Design and Simulation of a Ring Transducer Array for Ultrasound Retinal Stimulation
Abstract
1. Introduction
2. Methods
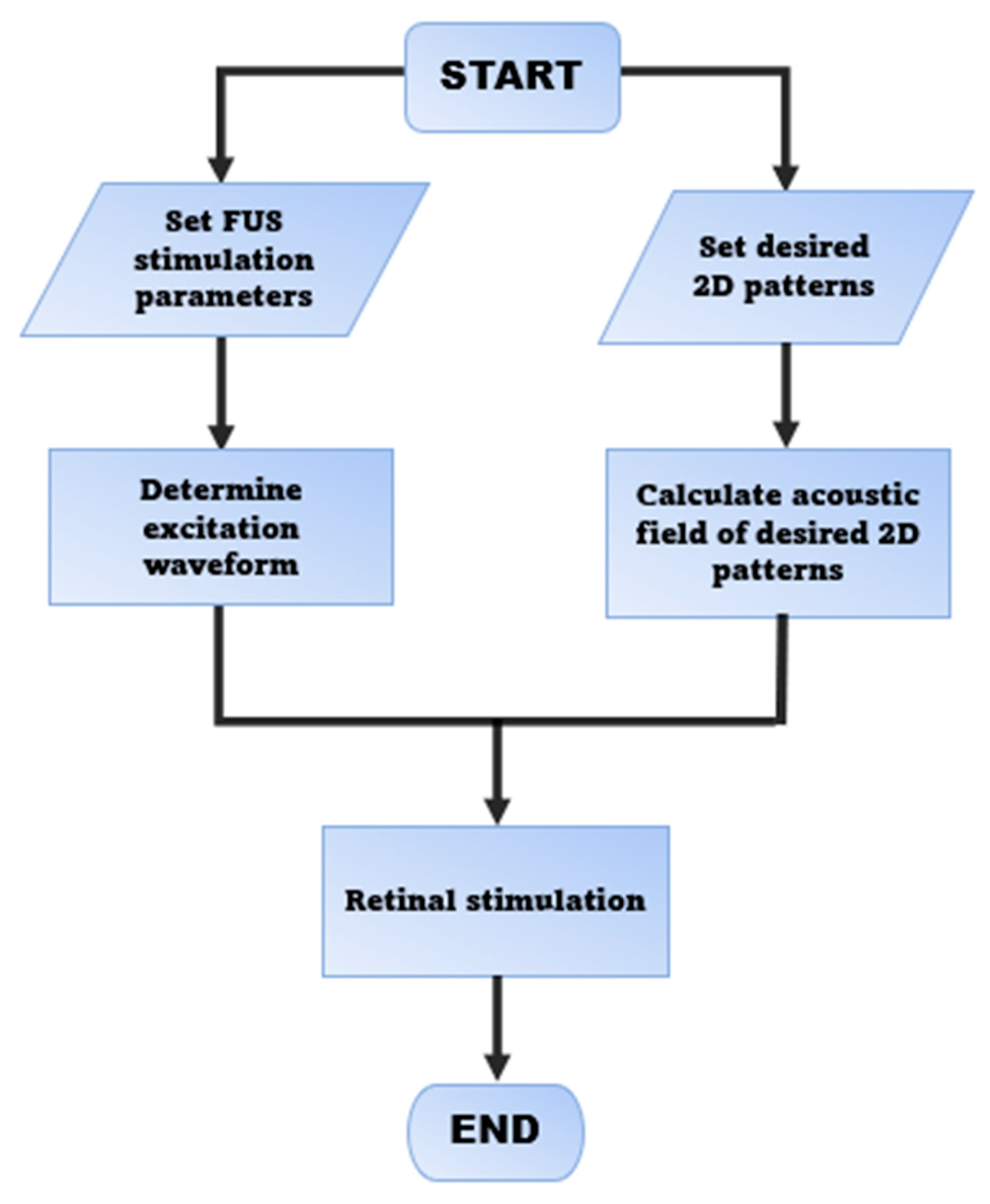
2.1. Strategy of FUS Retinal Stimulation
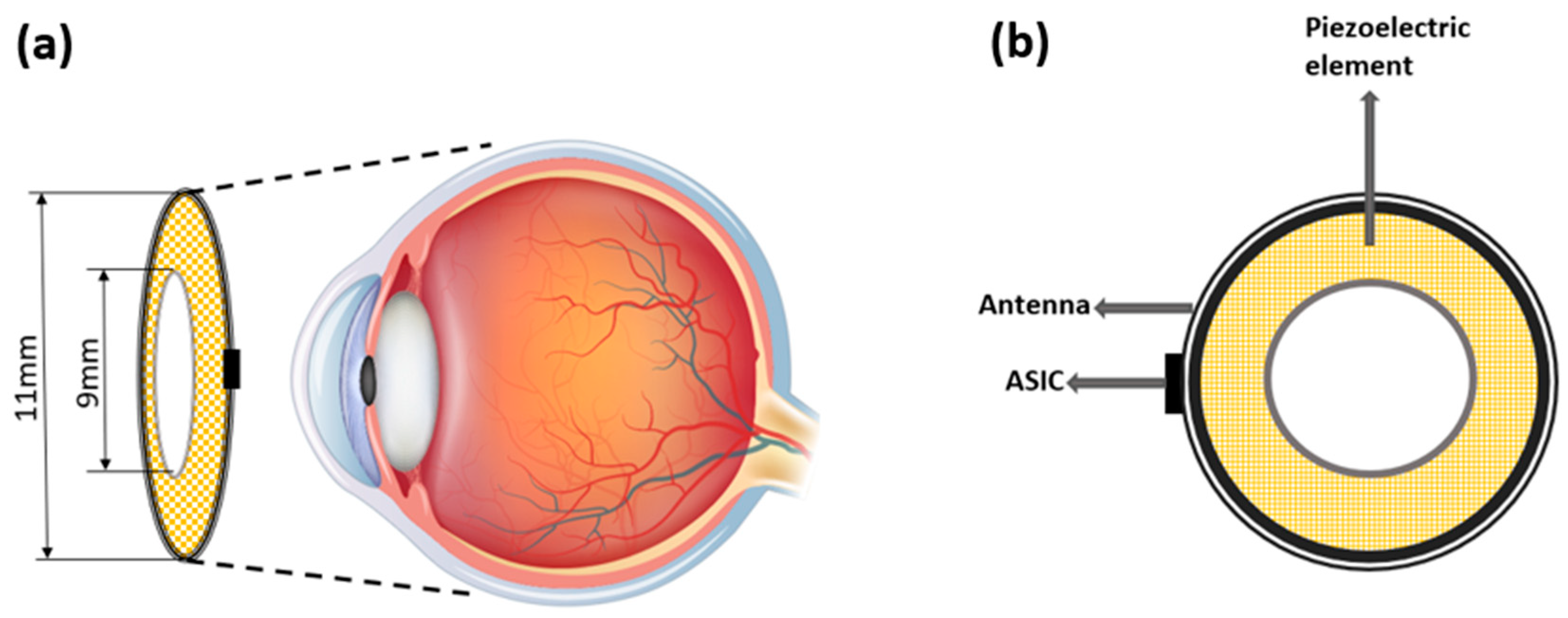
2.2. Design of the Ring Array Transducer
2.3. Simulation of the Ultrasonic Excitation Waveform and Pattern
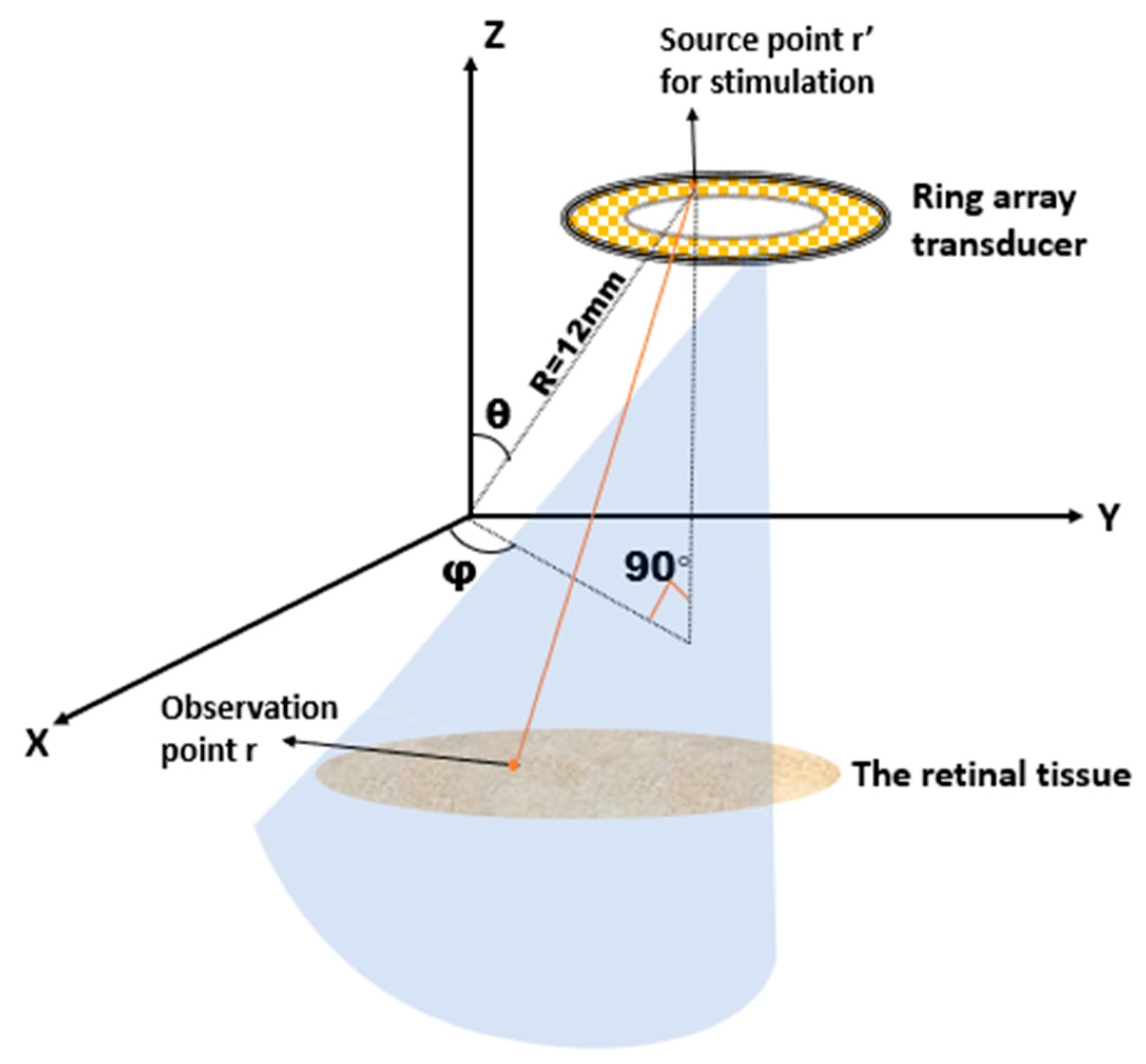
2.3.1. Determine the Excitation Waveform of Each PZT Element
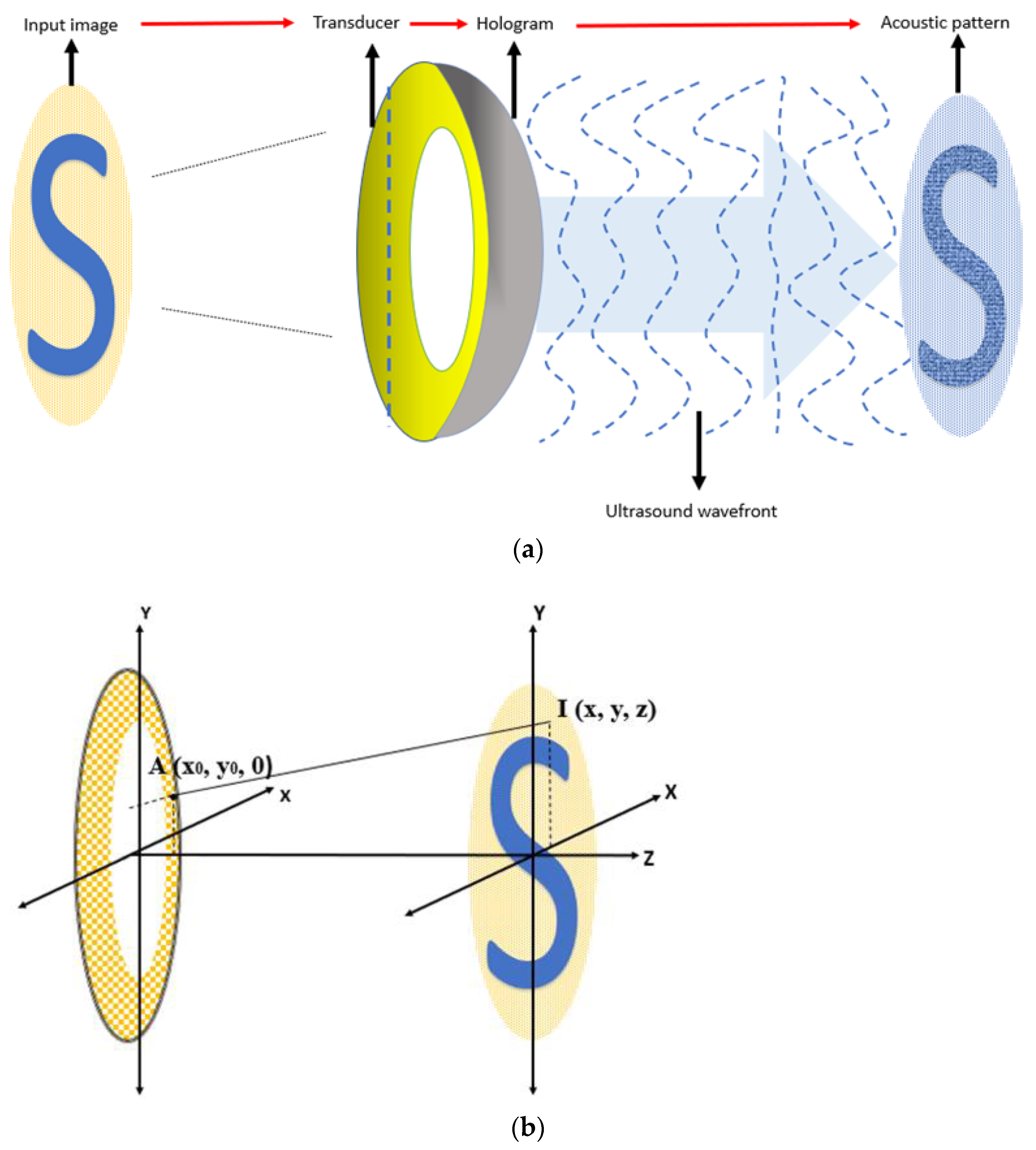
2.3.2. Determine the Pattern Generation Algorithm
2.3.3. Determine the Ultrasonic Frequency for Stimulation and Imaging
3. Result
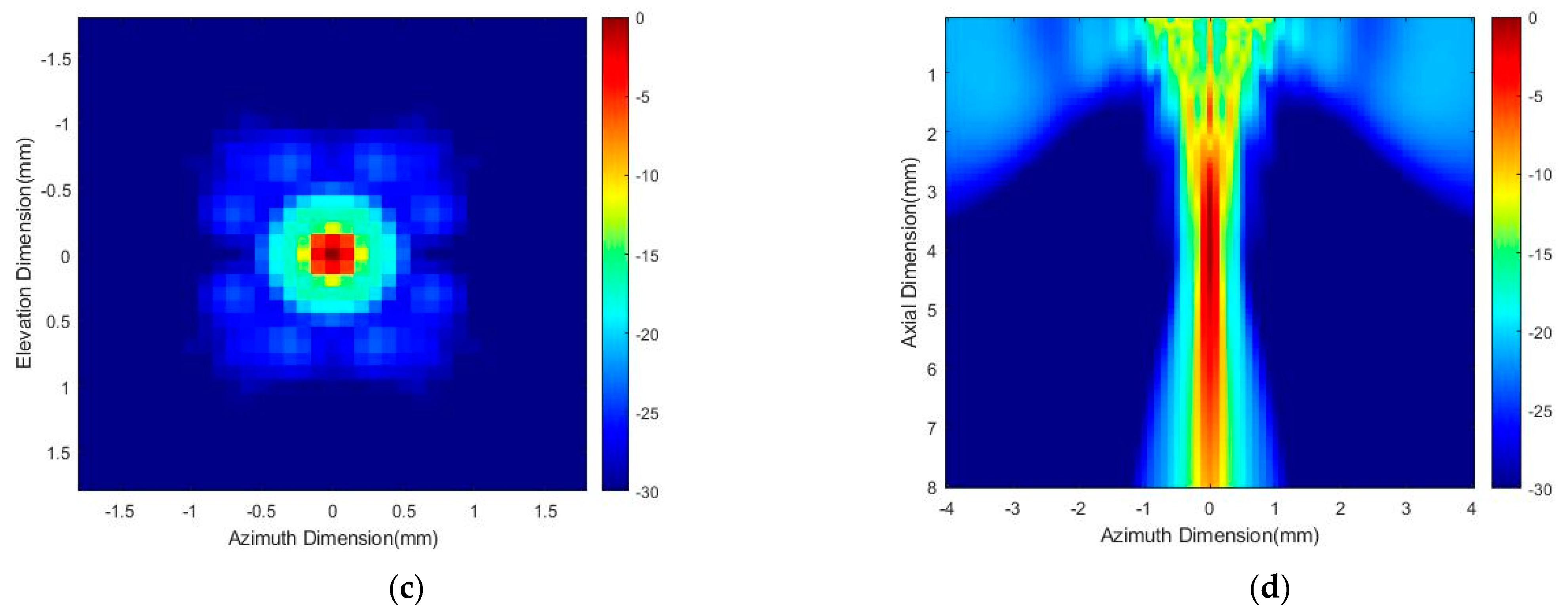
3.1. Acoustic Power Distribution
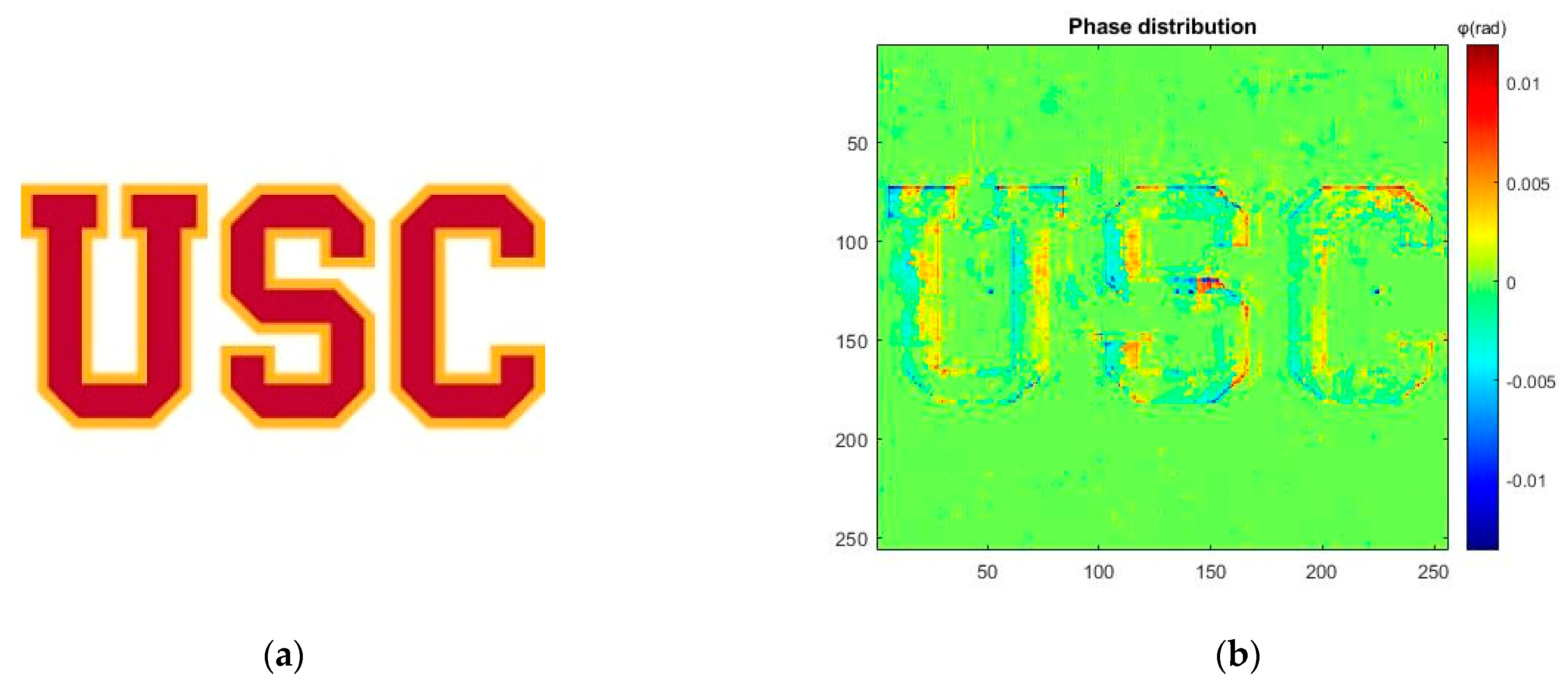
