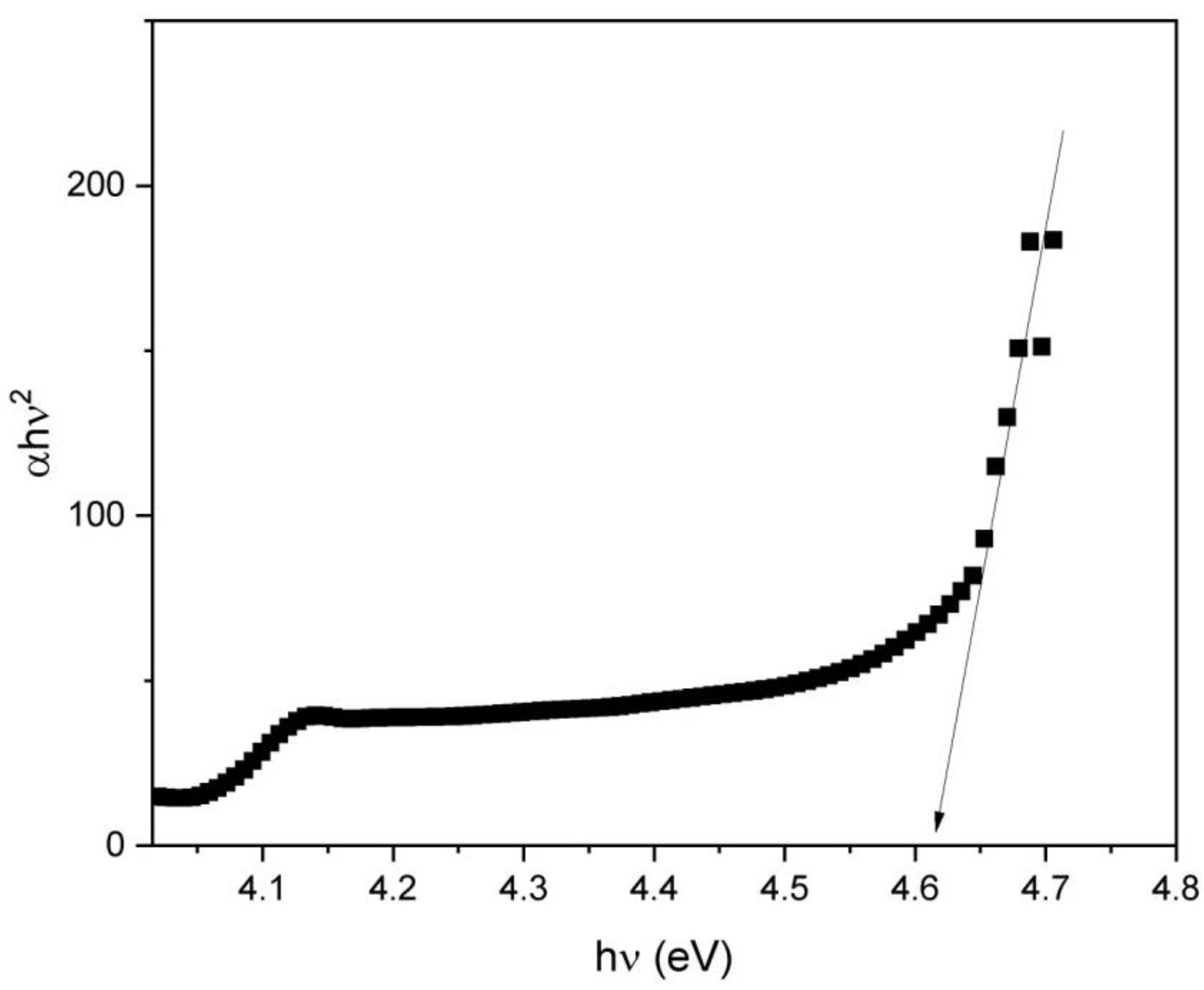
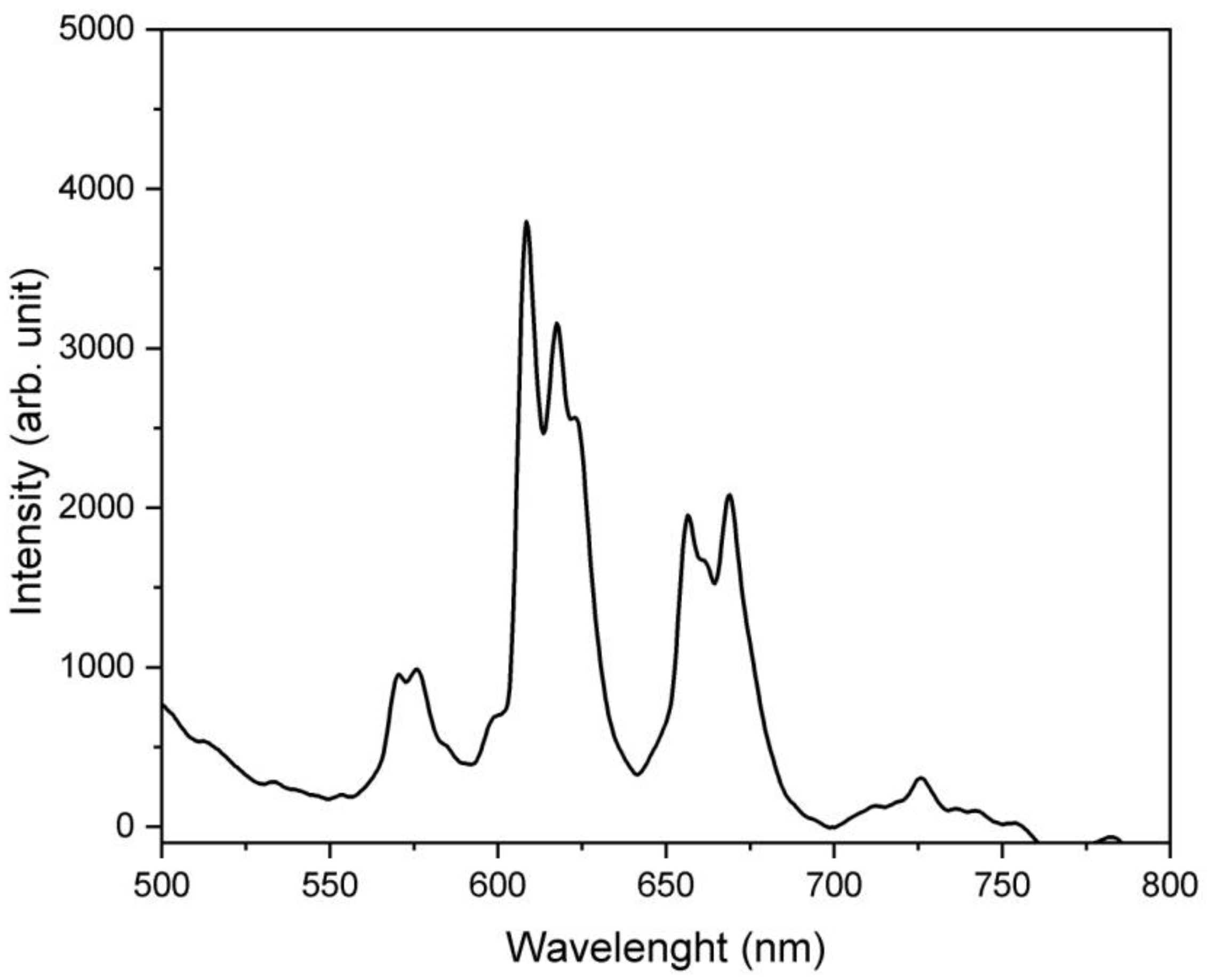
3.2. Bulk Sample Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hibino, Y.; Koyama, T.; Sumi, S.; Awano, H.; Miwa, K.; Ono, S.; Kohda, M.; Chiba, D. Control of magnetism by electrical charge doping or redox reactions in a surfaceoxidized Co thin film with a solid-state capacitor structure. Appl. Phys. Express. 2017, 10. [Google Scholar]
- Wang, H.; Jin, Z.; Liu, X.; Zhang, Z.; Lin, X.; Cheng, Z.; Ma, G. Photoinduced ultrafast optical anisotropy encountered by spin-flip transition in La0.67Ca0.33MnO3. Appl. Phys. Lett. 2017, 110, 252407. [Google Scholar] [CrossRef]
- Musorin, A.I.; Sharipova, M.I.; Dolgova, T.V.; Inoue, M.; Fedyanin, A.A. Ultrafast faraday rotation of slow light. Phys. Rev. Appl. 2016, 6, 024012. [Google Scholar] [CrossRef]
- Lvovsky, A.I.; Sanders, B.C.; Tittel, W. Optical quantum memory. Nat. Photonics 2009, 3, 706–714. [Google Scholar] [CrossRef]
- Savukov, I.M.; Lee, S.-K.; Romalis, M.V. Optical detection of liquid-state NMR. Nature 2006, 442, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-K.; Chiu, C.-Y.; Morris, S.M.; Tsai, M.-C.; Chen, C.-C.; Cheng, K.-T. Optically controllable linear-polarization rotator using chiral-azobenzene-doped liquid crystals. Materials 2017, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Zvezdin, A.K.; Kotov, V.A. Modern Magnetooptics and Magnetooptical Materials; Taylor & Francis: Abingdon-on-Thames, UK, 1997; ISBN 075030362X. [Google Scholar]
- Xu, Y.-N.; Gu, Z.-q.; Ching, W.Y. Electronic, structural, and optical properties of crystalline yttria. Phys. Rev. 1997, B56, 14993. [Google Scholar] [CrossRef]
- Biljan, T.; Gajović, A.; Meić, Z.; Meštrović, E. Preparation, characterization and luminescence of nanocrystalline Y2O3:Ho. J. Alloys Compd. 2007, 431, 217–220. [Google Scholar] [CrossRef]
