Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms
Abstract
1. Introduction
2. Materials and Methods
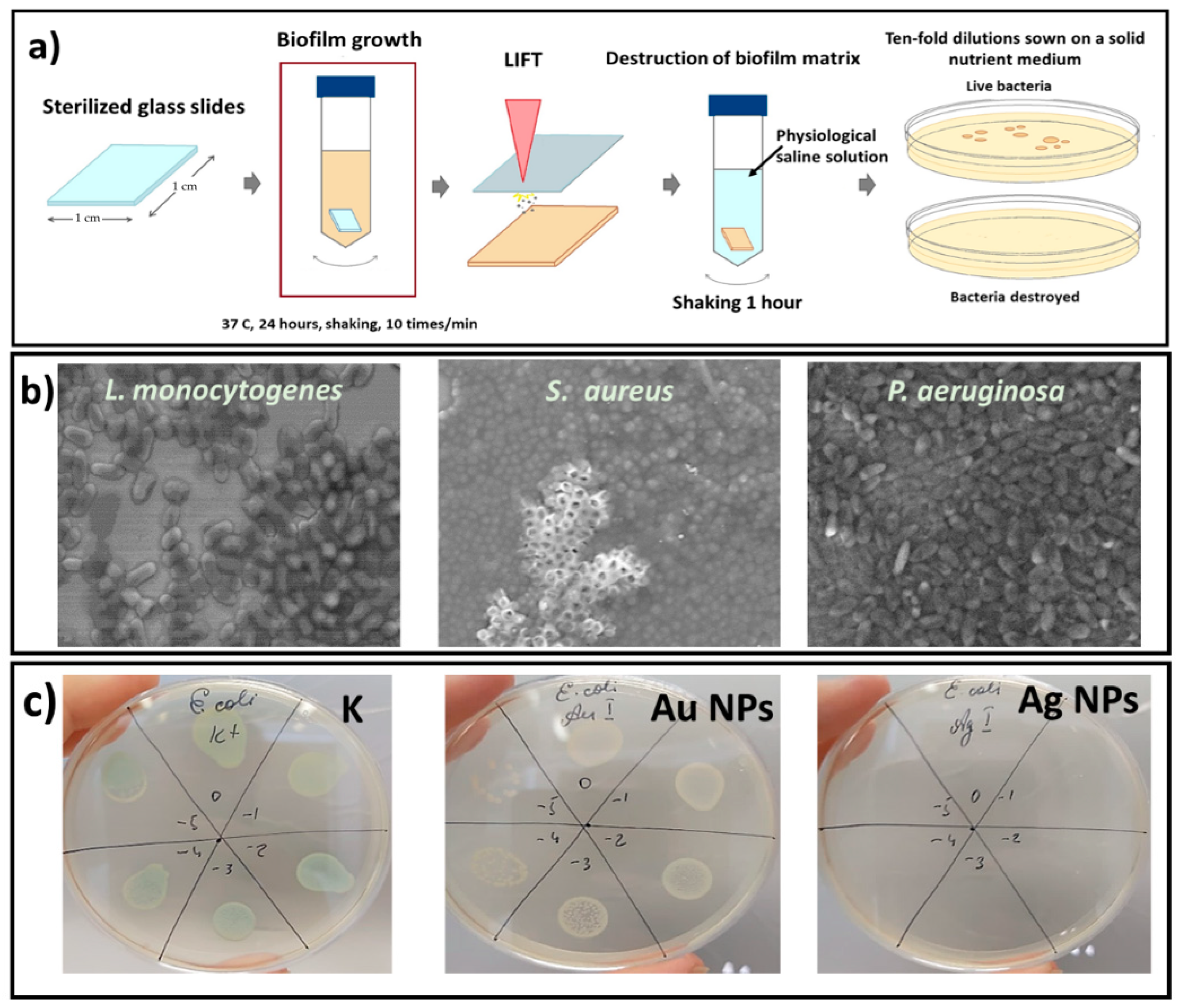
2.1. Materials and Bacterial Strains
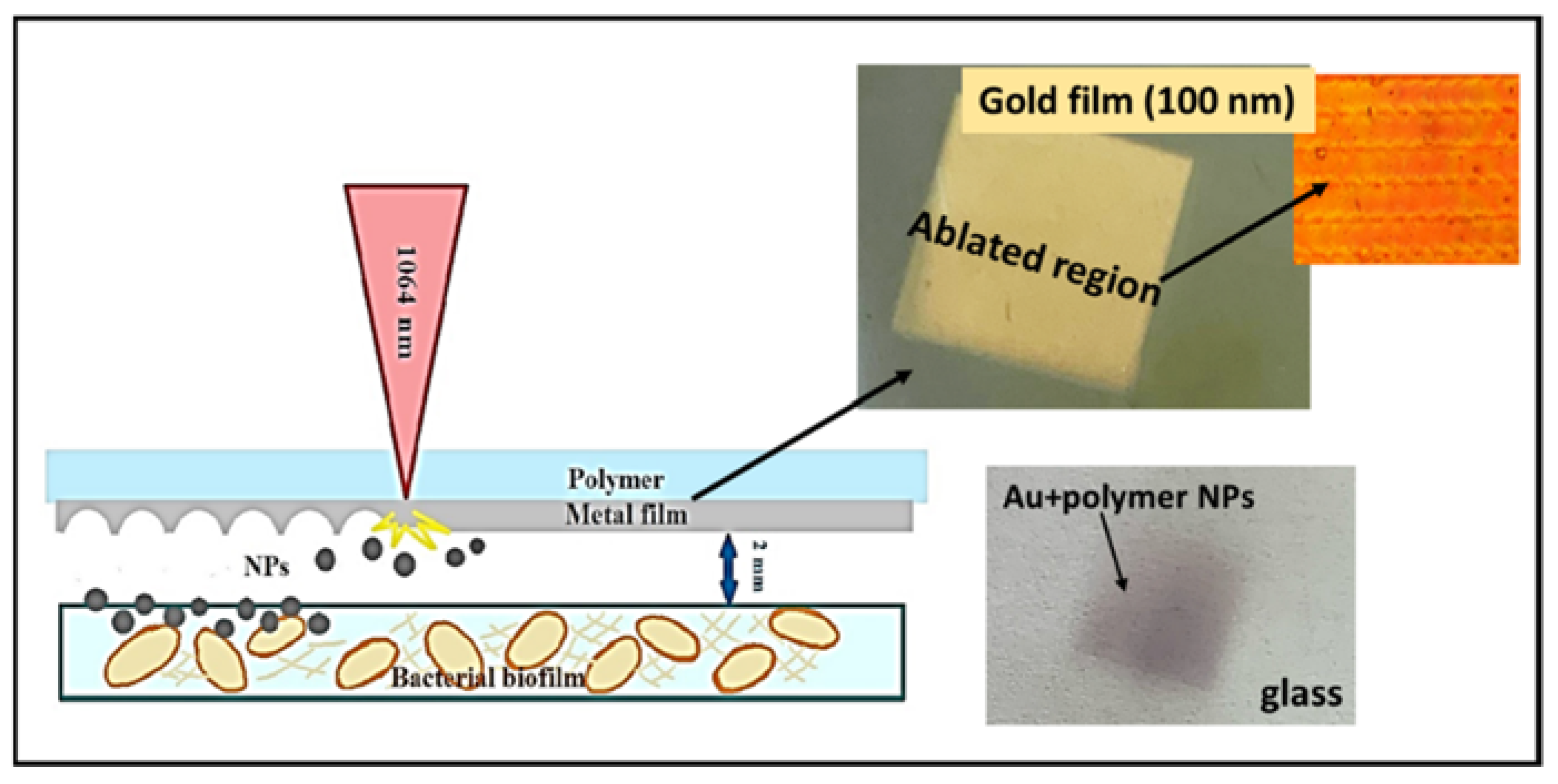
2.2. Nanoparticles by the Method LIFT
2.3. Sample Characterization
3. Results and Discussions