3.2. Pattern Generation Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wandell, B.A. Foundations of Vision; Sinauer Associates Inc.: Sunderland, MA, USA, 1995; pp. 13–54. [Google Scholar]
- Wells, J.; Wroblewski, J.; Keen, J.; Inglehearn, C.; Jubb, C.; Eckstein, A.; Jay, M.; Arden, G.; Bhattacharya, S.; Fitzke, F.; et al. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat. Genet. 1993, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Berson, E.L. Retinitis pigmentosa. The Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1659–1676. [Google Scholar]
- Klein, R.; Klein, B.E.; Jensen, S.C.; Meuer, S.M. The five-year incidence and progression of age-related maculopathy: The Beaver dameye study. Ophthalmology 1997, 104, 7–21. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sc, L.M.; Ehinger, B. Management of Hereditary Retinal Degenerations: Present Status and Future Directions. Surv. Ophthalmol. 1999, 43, 427–444. [Google Scholar] [CrossRef]
- Humayun, M.S.; Weiland, J.D.; Fujii, G.Y.; Greenberg, R.; Williamson, R.; Little, J.; Mech, B.; Cimmarusti, V.; Van Boemel, G.; Dagnelie, G.; et al. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vis. Res. 2003, 43, 2573–2581. [Google Scholar] [CrossRef]
- Picaud, S.; Sahel, J.-A. Retinal prostheses: Clinical results and future challenges. Comptes Rendus. Biol. 2014, 337, 214–222. [Google Scholar] [CrossRef]
- Ong, J.M.; da Cruz, L. The bionic eye: A review. Clin. Experim. Ophthalmol. 2012, 40, 6–17. [Google Scholar] [CrossRef]