- Kruk, A. Structural and magneto-optical characterization of La, Nd:Y2O3 Powders obtained via a Modified EDTA Sol-Gel Process and HIP-Treated ceramics. Materials 2020, 13, 4928. [Google Scholar] [CrossRef]
- Douillard, L.; Gautier, M.; Thromat, N.; Henriot, M.; Guittet, M.J. Local electronic structure of Ce-doped Y203. An XPS and XAS study. Phys. Rev. B 1994, 49, 16171–16180. [Google Scholar] [CrossRef]
- Tropf, W.J.; Thomas, M.E. Handbook of Optical Constants of Solids; Elsevier Inc.: Amsterdam, The Netherlands, 1998; Volume 2. [Google Scholar]
- Zhang, L.; Pan, W. Structural and thermo-mechanical properties of Nd:Y2O3 transparent ceramics. J. Am. Ceram. Soc. 2015, 98, 3326–3331. [Google Scholar] [CrossRef]
- Rodionov, E.; Shmidko, I.; Zolotovsky, A.; Kruchinin, S. Electroluminescence of Y2O3:Eu and Y2O3:Sm films. Mater. Sci. Pol. 2013, 232–239. [Google Scholar] [CrossRef]
- Kruk, A.; Wajler, A.; Bobruk, M.; Adamczyk, A.; Mrózek, M.; Gawlik, W.; Brylewski, T. Preparation of yttria powders co-doped with Nd3+, and La3+ using EDTA gel processes for application in transparent ceramics. J. Eur. Ceram. Soc. 2017, 37, 4139–4140. [Google Scholar] [CrossRef]
- Martel, J.F.; Jandl, S.; Viana, B.; Vivien, D. Crystal-field study of Sm3+ ions in Sm2O3, Sm3+:Gd2O3 and Sm3+:Y203. J. Phys. Chem. Solids 2000, 61, 1455–1463. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, X.; Wang, J.; Liu, P.; Li, D.; Wang, X.; An, L.; Zhang, J.; Xu, J.; Tang, D. Spark plasma sintering of Sm3+ doped Y2O3 transparent ceramics for visible light lasers. Ceram. Int. 2017, 43, 12057–12060. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Q.; Ren, B.; You, L.; Sun, Y. Facile synthesis and luminescence properties of Y2O3:Ln(3+) (Ln(3+) = Eu(3+), Tb(3+), Dy(3+), Sm(3+), Er(3+), Ho(3+), Tm(3+), Yb(3+)/Er(3+), Yb(3+)/Tm(3+), Yb(3+)/Ho(3+)) microspheres. J. Nanosci. Nanotechnol. 2014, 14, 5781–5789. [Google Scholar] [CrossRef]