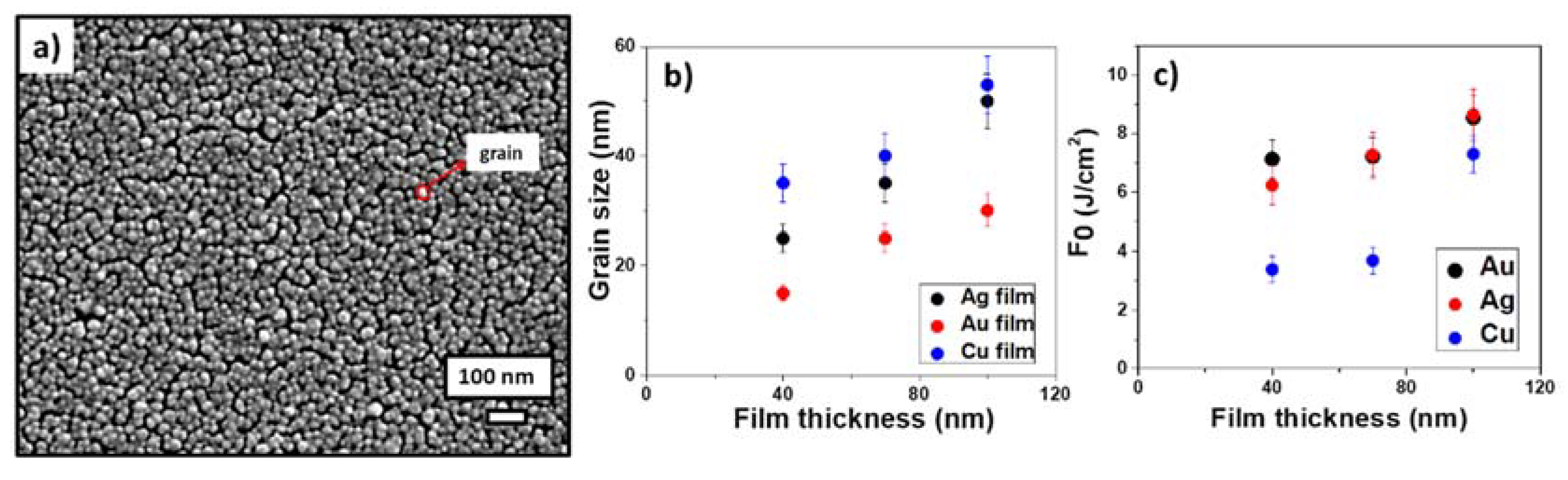
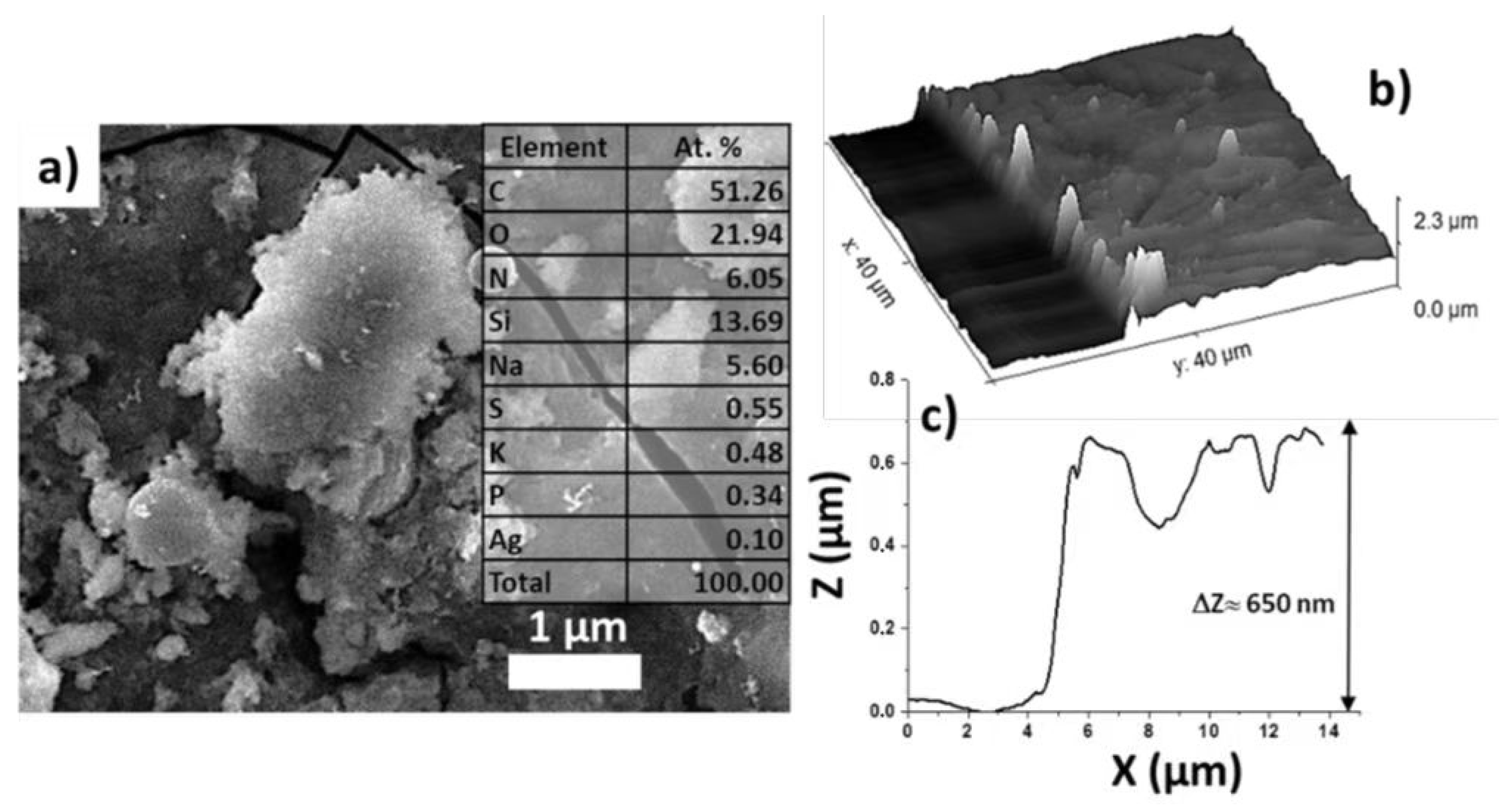
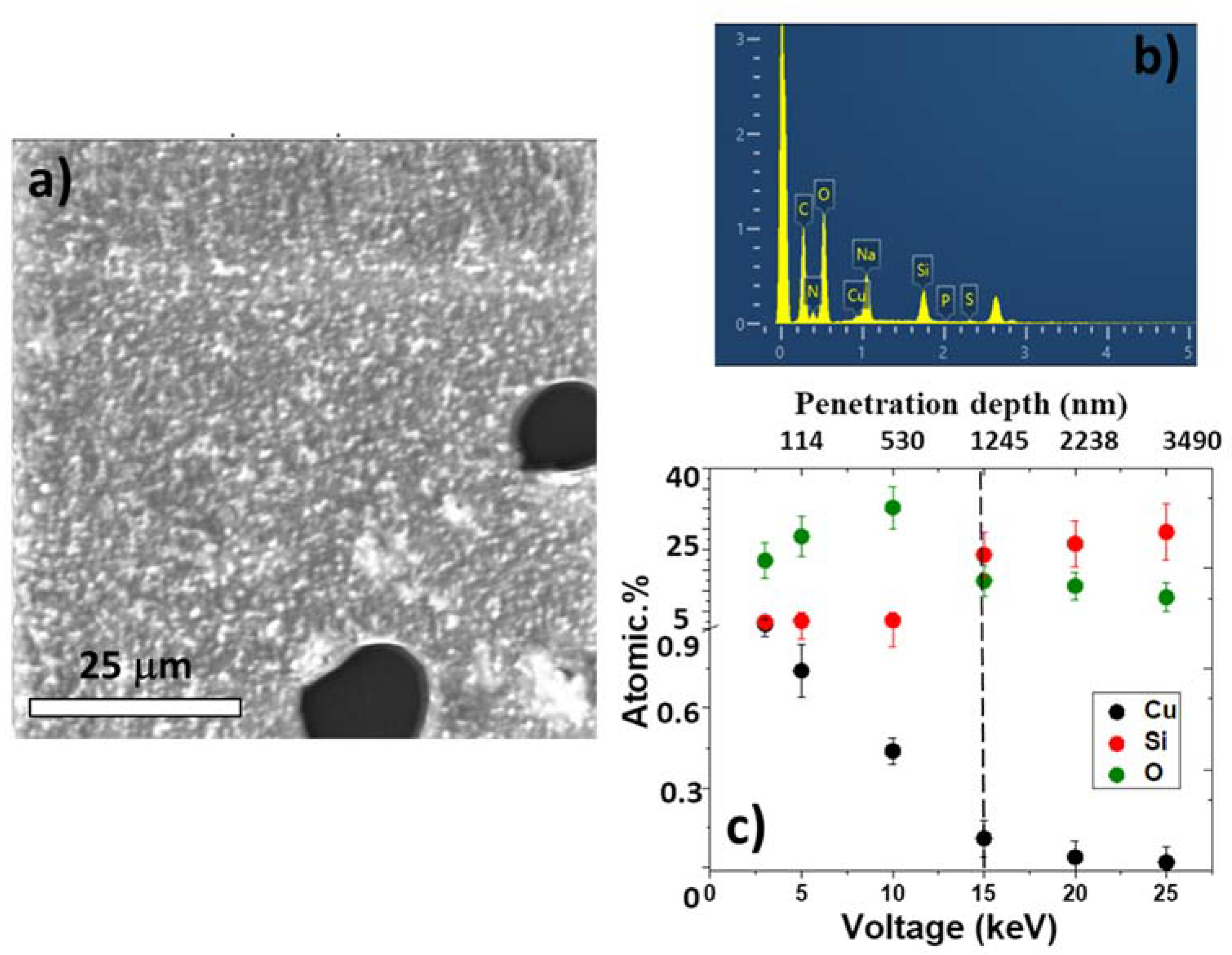
3.1. Characterization of LIFT Parameters
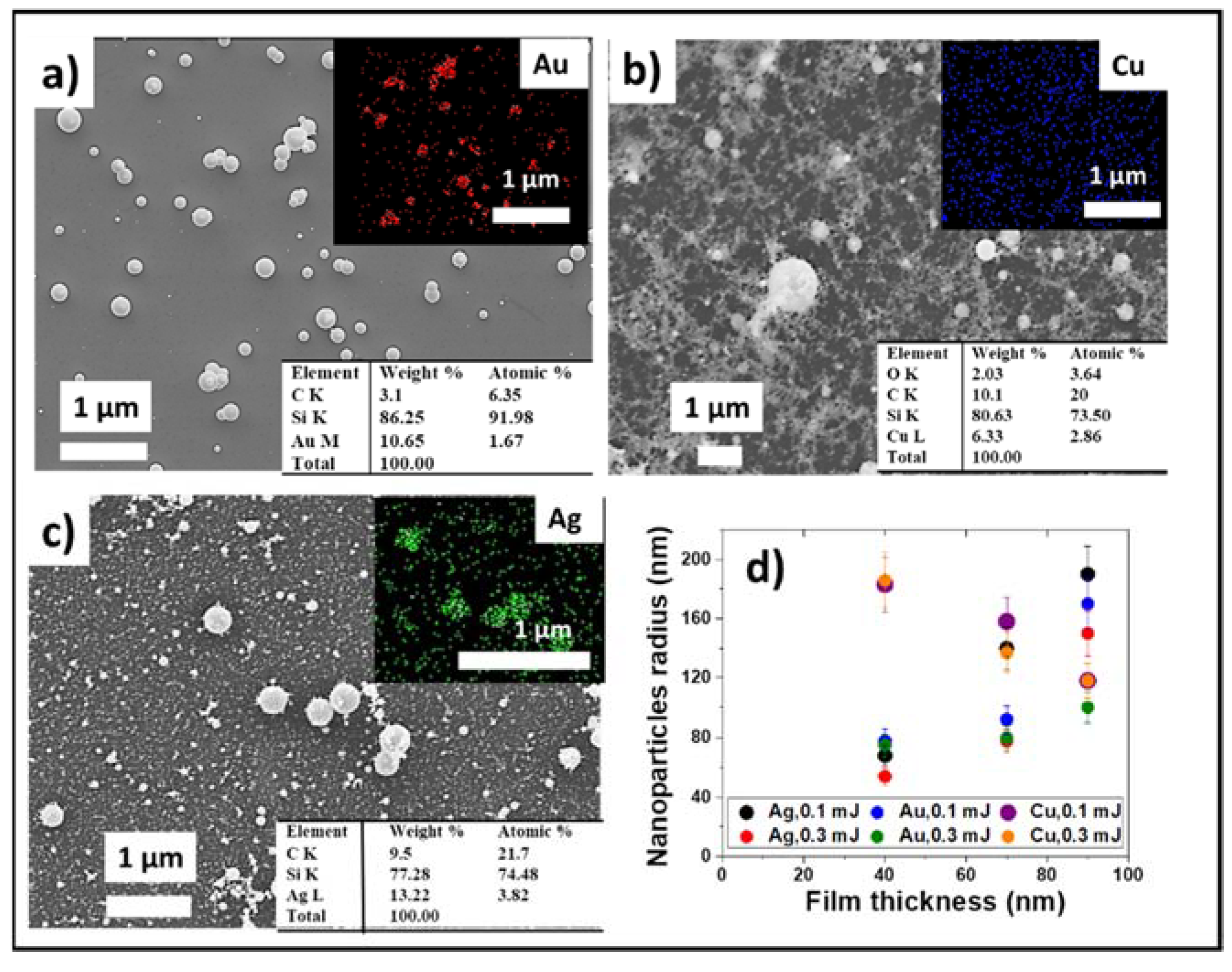
3.2. Optimization of LIFT Efficiency and Nanoparticle Sizes
3.3. Antibacterial LIFT Action on Biofilms and Its Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Silva, V.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Implications of antibiotics use during the COVID-19 pandemic: Present and future. J. Antimicrob. Chemother. 2020, 75, 3413–3416. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Microbiol. Immunol. 2008, 52, 13–22. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.I.; Ficai, D.; Ficai, A.; Andronescu, E. Chitosan-based nanocomposite polymeric membranes for water purification—A review. Materials 2021, 14, 2091. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative antimicrobial chitosan/ZnO/Ag NPs/citronella essential oil nanocomposite—Potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial biodegradable films based on alginate with silver nanoparticles and lemongrass essential oil–innovative packaging for cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Gibson, H.; Taylor, J.H.; Hall, K.E.; Holah, J.T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 1999, 87, 41–48. [Google Scholar] [CrossRef]
- Holah, J.T.; Kearney, L.R. Introduction to biofilms in the food industry. In Biofilms—Science and Technology; Springer: Dordrecht, The Netherlands, 1992; pp. 35–41. [Google Scholar]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 28 June 2022).
