Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an AlN-Based pMUT as Receiver
Abstract
:1. Introduction
2. Materials and Methods
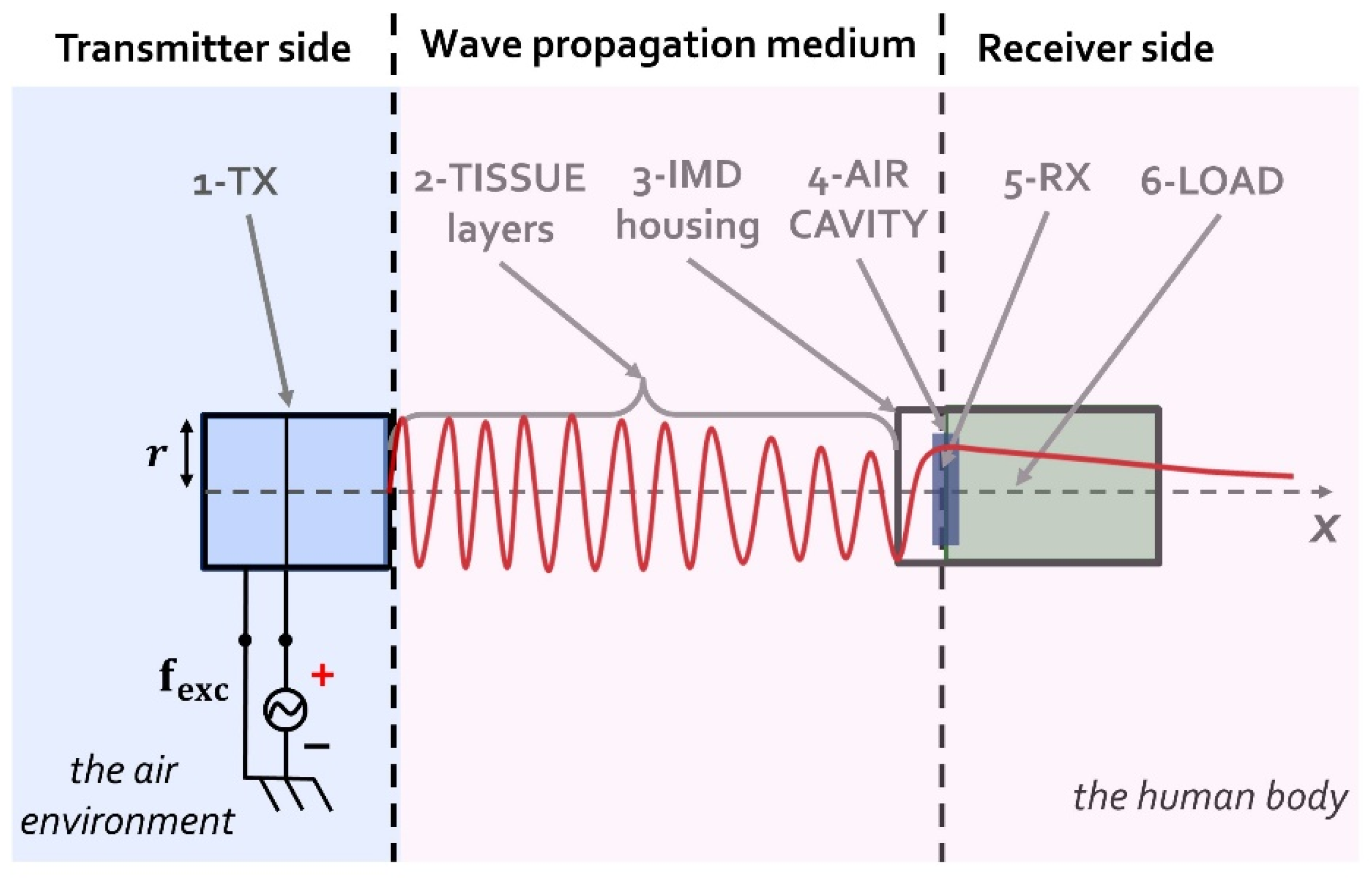
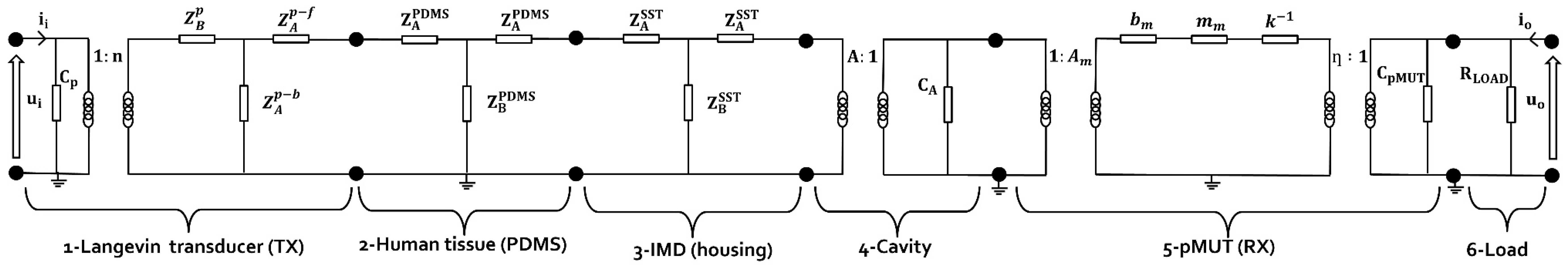
2.1. The UPD System Model
2.2. Elements Constituting the UPD System
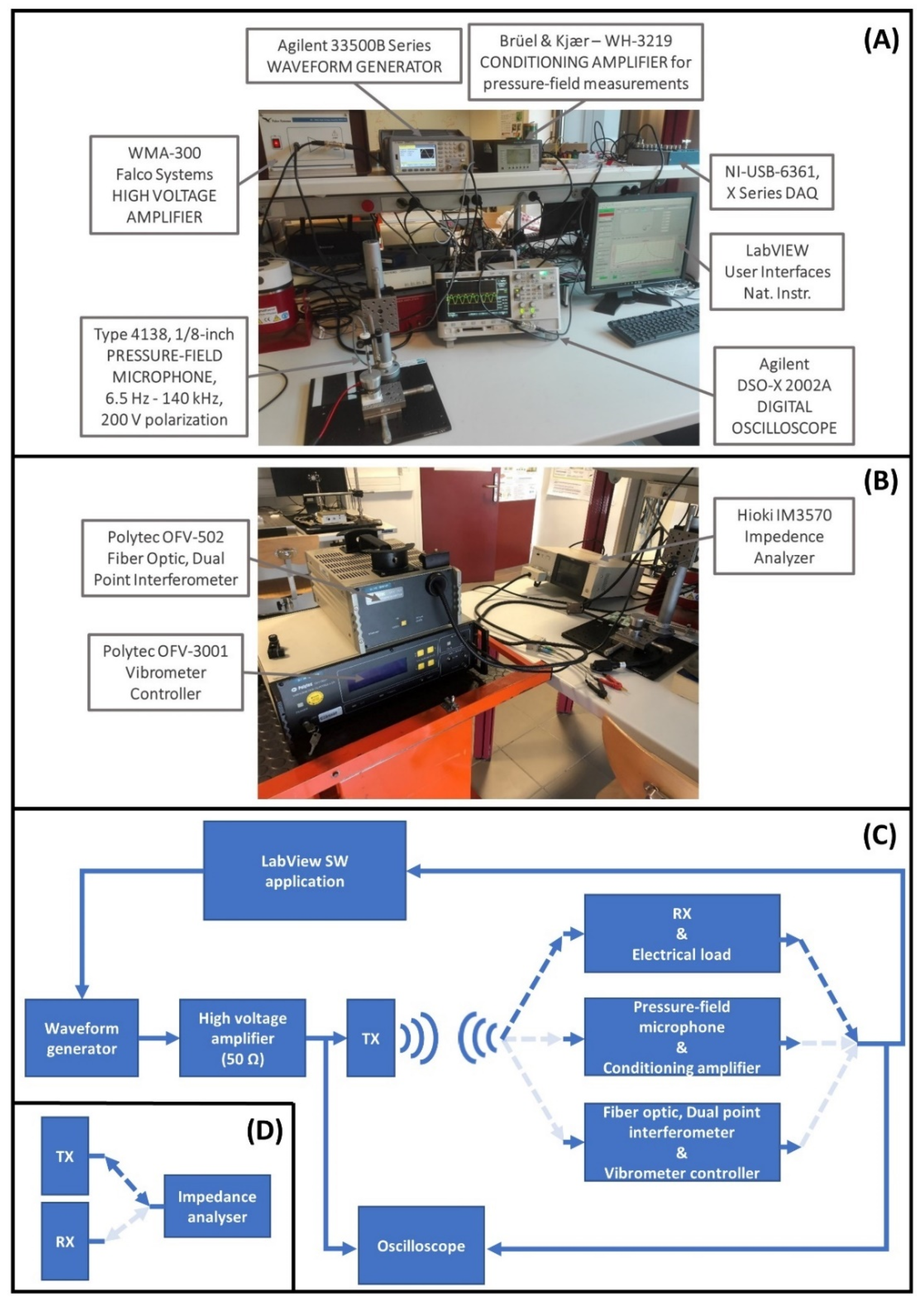
2.3. Measurement Setup
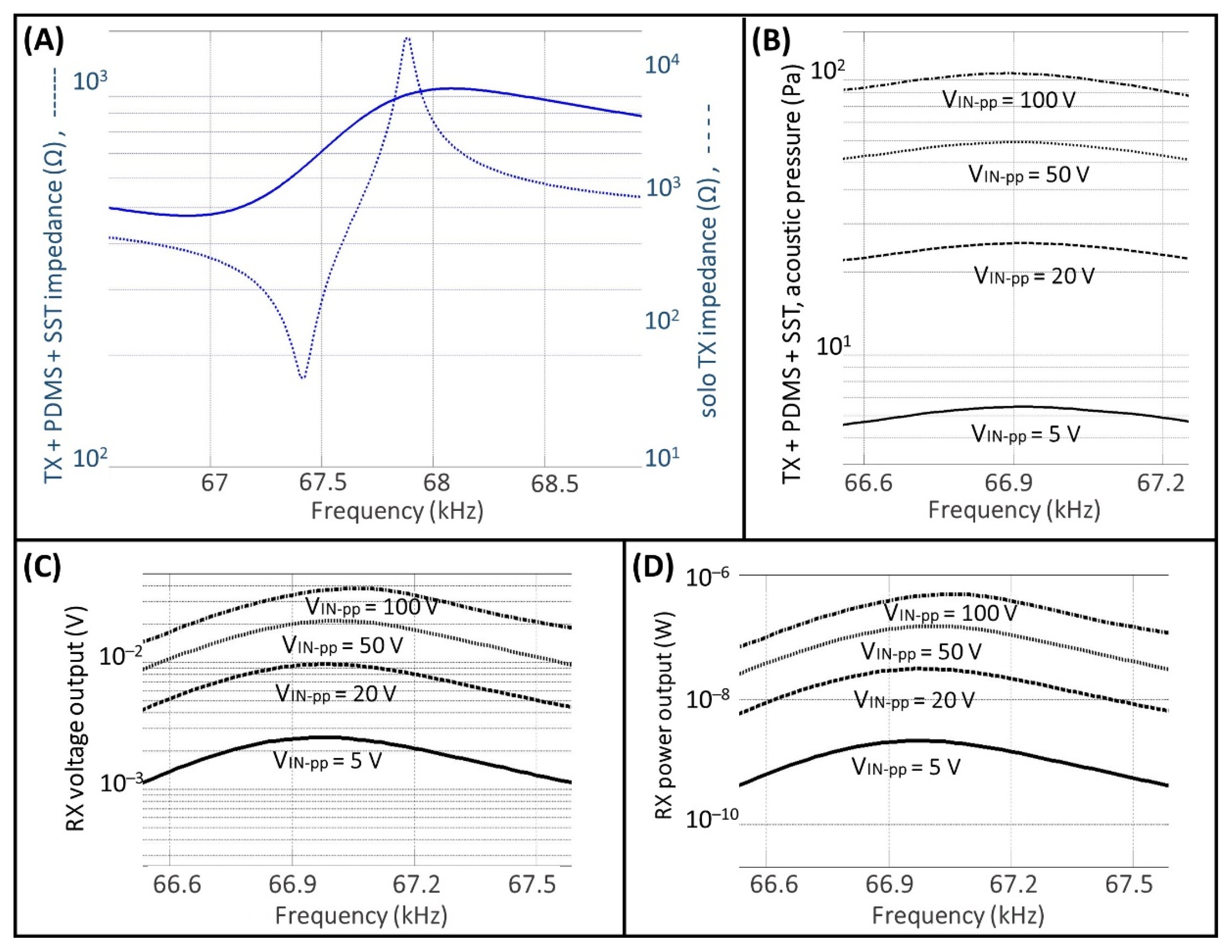
3. Results
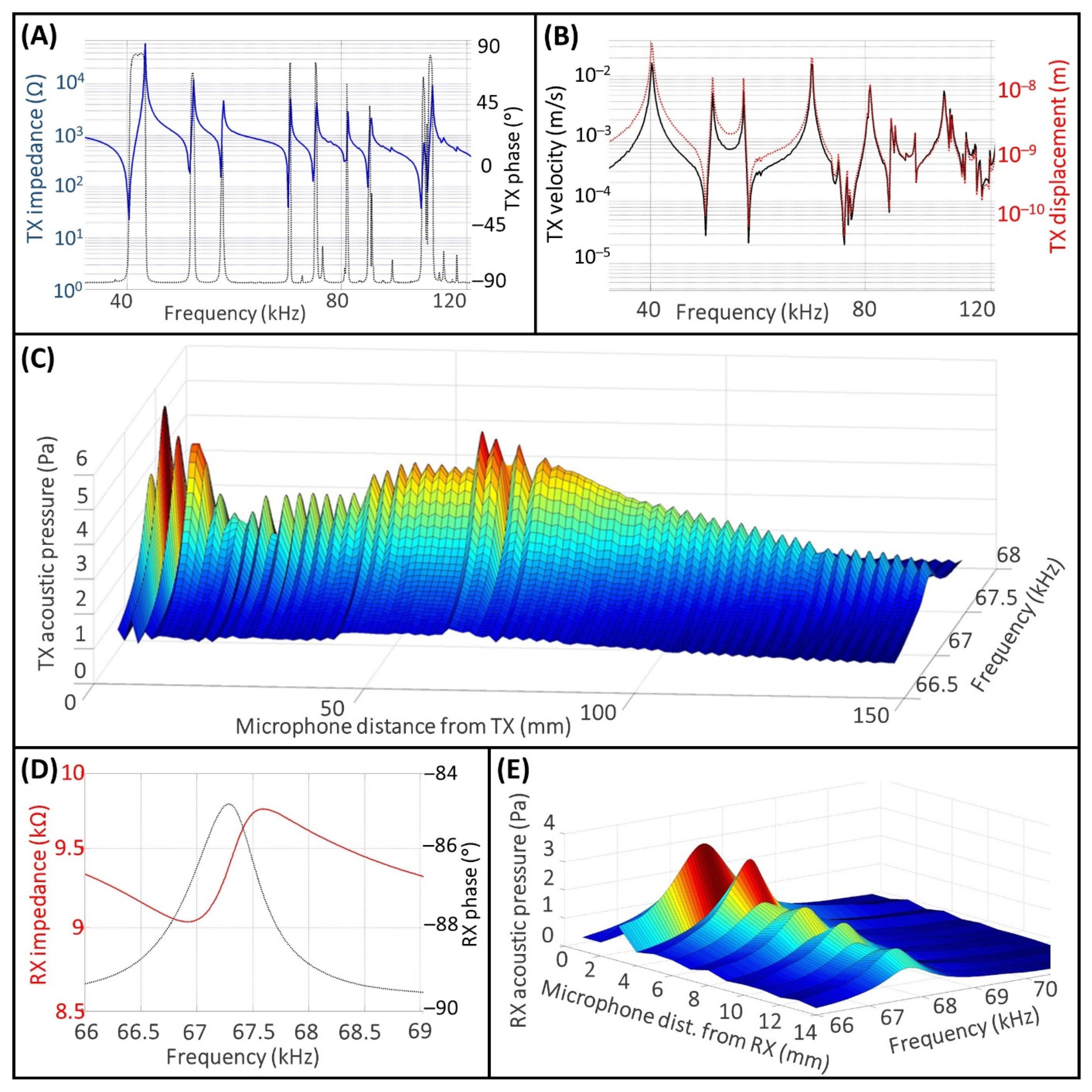
3.1. Measurements for the TX and RX Characterization
3.2. UPD System Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amar, A.B.; Kouki, A.B.; Cao, H. Power approaches for implantable medical devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, X.; Liang, J.; Shao, M.; Xie, E.; Yu, C.; Lan, W. Recent