- Dupont, A.; Parent, C.; Garrec, B.L.; Heintz, J.M. Size and morphology control of Y2O3 nanopowders via a solgel route. J. Solid State Chem. 2003, 171, 152–160. [Google Scholar] [CrossRef]
- Back, M.; Massari, A.; Boffelli, M.; Gonella, F.; Riello, P.; Cristofori, D.; Riccò, R.; Enrichi, F. Optical investigation of Tb3+-doped Y2O3 nanocrystals prepared by Pechini-type sol–gel process. J. Nanopart. Res. 2012, 792, 10. [Google Scholar] [CrossRef]
- Ramasamy, S.; Yogamalar, R.; Bose, A.C. Structural and optical studies of yttrium oxide nanoparticles synthesized by Co-Precipitation method. Mater. Res. Bull. 2010, 45, 1165–1170. [Google Scholar]
- Mangalaraja, R.V.; Mouzon, J.; Hedström, P.; Kero, I.; Ramam, K.V.S.; Camurri, C.P.; Odén, M. Combustion synthesis of Y2O3 and Yb–Y2O3: Part, I. Nanopowders Charact. 2008, 208, 415–422. [Google Scholar]
- Tanner, P.A.; Fu, L. Morphology of Y2O3:Eu3+ prepared by hydrothermal synthesis. Chem. Phys. Lett. 2009, 470, 75–79. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Zuo, H. Interactions between Y2O3–Al mixture studied by solid-state reaction method. Vacuum 2013, 87, 7–10. [Google Scholar]
- Ikesue, A.; Kamata, K.; Yoshida, K. Synthesis of transparent Nd-doped HfO2- Y2O3 ceramics using HIP. J. Am. Ceram. Soc. 1996, 79, 359–364. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Pan, W. Vacuum sintering of transparent Cr: Y2O3 ceramics. Ceram. Int. 2015, 41, 8755–8760. [Google Scholar] [CrossRef]
- Li, X.; Xia, X.; Xu, H.; Zhong, S.; He, D. He High-temperature high pressure synthesis of monoclinic Y2O3. Mater. Lett. 2018, 239, 82–85. [Google Scholar] [CrossRef]
- Ghaderi, M.; Razavi, R.S.; Loghman-Estarki, M.; Ghorbani, S. Spark plasma sintering of transparent Y2O3 ceramic using hydrothermal synthesized nanopowders. Ceram. Int. 2016, 42, 14403–14410. [Google Scholar] [CrossRef]
- Kruk, A. Optical and structural properties of arc melted Ce or Pr –doped Y2O3 transparent ceramics. Ceram. Int. 2017, 43, 16909–16914. [Google Scholar] [CrossRef]