- de W Blackburn, C.; McClure, P.J. Foodborne Pathogens: Hazards, Risk Analysis and Control, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Crum, N.F. Update on Listeria monocytogenes infection. Curr. Gastroenterol. Rep. 2002, 4, 287–296. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Immobilization of lysozyme on food contact polymers as potential antimicrobial films. Packag. Technol. Sci. 1997, 10, 271–279. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, T.; Jenal, U. Structural and mechanistic determinants of c-di-GMP signalling. Nat. Rev. Microbiol. 2009, 7, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.A. Bacterial biofilms in food processing environments: A review of recent developments in chemical and biological control. Int. J. Food Sci. Technol. 2016, 51, 1731–1743. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Zheng, W.; Zhao, Y.F.; Jin, Y.F.; Tang, Z.X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. 2014, 31, 173–186. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Nastulyavichus, A.; Kudryashov, S.; Smirnov, N.; Saraeva, I.; Rudenko, A.; Tolordava, E.; Ionin, A.; Romanova, Y.; Zayarny, D. Antibacterial coatings of Se and Si nanoparticles. Appl. Surf. Sci. 2019, 469, 220–225. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and-mechanisms of action. SLJID 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Vasilache, V.; Popa, C.; Filote, C.; Cretu, M.A.; Benta, M. Nanoparticles applications for improving the food safety and food processing. In Proceedings of the 7th International Conference on Materials Science and Engineering–BRAMAT, Brasov, Romania, 24–26 February 2011; pp. 24–26. [Google Scholar]
- Rana, S.; Kalaichelvan, P.T. Antibacterial activities of metal nanoparticles. Biotechnol 2011, 11, 21–23. [Google Scholar]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and antibacterial activi-ties of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Nastulyavichus, A.; Shahov, P.; Khaertdinova, L.; Tolordava, E.; Saraeva, I.; Yushina, Y.; Rudenko, A.; Ionin, A.; Khmelnitskiy, R.; Khmelenin, D.; et al. Bactericidal impact of nickel-oxide nanoparticles on foodborne pathogens: Complementary microbiological and IR-spectroscopic insights. Appl. Surf. Sci. 2021, 558, 149857. [Google Scholar] [CrossRef]
- Chrzanowska, N.; Załeska-Radziwiłł, M. The impacts of aluminum and zirconium nano-oxides on planktonic and biofilm bacteria. Desalin. Water Treat. 2014, 52, 3680–3689. [Google Scholar] [CrossRef]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.; Youn, H.S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnology 2010, 8, 16. [Google Scholar] [CrossRef]
- Eleftheriadou, M.; Pyrgiotakis, G.; Demokritou, P. Nanotechnology to the rescue: Using nano-enabled approaches in microbiological food safety and quality. Curr. Opin. Biotechnol. 2017, 44, 87–93. [Google Scholar] [CrossRef]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Haghighi, F.; Roudbar Mohammadi, S.; Mohammadi, P.; Hosseinkhani, S.; Shipour, R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infect. Epidemiol. Med. 2013, 1, 33–38. [Google Scholar]
- Singh, N.; Rajwade, J.; Paknikar, K. Transcriptome analysis of silver nanoparticles treated Staphylococcus aureus reveals potential targets for biofilm inhibition. Colloids Surf. B 2019, 175, 487–497. [Google Scholar] [CrossRef]
- Al-jassani, M.J.; Raheem, H.Q. Anti-bacterial activity of CuO nanoparticles against some pathogenic bacteria. Int. J. Chem. Tech. Res. 2017, 10, 818–822. [Google Scholar]