Advances of Energy Solutions for Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, 2100199. [Google Scholar] [CrossRef]
- Sette, A.L.; Seigneuret, E.; Reymond, F.; Chabardes, S.; Castrioto, A.; Boussat, B.; Moro, E.; Francois, P.; Fraix, V. Battery longevity of neurostimulators in Parkinson disease: A historic cohort study. Brain Stimul. 2019, 12, 851–857. [Google Scholar] [CrossRef]
- Alam, M.B.; Munir, M.B.; Rattan, R.; Flanigan, S.; Adelstein, E.; Jain, S.; Saba, S. Battery longevity in cardiac resynchronization therapy implantable cardioverter defibrillators. Europace 2014, 16, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M. Ultrasound-powered tiny neural stimulators. Nat. Biomed. Eng. 2020, 4, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Chakrabartty, S.; Lajnef, N.; Elvin, N.G.; Elvin, A. Toward Self-Powered Sensors and Circuits for Biomechanical Implants. In VLSI Circuits for Biomedical Applications; Iniewski, K., Ed.; Artech House Inc.: Norwood, MA, USA, 2008; pp. 75–109. [Google Scholar]
- Zebda, A.; Alcaraz, J.-P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Thimot, J.; Shepard, K.L. Bioelectronic devices: Wirelessly powered implants. Nat. Biomed. Eng. 2017, 1, 0051. [Google Scholar] [CrossRef]
- Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Available online: https://www.fda.gov/media/71100/download (accessed on 27 September 2022).