- Kruk, A. Fabrication of MgO high transparent ceramics by arc plasma synthesis. Opt. Mater. 2018, 84, 360–366. [Google Scholar] [CrossRef]
- Kruk, A.; Madej, D. Structural properties and Faraday effect of arc melted magnesia transparent polycrystal. Opt. Mater. 2020, 108, 110245. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Liu, Z.; Li, X.; Tian, F.; Zhu, D.; Yang, Z.; Wu, L.; Li, J. Fabrication of Dy2O3 transparent ceramics by vacuum sintering using precipitated powders. Magnetochemistry 2021, 7, 6. [Google Scholar] [CrossRef]
- Feng, S.; Qin, H.; Wu, G.; Jiang, H.; Zhao, J.; Liu, Y.; Luo, Z.; Qiao, J.; Jiang, J. Spectrum regulation of YAG: Ce transparent ceramics with Pr, Cr doping for white light emitting diodes application. J. Eur. Ceram. Soc. 2017, 37, 10. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, B.-N.; Jang, B.-K. Fabrication of transparent Y2O3 ceramics by two-step spark plasma sintering. J. Am. Ceram. Soc. 2021, 104, 5501–5508. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, H.; Huo, D.; Tan, W. Room-temperature magnetoresistive and magnetocaloric effect in La1−xBaxMnO3 compounds: Role of Griffiths phase with ferromagnetic metal cluster above Curie temperature. J. Appl. Phys. 2022, 131, 043901. [Google Scholar] [CrossRef]
- Snetkov, I.L.; Permin, D.A.; Balabanov, S.S.; Palashov, O.V. Wavelength dependence of Verdet constant of Tb3+:Y2O3 ceramics. Appl. Phys. Lett. 2016, 108, 161905. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Youvanidha, A.; Vidhya, B.; Nelson, P.I. Investigation on the structural, optical and electrical properties of ZnO-Y2O3 (YZO) thin films prepared by PLD for TCO layer applications. AIP Conf. Proc. 2019, 2166, 020023. [Google Scholar] [CrossRef]
- Kruk, A.; Jany, B.R.; Owczarczyk, K.; Madej, D. On the possibility of using arc plasma melting technique in preparation of transparent yttria ceramics. Opt. Appl. 2019, 49, 355–364. [Google Scholar]