- Taran, M.; Rad, M.; Alavi, M. Antibacterial Activity of Copper Oxide (CuO) Nanoparticles Biosynthesized by Bacillus sp. FU4: Optimization of Experiment Design. Pharm. Sci. 2017, 23, 198–206. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Muzammil, S.; Rasool, M.H.; Nisar, Z.; Hussain, S.Z.; Sabri, A.N.; Jamil, S. In vitro antibiofilm and antiadhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria Running. Microbiol. Immunol. 2017, 26, 211–220. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterialaction of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. J. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Díaz-Visurraga, J.; Gutiérrez, C.; Von Plessing, C.; García, A. Metal nanostructures as antibacterial agents. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 1, 210–218. [Google Scholar]
- Ahmad, S.A.; Das, S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Samokhvalov, A.A.; Nastulyavichus, A.A.; Saraeva, I.N.; Mikhailovskii, V.Y.; Ionin, A.A.; Veiko, V.P. Nanosecond-Laser Generation of Nanoparticles in Liquids: From Ablation through Bubble Dynamics to Nanoparticle Yield. Materials 2019, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Nastulyavichus, A.A.; Kudryashov, S.I.; Smirnov, N.A.; Khmel’nitskii, R.A.; Rudenko, A.A.; Mel’nik, N.N.; Kirilenko, D.A.; Brunkov, P.N.; Ionin, A.A. Laser Formation of Colloidal Sulfur-and Carbon-Doped Silicon Nanoparticles. Opt. Spectrosc. 2020, 128, 897–901. [Google Scholar] [CrossRef]
- Willis, D.A.; Grosu, V. Microdroplet deposition by laser-induced forward transfer. Appl. Phys. Lett. 2005, 86, 244103. [Google Scholar] [CrossRef]
- Piqué, A.; Chrisey, D.B.; Auyeung, R.C.Y.; Fitz-Gerald, J.; Wu, H.D.; McGill, R.A.; Lakeou, S.; Wu, P.K.; Nguen, V.; Duignan, M. A novel laser transfer process for direct writing of electronic and sensor materials. Appl. Phys. A 1999, 69, S279–S284. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F.; Adrian, F.J.; Jette, A.N. Metal deposition at 532 nm using a laser transfer technique. J. Appl. Phys. 1988, 63, 1158–1162. [Google Scholar] [CrossRef]
- Bezhanov, S.G.; Danilov, P.A.; Ionin, A.A.; Kiseleva, I.V.; Kudryashov, S.I.; Uryupin, S.A.; Zayarnyi, D.A. Femtosecond laser induced nanostructuring of aluminum films of variable thickness. Laser Phys. Lett. 2018, 15, 01590. [Google Scholar] [CrossRef]
- Nath, P.; Chopra, K.L. Thermal conductivity of copper films. Thin Solid Films 1974, 20, 53–62. [Google Scholar] [CrossRef]
- Nastulyavichus, A.; Tolordava, E.; Rudenko, A.; Zazymkina, D.; Shakhov, P.; Busleev, N.; Romanova, Y.; Ionin, A.; Kudryashov, S. In Vitro Destruction of Pathogenic Bacterial Biofilms by Bactericidal Metallic Nanoparticles via Laser-Induced Forward Transfer. Nanomaterials 2020, 10, 2259. [Google Scholar] [CrossRef]
- Dorranian, D.; Afshar, S.A.A.; Tahmasebi, N.; Eskandari, A.F. Effect of laser pulse energy on the characteristics of Cu nanoparticles produced by laser ablation method in acetone. J. Clust. Sci. 2014, 25, 1147–1156. [Google Scholar] [CrossRef]
- Dorranian, D.; Tajmir, S.; Khazanehfar, F. Effect of laser fluence on the characteristics of Ag nanoparticles produced by laser ablation. SNL 2013, 3, 93–100. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastulyavichus, A.; Khaertdinova, L.; Tolordava, E.; Yushina, Y.; Ionin, A.; Semenova, A.; Kudryashov, S. Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms. Micromachines 2022, 13, 2170. https://doi.org/10.3390/mi13122170
Nastulyavichus A, Khaertdinova L, Tolordava E, Yushina Y, Ionin A, Semenova A, Kudryashov S. Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms. Micromachines. 2022; 13(12):2170. https://doi.org/10.3390/mi13122170
Chicago/Turabian StyleNastulyavichus, Alena, Liliana Khaertdinova, Eteri Tolordava, Yulia Yushina, Andrey Ionin, Anastasia Semenova, and Sergey Kudryashov. 2022. "Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms" Micromachines 13, no. 12: 2170. https://doi.org/10.3390/mi13122170
APA StyleNastulyavichus, A., Khaertdinova, L., Tolordava, E., Yushina, Y., Ionin, A., Semenova, A., & Kudryashov, S. (2022). Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms. Micromachines, 13(12), 2170. https://doi.org/10.3390/mi13122170