- Guidelines for Evaluating the Environmental Effects of Radio Frequency Radiation. Available online: https://transition.fcc.gov/Bureaus/Engineering_Technology/Orders/1996/fcc96326.pdf (accessed on 27 September 2022).
- IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz. Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=1626482 (accessed on 27 September 2022).
- Harvey, G.; Gachagan, A.; Mutasa, T. Review of high-power ultrasound-industrial applications and measurement methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 481–495. [Google Scholar] [CrossRef] [Green Version]
- Medan, M.S.; Abd El-Aty, A.M. Advances in ultrasonography and its applications in domestic ruminants and other farm animals reproduction. J. Adv. Res. 2010, 1, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Eshky, A.; Cleland, J.; Ribeiro, M.S.; Sugden, E.; Richmond, K.; Renals, S. Automatic audiovisual synchronisation for ultrasound tongue imaging. Speech Commun. 2021, 132, 83–95. [Google Scholar] [CrossRef]
- Ozeri, S.; Shmilovitz, D. Ultrasonic transcutaneous energy transfer for powering implanted devices. Ultrasonics 2010, 50, 556–566. [Google Scholar] [CrossRef]
- Mazzilli, F.; Lafon, C.; Dehollain, C. A 10.5 cm ultrasound link for deep implanted medical devices. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 738–750. [Google Scholar] [CrossRef]
- Radziemski, L.; Makin, I.R.S. In vivo demonstration of ultrasound power delivery to charge implanted medical devices via acute and survival porcine studies. Ultrasonics 2016, 64, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, J.; Sun, W.; Yang, H.; Liu, Y. Investigation and Modeling of Multi-Node Body Channel Wireless Power Transfer. Sensors 2019, 20, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.C.; Weber, M.J.; Charthad, J.; Baltsavias, S.; Arbabian, A. End-to-end design of efficient ultrasonic power links for scaling towards submillimeter implantable receivers. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1100–1111. [Google Scholar] [CrossRef]
- Arbabian, A.; Chang, T.C.; Wang, M.L.; Charthad, J.; Baltsavias, S.; Fallahpour, M.; Weber, M.J. Sound Technologies, Sound Bodies. IEEE Microw. Mag. 2016, 17, 39–54. [Google Scholar] [CrossRef]
- Basaeri, H.; Christensen, D.B.; Roundy, S. A review of acoustic power transfer for bio-medical implants. Smart Mater. Struct. 2016, 25, 123001. [Google Scholar] [CrossRef]
- Laursen, K.; Rashidi, A.; Hosseini, S.; Mondal, T.; Corbett, B.; Moradi, F. Ultrasonically Powered Compact Implantable Dust for Optogenetics. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Weber, M.J.; Wang, M.L.; Charthad, J.; Khuri-Yakub, B.T.; Arbabian, A. Design of Tunable Ultrasonic Receivers for Efficient Powering of Implantable Medical Devices With Reconfigurable Power Loads. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, T.; Lee, C. MEMS Based Broadband Piezoelectric Ultrasonic Energy Harvester (PUEH) for Enabling Self-Powered Implantable Biomedical Devices. Sci. Rep. 2016, 6, 24946. [Google Scholar] [CrossRef]
- Eovino, B.E.; Liang, Y.; Lin, L. Concentric PMUT Arrays for Focused Ultrasound and High Intensity Applications. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Republic of Korea, 25–29 January 2019. [Google Scholar] [CrossRef]
- Wang, L.; Chiu, Y.; Gong, D.; Ma, S.; Yang, Y.; Li, H.; Lee, H.; Liu, H.; Jin, Y. Fabrication Process and Performance Analysis of AlN based Piezoelectric Micromachined Ultrasonic Transducer with a Suspended Structure. In Proceedings of the 14th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Bangkok, Thailand, 11–14 April 2019. [Google Scholar] [CrossRef]
- Alasatri, S.; Rufer, L.; Lee, J.E.-Y. AlN-on-Si Square Diaphragm Piezoelectric Micromachined Ultrasonic Transducer with Extended Range of Detection. Proceedings 2018, 2, 913. [Google Scholar] [CrossRef] [Green Version]