- Bersons, I.; Veilande, R.; Balcers, O. Reflection and refraction of photons. Phys. Scr. 2022, 97, 3. [Google Scholar] [CrossRef]
- Mie, G. Beitrage zur optik truber medien, speziell kolloidaler metallosungen. Ann. Der Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, W.; Feng, J. Dependence of spectroscopic and thermal properties on concentration and temperature for Yb:Y2O3 transparent ceramics. J. Eur. Ceram. Soc. 2015, 35, 2547–2554. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic Energy Levels in the Trivalent Lanthanide Aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Bakovets, V.V.; Yushina, I.V.; Antonova, O.V.; Pomelova, T. Correction of the band gap of Y2O3:Eu3+ phosphor. Opt. Spectrosc. 2016, 121, 862–866. [Google Scholar] [CrossRef]
- Quah, H.J.; Cheong, K.Y. Effects of post-deposition annealing ambient on band alignment of RF magnetron-sputtered Y2O3 film on gallium nitrid. Nanoscale Res. Lett. 2013, 8, 53. [Google Scholar] [CrossRef]
- Som, S.; Sharma, S.; Lochab, S. Ion induced modification of bandgap and CIE parameters in Y2O3:Dy3+ phosphor. Ceram. Int. 2013, 39, 7693–7701. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Electronic Oxide Polarizability and Optical Basicity of Simple Oxide. J. Appl. Phys. 1996, 79, 1736–1740. [Google Scholar] [CrossRef]
- Nigara, Y. Measurement of the optical constants of yttrium oxide. Jpn. J. Appl. Phys. 1968, 7, 404–408. [Google Scholar] [CrossRef]
- Yamaga, M.; Uno, H.; Tsuda, S.-I.; Wells, J.-P.R.; Han, T.P. Resonant energy transfer and cross relaxation between Sm3+ ions in LiYF4 crystals. J. Lumin. 2012, 132, 1608–1617. [Google Scholar] [CrossRef]
- Kruk, A.; Brylewski, T.; Mrózek, M. Optical and magneto-optical properties of Nd0.1La0.1Y1.8O3 transparent ceramics. J. Lumin. 2019, 209, 333–339. [Google Scholar] [CrossRef]
- Cheng, H.; Lu, B.; Liu, Y.; Zhao, Y.; Sakka, Y.; Li, J.-G. Transparent magneto-optical Ho2O3 ceramics: Role of self-reactive resultant oxyfluoride additive and investigation of vacuum sintering kinetics. Ceram. Int. 2019, 45, 14761–14767. [Google Scholar] [CrossRef]
- Wang, M.; Lu, B.; Li, H. Fantastic valence impacts of Pr on microstructures and Faraday magneto-optical effects of transparent (Ho,Pr)2O3 ceramics. J. Eur. Ceram. Soc. 2021, 41, 5258–5263. [Google Scholar] [CrossRef]
- Snetkov, I.; Palashov, O. Cryogenic temperature characteristics of Verdet constant of terbium sesquioxide ceramics. Opt. Mater. 2016, 62, 697–700. [Google Scholar] [CrossRef]
- Zhuang, N.; Song, C.; Guo, L.; Wang, R.; Hu, X.; Zhao, B.; Lin, S.; Chen, J. Growth of terbium gallium garnet (TGG) magneto-optic crystals by edge-defined film-fed growth method. J. Cryst. Growth 2013, 381, 27–32. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Z. Magneto-optical glass mixed with Tb3+ ions: High Verdet constant and luminescence properties. J. Lumin. 2021, 231, 117804. [Google Scholar] [CrossRef]
- Chen, Q.L.; Zhang, M.; Wang, H.; Wang, Q.W.; Ma, Q.H.; Li, J. Structures and magneto optical property of diamagnetic TiO2-TeO2-PbO-B2O3 glass. J. Non-Cryst. Solids 2017, 468, 58–66. [Google Scholar] [CrossRef]
- Chen, Q.; Su, K.; Wang, H.; Wang, Q. Multiferroic BiFeO3 enhanced Faraday rotation effect in magneto optical glasses. J. Non-Cryst. Solids 2018, 495, 75–84. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, H.; Xiao, X.; Wang, P.; Cui, X.; Lu, M.; Lin, C.; Dai, S.; Peng, B. High Verdet constants and diamagnetic responses of GeS2-In2S3-PbI2 chalcogenide glasses for integrated optics applications. Opt. Express 2017, 25, 20410–20420. [Google Scholar] [CrossRef]