- Sammoura, F.; Shelton, S.; Akhbari, S.; Horsley, D.; Lin, L. A Two-Port Piezoelectric Micromachined Ultrasonic Transducer. In Proceedings of the Joint IEEE International Symposium on the Applications of Ferroelectric, International Workshop on Acoustic Transduction Materials and Devices and Workshop on Piezoresponse Force Microscopy (ISAF/IWATMD/PFM), State College, PA, USA, 7–11 May 2014. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, T.; Liu, H.; Sun, L.; Wang, T.; Lee, C.; Le, X.; Xie, J. An AlN-based piezoelectric micro-machined ultrasonic transducer (pMUT) array. In Proceedings of the 16th IEEE International Conference on Nanotechnology (IEEE NANO), Sendai, Japan, 22–25 August 2016. [Google Scholar] [CrossRef]
- Halbach, A.; Gijsenbergh, P.; Jeong, Y.; Billen, M.; Chare, C.; Gao, H.; Torri, G.B.; Cheyns, D.; Rottenberg, X.; Rochus, V. Modelling of display-compatible piezoelectric micromachined ultrasonic transducers for haptic feedback. In Proceedings of the 20th International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), Hannover, Germany, 24–27 March 2019. [Google Scholar] [CrossRef]
- Huang, C.H.; Demi, L.; Torri, G.B.; Mao, S.; Billen, M.; Jeong, Y.; Cheyns, D.; Rottenberg, X.; Rochus, V. Display compatible pMUT device for mid air ultrasound gesture recognition. In Proceedings of the 11th Annual TechConnect World Innovation Conference and Expo, Held Jointly with the 20th Annual Nanotech Conference and Expo, the SBIR/STTR Spring Innovation Conference, and the Defense TechConnect DTC Spring Conference, Anaheim, CA, USA, 13–16 May 2018; ISBN 978-099887825-6. [Google Scholar]
- Griffin, B.A.; Williams, M.D.; Coffman, C.S.; Sheplak, M. Aluminum nitride ultrasonic air-coupled actuator. J. Microelectromechanical Syst. 2011, 20, 476–486. [Google Scholar] [CrossRef]
- Przybyla, R.J.; Shelton, S.E.; Guedes, A.; Izyumin, I.I.; Kline, M.H.; Horsley, D.A.; Boser, B.E. In-air rangefinding with an AlN piezoelectric micromachined ultrasound transducer. IEEE Sens. J. 2011, 11, 2690–2697. [Google Scholar] [CrossRef]
- Alasatri, S.; Mak, K.L.; Benserhir, J.; Lee, J.E.-Y.; Rufer, L. Air-coupled Ultrasonic Rangefinder with Meter-long Detection Range Based on a Dual-electrode PMUT Fabricated Using a Multi-user MEMS Process. In Proceedings of the 18th IEEE Sensors Conference, Montreal, QC, Canada, 27–30 October 2019. [Google Scholar] [CrossRef]
- Basaeri, H.; Yu, Y.; Young, D.; Roundy, S. A MEMS-Scale Ultrasonic Power Receiver for Biomedical Implants. IEEE Sens. Lett. 2019, 3, 8664093. [Google Scholar] [CrossRef]
- Rebelo, R.; Barbosa, A.I.; Correlo, V.M.; Reis, R.L. An outlook on implantable biosensors for personalized medicine. Engineering 2021, 7, 1696–1699. [Google Scholar] [CrossRef]
- Horsley, D.A.; Rozen, O.; Lu, Y.; Shelton, S.; Guedes, A.; Przybyla, R.; Tang, H.-Y.; Boser, B.E. Piezoelectric Micromachined Ultrasonic Transducers for Human-Machine Interfaces and Biometric Sensing. In Proceedings of the IEEE Sensors Conference, Busan, Republic of Korea, 1–4 November 2015. [Google Scholar] [CrossRef]
- Cowen, A.; Hames, G.; Glukh, K.; Hardy, B. PiezoMUMPs Design Handbook; MEMSCAP Inc.: Windsor, ON, Canada, 2013. [Google Scholar]
- Bolt Clamped Langevin Transducer 40 KHz. Available online: https://www.steminc.com/PZT/en/bolt-clamped-langevin-tranducer-40-khz (accessed on 27 September 2022).
- Yoo, S.; Lee, J.; Joo, H.; Sunwoo, S.-H.; Kim, S.; Kim, D.-H. Wireless Power Transfer and Telemetry for Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, 2100614. [Google Scholar] [CrossRef]
- Khan, S.R.; Pavuluri, S.K.; Cummins, G.; Desmulliez, M.P.Y. Wireless power transfer techniques for implantable medical devices: A review. Sensors 2020, 20, 3487. [Google Scholar] [CrossRef]
- Todar, M.T.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Desmaele, D.; Epifani, G.; De Vittorio, M. Biocompatible, flexible, and compliant energy harvesters based on piezoelectric thin films. IEEE Trans. Nanotechnol. 2018, 17, 220–230. [Google Scholar] [CrossRef]
- Fei, C.; Liu, X.; Zhu, B.; Li, D.; Yang, X.; Yang, Y.; Zhou, Q. AlN piezoelectric thin films for energy harvesting and acoustic devices. Nano Energy 2018, 51, 146–161. [Google Scholar] [CrossRef]