- Yu, J.B.; Yi, G.; Zhang, Q.; Luo, Z.W.; Lu, A.X. A new type of NiO-doped phosphate glass with excellent faraday effects. Mater. Lett. 2018, 212, 25–27. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Hang, Y.; Wang, X. Effective development of Faraday rotator based on the highly transparent Tb2.96Pr0.04Ga5O12 single crystal. Appl. Phys. B 2016, 122, 61. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Hang, Y.; Wang, X. Improving characteristic of Faraday effect based on the Tm3+ doped terbium gallium garnet single crystal. J. Alloys Compd. 2016, 661, 62–65. [Google Scholar] [CrossRef]
- Lu, B.; Cheng, H.M.; Xu, X.X.; Chen, H.B. Preparation and characterization of transparent magneto-optical Ho2O3 ceramics. J. Am. Ceram. Soc. 2019, 102. [Google Scholar]
- Snetkov, I.L.; Yakovlev, A.I.; Permin, D.A.; Balabanov, S.S.; Palashov, O.V. Magneto-optical Faraday effect in dysprosium oxide (Dy2O3) based ceramics obtained by vacuum sintering. Opt. Lett. 2018, 43, 4041–4044. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.A.; Ikesue, R. Magneto-optic pyrochlore ceramics of Tb2Hf2O7 for Faraday rotator. Opt. Express 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Tokita, S.; Kawanaka, J.; Kawashima, T.; Kan, H.; Yagi, H.; Nozawa, H.; Yanagitani, T.; Fujimoto, Y.; Yoshida, H.; et al. Cryogenic temperature characteristics of Verdet constant on terbium gallium garnet ceramics. Opt. Express 2007, 15, 11255–11261. [Google Scholar] [CrossRef]
- Ivanov, I.; Karimov, D.; Snetkov, I.; Palashov, O.; Kochurikhin, V.; Masalov, A.; Fedorov, V.; Ksenofontov, D.; Kabalov, Y. Study of the influence of Tb-Sc-Al garnet crystal composition on Verdet constant. Opt. Mater. 2017, 66, 106–109. [Google Scholar] [CrossRef]

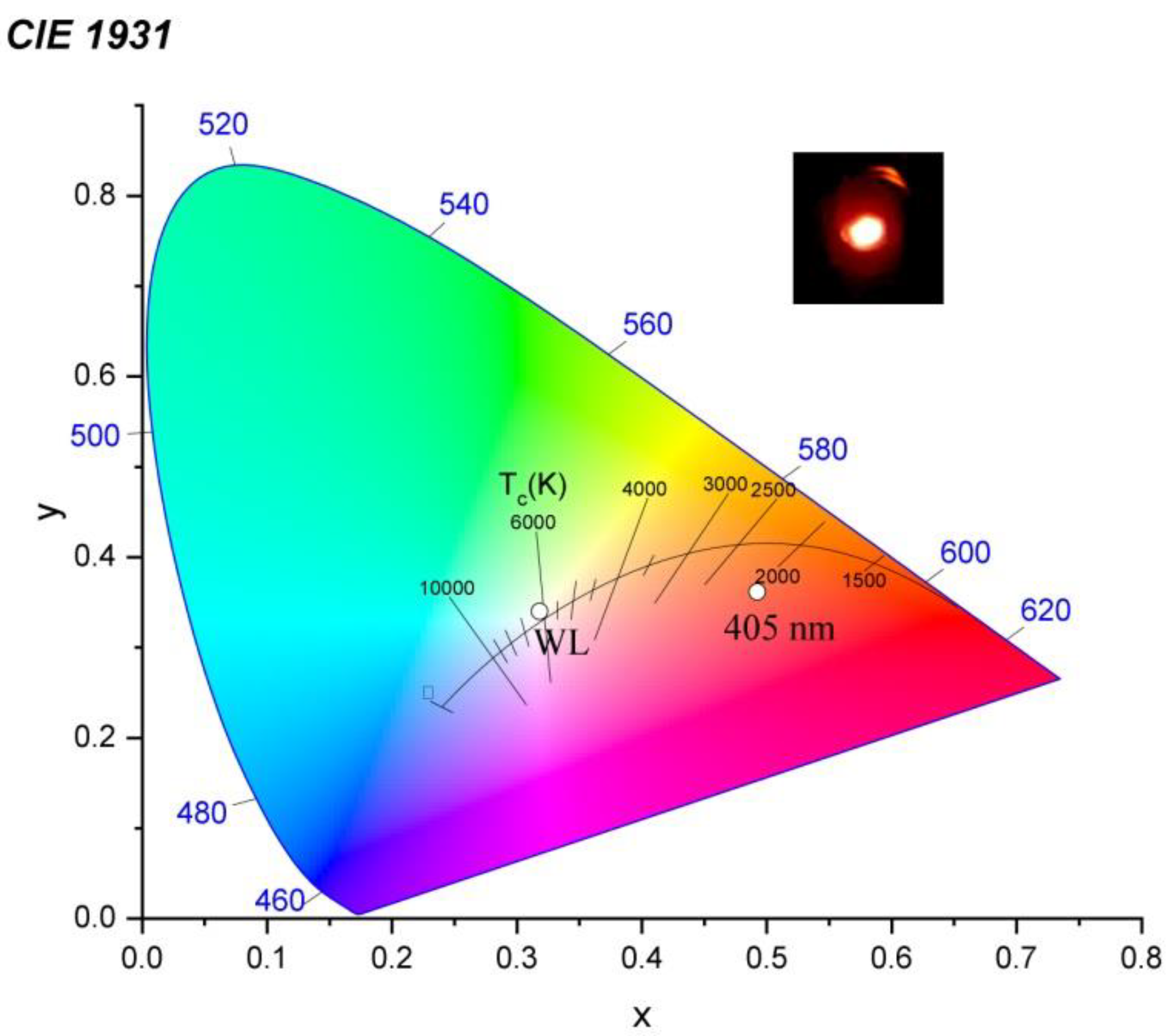
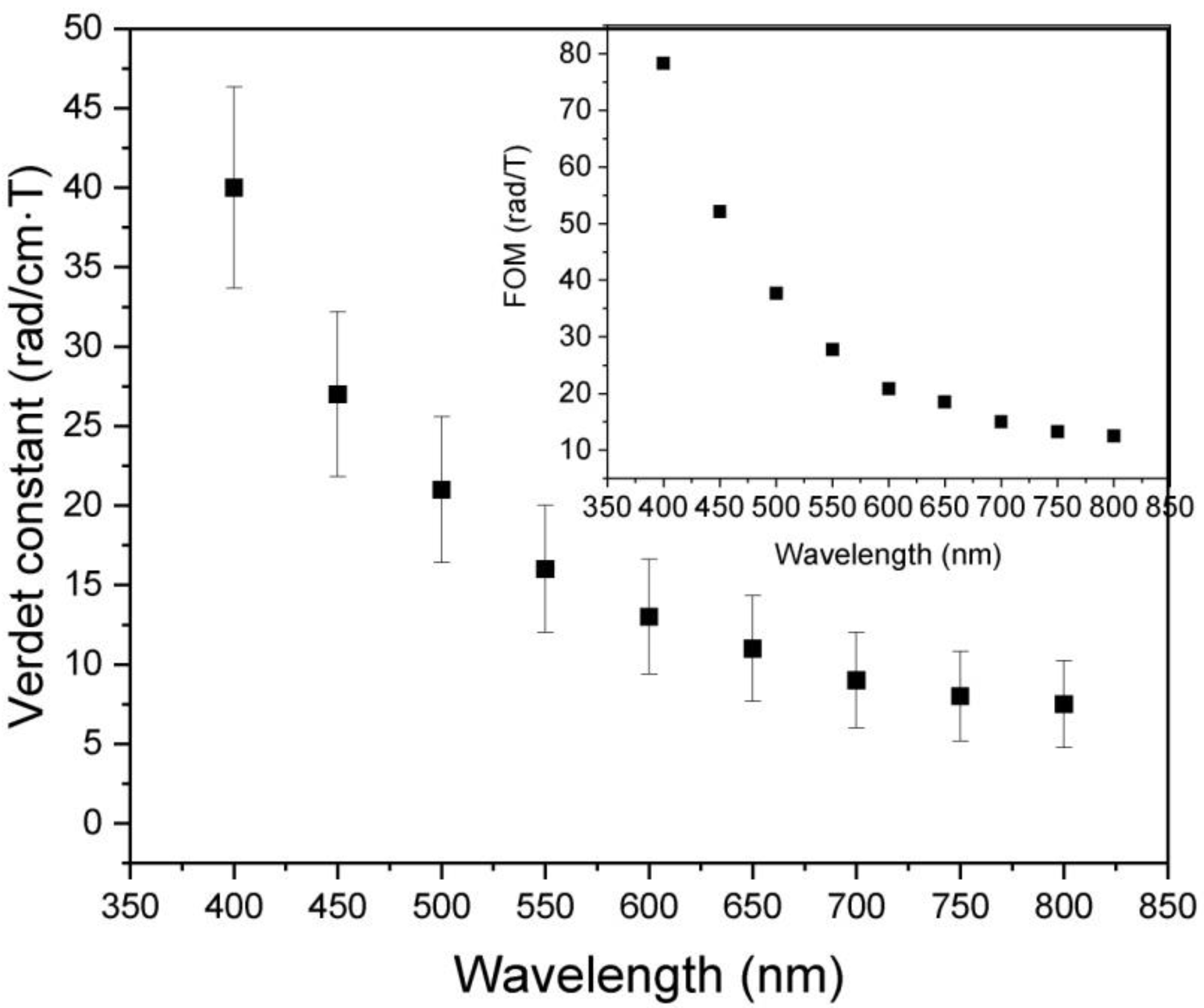
| Y2O3 | Sm0.01Y1.99O3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| d222 [nm] | A [Å] | V [Å3] | Dxrd [nm] | ρ [1014 m−2] | d222 [nm] | A [Å] | V [Å3] | Dxrd [nm] | ρ [1014 m2] |
| 27.01 | 10.6 | 1191.02 | 15.05 | 4.14 | 31.2 | 10.62 | 1197.77 | 28.62 | 12.2 |
| Sample | State | Verdet Constant | Ref | ||
|---|---|---|---|---|---|
| Ho2O3 | ceramic | −180 rad/Tm (632.8 nm) | −46 rad/Tm (1064nm) | 20 rad/Tm (1550 nm) | [63] |
| (Ho0.9Pr0.1)2O3 | ceramic | −235 rad/Tm (635 nm) | −82 rad/Tm (1064 nm) | -36 rad/Tm (1550 nm) | [53] |
| (Dy0.9Y0.05La0.05)2O3 | ceramic | 886 rad/Tm (405 nm) | 444 rad/Tm (532 nm) | 101 rad/Tm (1064 nm) | [64] |
| Tb2O3 | ceramic | ~480 rad/Tm (632nm) | ~130 rad/Tm (1075 nm) | ~40 rad/Tm (1561nm) | [36] |
| 30%Tb2O3:Y2O3 | ceramic | ~220 rad/Tm (632nm) | ~42 rad/Tm (1075 nm) | ~17 rad/Tm (1561nm) | [36] |
| Tb2Hf2O7 | ceramic | ~270 rad/Tm (532nm) | ~150 rad/Tm (650 nm) | 50 rad/Tm (1064 nm) | [65] |
| T40 | glass | −158 rad/(T⋅m) (633 nm) | − 48.87 rad/Tm (1064 nm) | [56] | |
| 15%TiO2 | glass | ~0.225 min/G·cm (532 nm) | ~0.173 min/G·cm (632 nm) | ~0.168 min/G·cm (650 nm) | [57] |
| TGG | monocrystalline | ~1.2 min/G·cm (460 nm) | ~0.75 min/G·cm (532 nm) | ~0.375 min/G·cm (695 nm) | [66] |
| TSAG | monocrystalline | ~2 min/Oe·cm (405 nm) | ~1 min/Oe·cm (500 nm) | ~0.2 min/Oe·cm (500 nm) | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, A.; Ziewiec, K. Preparation, Characterization and Magneto-Optical Properties of Sm-Doped Y2O3 Polycrystalline Material. Micromachines 2022, 13, 2254. https://doi.org/10.3390/mi13122254
Kruk A, Ziewiec K. Preparation, Characterization and Magneto-Optical Properties of Sm-Doped Y2O3 Polycrystalline Material. Micromachines. 2022; 13(12):2254. https://doi.org/10.3390/mi13122254
Chicago/Turabian StyleKruk, Andrzej, and Krzysztof Ziewiec. 2022. "Preparation, Characterization and Magneto-Optical Properties of Sm-Doped Y2O3 Polycrystalline Material" Micromachines 13, no. 12: 2254. https://doi.org/10.3390/mi13122254
APA StyleKruk, A., & Ziewiec, K. (2022). Preparation, Characterization and Magneto-Optical Properties of Sm-Doped Y2O3 Polycrystalline Material. Micromachines, 13(12), 2254. https://doi.org/10.3390/mi13122254