- Peres, P.L.D.; de Souza, C.R.; Bonatti, I.S. ABCD matrix: A unique tool for linear two-wire transmission line modelling. Int. J. Electr. Eng. Educ. 2003, 40, 220–229. [Google Scholar] [CrossRef]
- Frickey, D.A. Conversions between S, Z, Y, H, ABCD, and T parameters which are valid for complex source and load impedances. IEEE Trans. Microw. Theory Tech. 1994, 42, 205–211. [Google Scholar] [CrossRef]
- Turner, B.L.; Senevirathne, S.; Kilgour, K.; McArt, D.; Biggs, M.; Menegatti, S.; Daniele, M.A. Ultrasound-Powered Implants: A Critical Review of Piezoelectric Material Selection and Applications. Adv. Healthc. Mater. 2021, 10, 2100986. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, X.; Wang, H.; Chen, Z.; Yang, Z. A wideband ultrasonic energy harvester using 1-3 piezoelectric composites with non-uniform thickness. Appl. Phys. Lett. 2018, 112, 043903. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Yang, B.; Wang, X.; Chen, X.; Yang, C. MEMS-based ultrasonic transducer as the receiver for wireless power supply of the implantable microdevices. Sens. Actuator A Phys. 2014, 219, 65–72. [Google Scholar] [CrossRef]
- Joseph, J.; Singh, S.G.; Vanjari, S.R.K. Piezoelectric Micromachined Ultrasonic Transducer Using Silk Piezoelectric Thin Film. IEEE Electron. Device Lett. 2018, 39, 749–752. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, Y.; Chen, R.; Lu, G.; Li, R.; Xing, J.; Shung, K.K.; Humayun, M.S.; Zhu, J.; Chen, Y.; et al. Ultrasound-induced wireless energy harvesting for potential retinal electrical stimulation application. Adv. Funct. Mater. 2019, 29, 1902522. [Google Scholar] [CrossRef]
- Al-Shidaifat, A.; Kumar, S.; Chakrabartty, S.; Song, H.J. A Conceptual Investigation at the Interface between Wireless Power Devices and CMOS Neuron IC for Retinal Image Acquisition. Appl. Sci. 2020, 10, 6154. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Yang, D.; Chen, X.; Le, X.; Xie, J. A Computational Piezoelectric Micro-Machined Ultrasonic Transducer Toward Acoustic Communication. IEEE Electron. Device Lett. 2019, 40, 965–968. [Google Scholar] [CrossRef]
- Defoort, M.; Rufer, L.; Fesquet, L.; Basrour, S. A dynamical approach to generate chaos in a micromechanical resonator. Microsyst. Nanoeng. 2021, 7, 17. [Google Scholar] [CrossRef]
- Ahmadi, F.; McLoughlin, I.V.; Chauhan, S.; ter-Haar, G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog. Biophys. Mol. Biol. 2012, 108, 119–138. [Google Scholar] [CrossRef]
- Boucaud, A.; Montharu, J.; Machet, L.; Arbeille, B.; Machet, M.C.; Patat, F.; Vaillant, L. Clinical, histologic, and electron microscopy study of skin exposed to low-frequency ultrasound. Anat. Rec. 2001, 264, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ho, J.S.; Chen, L.Y.; Poon, A.S.Y. Wireless power transfer to a cardiac implant. Appl. Phys. Lett. 2012, 101, 073701. [Google Scholar] [CrossRef] [Green Version]
- Kazanc, O.; Maloberti, F.; Dehollain, C. Simulation oriented rectenna design methodology for remote powering of wireless sensor systems. In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Seoul, Republic of Korea, 20–23 May 2012; pp. 2877–2880. [Google Scholar] [CrossRef]
- Ko, Y.Y.; Ho, S.L.; Fu, W.N.; Zhang, X. A novel hybrid resonator for wireless power delivery in bio-implantable devices. IEEE Trans. Magn. 2012, 48, 4518–4521. [Google Scholar] [CrossRef]
- Cong, P.; Suster, M.A.; Chaimanonart, N.; Young, D.J. Wireless power recharging for implantable bladder pressure sensor. In Proceedings of the IEEE Sensors Conference (SENSORS), Christchurch, New Zealand, 25–28 October 2009; pp. 1670–1673. [Google Scholar] [CrossRef]
- Sanni, A.; Vilches, A.; Toumazou, C. Inductive and ultrasonic multi-tier interface for low-power, deeply implantable medical devices. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, F.; Thoppay, P.E.; Praplan, V.; Dehollaini, C. Ultrasound energy harvesting system for deep implanted-medical-devices (IMDs). In Proceedings of the IEEE International Symposium on Circuits and Systems (ISCAS), Seoul, Republic of Korea, 20–23 May 2012; pp. 2865–2868. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Yang, Y.; Chen, R.; Lu, G.; Li, R.; Li, D.; Humayun, M.S.; Shung, K.K.; Zhu, J.; Chen, Y. Flexible piezoelectric ultrasonic energy harvester array for bio-implantable wireless generator. Nano Energy 2019, 56, 216–224. [Google Scholar] [CrossRef]
- Herrera, B.; Pop, F.; Cassella, C.; Rinaldi, M. AlN PMUT-based Ultrasonic Power Transfer Links for Implantable Electronics. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII, TRANSDUCERS 2019 and EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019; pp. 861–864. [Google Scholar] [CrossRef]
- Gong, D.; Ma, S.; Chiu, Y.; Lee, H.; Jin, Y. Study of the properties of AlN PMUT used as a wireless power receiver. In Proceedings of the IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 1503–1508. [Google Scholar] [CrossRef]
- Rong, Z.; Zhang, M.; Ning, Y.; Pang, W. An ultrasound-induced wireless power supply based on AlN piezoelectric micromachined ultrasonic transducers. Sci. Rep. 2022, 12, 16174. [Google Scholar] [CrossRef]
- Moerke, C.; Wolff, A.; Ince, H.; Ortak, J.; Oner, A. New strategies for energy supply of cardiac implantable devices. Herzschr. Elektrophys. 2022, 33, 224–231. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Long, Z.-L.; Ma, W.-J.; Hu, G.-H.; Li, Y.-M. Electromechanical dynamics model of ultrasonic transducer in ultrasonic machining based on equivalent circuit approach. Sensors 2019, 19, 1405. [Google Scholar] [CrossRef]
- Sherrit, S.; Leary, S.P.; Dolgin, B.P.; Bar-Cohen, Y. Comparison of the Mason and KLM equivalent circuits for piezoelectric resonators in the thickness mode. In Proceedings of the IEEE Ultrasonics Symposium, Tahoe, NV, USA, 17–20 October 1999; Volume 2, pp. 921–926. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, J. Theoretical Resonance Analysis of Langevin Transducers with Equivalent Circuit Models for Therapeutic Ultrasound. J. Electr. Eng. Technol. 2019, 14, 2437–2445. [Google Scholar] [CrossRef]
- Meeker, T.R. Publication and proposed revision of ANSI/IEEE standard 176-1987 “ANSI/IEEE standard on piezoelectricity”. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1996, 43, 717–771. [Google Scholar] [CrossRef]
- Uchino, K. High-Power Piezoelectrics and Loss Mechanisms. In Advanced Piezoelectric Materials: Science and Technology, 2nd ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 647–754. [Google Scholar]
- Ashby, M.F. Material Property Charts. In Materials Selection in Mechanical Design, 4th ed.; Elsevier Ltd.: London, UK, 2011; pp. 57–96. [Google Scholar]
- Horsley, D.; Lu, Y.; Rozen, O. Flexural Piezoelectric Resonators. In Piezoelectric MEMS Resonators; Bhurgra, H., Piazza, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 153–173. [Google Scholar]
- Marzencki, M.; Basrour, S. Modeling of Piezoelectric MEMS Vibration Energy Harvesters. In MEMS: Fundamental Technology and Applications; Choudhary, V., Iniewski, K., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 131–160. [Google Scholar]
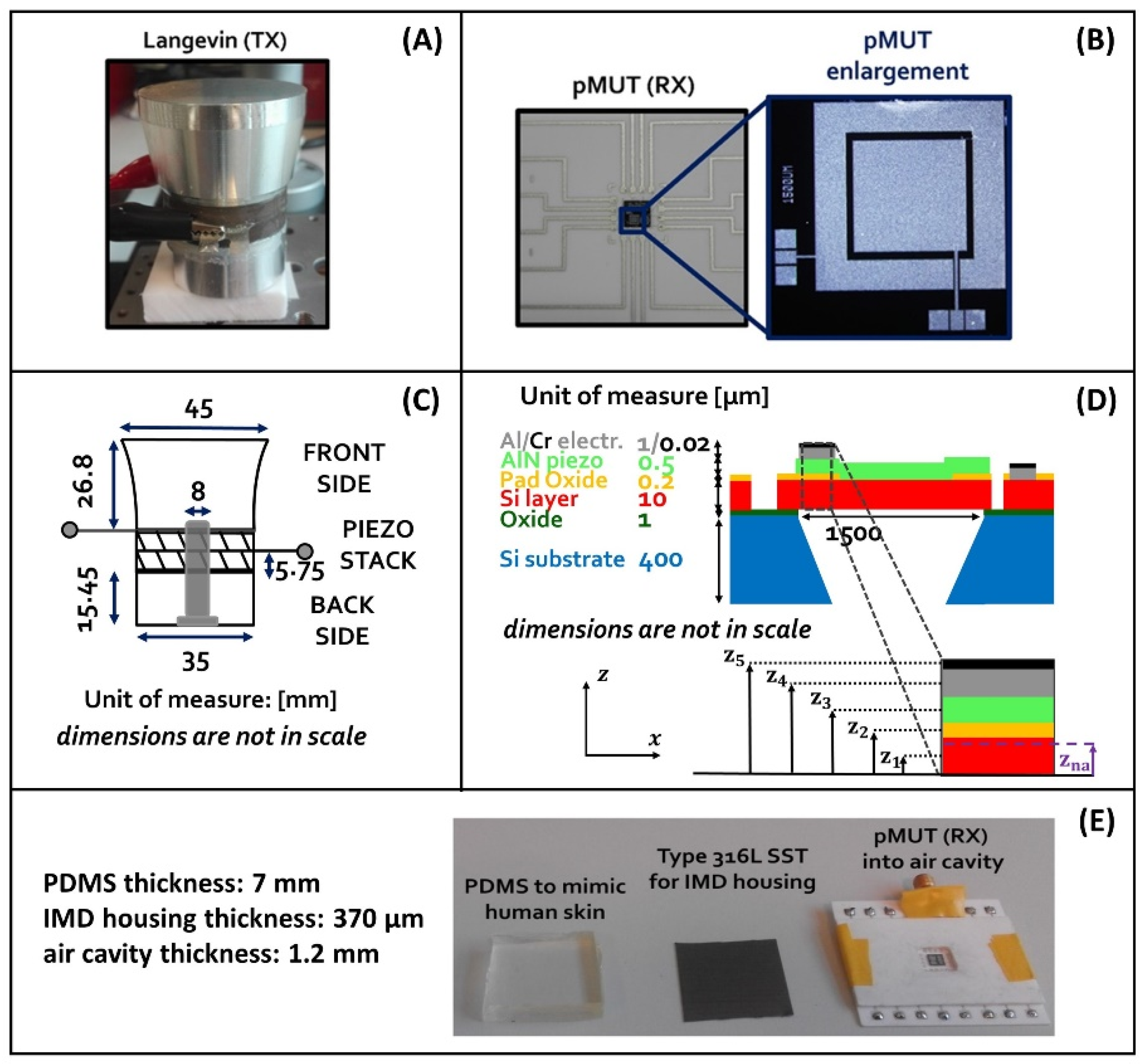

| Parameter | PZT-8 (TX) | AlN (RX) |
|---|---|---|
| Cross-section Area (S) [m2] | 9.1185·10−4 | 2.25·10−6 |
| Thickness (t) [m] | 5.75·10−3 | 5·10−7 |
| Length (l) [m] | - | 1.5·10−3 |
| Density (ρ) [kg m−3] | 7600 | 3260 |
| Young’s Modulus (E) [Pa] | 7.4·1010 | 3.45·1011 |
| Poisson’s Ratio (υ) | - | 0.32 |
| Plate Modulus (Y) [Pa] | - | 3.8436·1011 |
| Dielectric Constant (ε) | 1200 | 7.9059 |
| Piezo Charge Coeff. (d31, d33) [pC N−1] | -, 240 | −2.3259, - |
| Piezo Constant (e31, e33) [C m−2] | -, 14.6385 | −0.8024, - |
| Coupling Factor (k) | 0.48 | 0.17 |
| Quality Factor (Q) | 1000 | 231 1 |
| Dielectric Loss (tan δ′) | 0.004 | 0.001 |
| Parameter | Si Layer | Dioxide (SiO2) | AlN Diaphragm | Al Pad | Cr Pad |
|---|---|---|---|---|---|
| Cross-section Area (S) [m2] | 2.25·10−6 | 2.25·10−6 | 2.25·10−6 | 2.25·10−6 | 2.25·10−6 |
| Thickness (t) [m] | 10·10−6 | 2·10−7 | 5·10−7 | 1·10−6 | 20·10−9 |
| Length (l) [m] | 1.5·10−3 | 1.5·10−3 | 1.5·10−3 | 1.5·10−3 | 1.5·10−3 |
| Volume (V) [m3] | 2.25·10−11 | 4.5·10−13 | 1.125·10−12 | 2.25·10−12 | 4.5·10−14 |
| Density (ρ) [kg m−3] | 2330 | 2200 | 3260 | 2680 | 7140 |
| Mass (M) [kg] | 5.243·10−8 | 9.9·10−10 | 3.668·10−9 | 6.03·10−9 | 3.213·10−10 |
| Young’s Modulus (E) [Pa] | 1.65·1011 | 0.73·1011 | 3.45·1011 | 0.71·1011 | 2.45·1011 |
| Poisson’s Ratio (υ) | 0.22 | 0.17 | 0.32 | 0.33 | 0.2 |
| Plate Modulus (Y) [Pa] | 1.734·1011 | 0.752·1011 | 3.844·1011 | 0.797·1011 | 2.552·1011 |
| Mid-plane Location (z) [m] | 5·10−6 | 1.01·10−5 | 1.045·10−5 | 1.12·10−5 | 1.171·10−5 |
| Top-plane Location (h) [m] | 1·10−5 | 1.02·10−5 | 1.07·10−5 | 1.17·10−5 | 1.172·10−5 |
| RMS Input Voltage (V) | RMS Acoustic Pressure (Pa) | RMS Voltage Output (mV) | Sensitivity VOUT/VIN (%) | Power Input (mW cm−2) | Power Output (µW cm−2) | Efficiency POUT/PIN (%) |
|---|---|---|---|---|---|---|
| 1.768 | 6.5 | 2.5 | 0.141 | 0.97 | 0.1 | 0.010 |
| 7.071 | 25.5 | 9.6 | 0.136 | 15.72 | 1.4 | 0.009 |
| 17.678 | 59.4 | 21.2 | 0.120 | 91.70 | 6.7 | 0.007 |
| 35.355 | 105.6 | 38.1 | 0.108 | 372.50 | 21.6 | 0.006 |
| RMS Input Voltage (V) | Measured RMS Power Output (nW) | Simulated RMS Power Output (nW) |
|---|---|---|
| 1.768 | 2 | 3 |
| 7.071 | 31 | 35 |
| 17.678 | 150 | 158 |
| 35.355 | 486 | 497 |
| Parameter | Value |
|---|---|
| SST housing cross-sectional area (A) [m2] | 1·10−4 |
| Acoustic compliance (CA) [m5 N−1] | 7.85·10−13 |
| Back-air cavity cross-sectional area (Am) [m2] | 0.75·10−6 |
| Damping (bm) [N s m−1] | 9.09·10−6 |
| Mass (mm) [kg] | 5.01·10−9 |
| Compliance (k−1) [m N−1] | 1.16·10−3 |
| Coupling coefficient (η) [N V−1] | 8.31·10−5 |
| Equivalent capacitance (CpMUT) [F] | 290·10−12 |
| Solution | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Electromagnetic (far field) | Small antenna | Low efficiency | [56] |
| High loss in body | [57] | ||
| Electromagnetic (near field) | High performance | Short distance | [58] |
| Low loss in body | Coil size | [59] | |
| Ultrasound | High efficiency in body | High loss in air | [60] |
| Deeply implant | Special equipment | [61] |
| RX Struct. | RX csa (mm2) | TX-RX Distan. (mm) | Propagat. Medium | Oper. Freq. (kHz) | Power Input (mW cm−2) | Power Output (µW cm−2) | Effic. (%) | Lead Free | CMOS Comp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PZT | 2.25 | 23 | water | 350 | 65 | 4.1 | 0.0063 | NO | NO | [62] |
| PZT | 2.058 | 10 | water | 240 | 1 | 3.75 | 0.375 | NO | NO | [24] |
| PZT | 4 | 20 | water | 88 | 322 | 1063 | 0.33 | NO | NO | [35] |
| PZT | 314 | - | water | wide range 600–1200 | - | - | - | NO | NO | [47] |
| PNZT | 30 | 22 | fatty | 40 | - | - | 0.096 | NO | NO | [48] |
| KNNS | 2 | 20 | water | 304 | - | - | - | YES | NO | [50] |
| AlN | 16 | 40 | oil | 2000 | 77 | 71 | 0.009 | YES | YES | [63] |
| AlN | 1.44 | 127 | air | 500 | - | - | - | YES | YES | [64] |
| AlN | 2.55 | 25 | water | 3000 | 7 | 16.47 | 0.235 | YES | YES | [65] |
| AlN | 2.25 | 8.6 | PDMS | 67 | 1 | 0.1 | 0.010 | YES | YES | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proto, A.; Rufer, L.; Basrour, S.; Penhaker, M. Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an AlN-Based pMUT as Receiver. Micromachines 2022, 13, 2127. https://doi.org/10.3390/mi13122127
Proto A, Rufer L, Basrour S, Penhaker M. Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an AlN-Based pMUT as Receiver. Micromachines. 2022; 13(12):2127. https://doi.org/10.3390/mi13122127
Chicago/Turabian StyleProto, Antonino, Libor Rufer, Skandar Basrour, and Marek Penhaker. 2022. "Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an AlN-Based pMUT as Receiver" Micromachines 13, no. 12: 2127. https://doi.org/10.3390/mi13122127
APA StyleProto, A., Rufer, L., Basrour, S., & Penhaker, M. (2022). Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an AlN-Based pMUT as Receiver. Micromachines, 13(12), 2127. https://doi.org/10.3390/mi13122127