- Second Sight Medical Products Receives FDA Approval for Argus II System. 2013. Available online: https://investors.secondsight.com/news-releases/news-release-details/second-sight-medical-products-receives-fda-approval-argus-ii (accessed on 15 August 2022).
- Stronks, H.C.; Dagnelie, G. The functional performance of the Argus II retinal prosthesis. Expert Rev. Med. Devices 2013, 11, 23–30. [Google Scholar] [CrossRef]
- Winter, J.O.; Cogan, S.F.; Rizzo, J.F. Retinal prostheses: Current challenges and future outlook. J. Biomater. Sci. Polym. Ed. 2007, 18, 1031–1055. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Non-invasive human brain stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef]
- Behrend, M.R.; Ahuja, A.K.; Humayun, M.S.; Chow, R.H.; Weiland, J.D. Resolution of the Epiretinal Prosthesis is not Limited by Electrode Size. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 436–442. [Google Scholar] [CrossRef]
- Weitz, A.C.; Nanduri, D.; Behrend, M.R.; Gonzalez-Calle, A.; Greenberg, R.J.; Humayun, M.S.; Chow, R.H.; Weiland, J.D. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration. Sci. Transl. Med. 2015, 7, 203–318. [Google Scholar] [CrossRef]
- Shah, N.P.; Chichilnisky, E.J. Computational challenges and opportunities for a bi-directional artificial retina. J. Neural Eng. 2020, 17, 055002. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.-P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.-H. Ectopic Expression of a Microbial-Type Rhodopsin Restores Visual Responses in Mice with Photoreceptor Degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic Reactivation of Cone Photoreceptors Restores Visual Responses in Retinitis Pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef]
- Legal, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON-bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Degli Esposti, S.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.H.; Tyler, W.J. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011, 6, 1453–1470. [Google Scholar] [CrossRef]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef]
- Yoo, S.-S.; Bystritsky, A.; Lee, J.-H.; Zhang, Y.; Fischer, K.; Min, B.-K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. NeuroImage 2011, 56, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, T.; Younan, Y.; Wattiez, N.; Tanter, M.; Pouget, P.; Aubry, J.-F. Low-Intensity Focused Ultrasound Modulates Monkey Visuomotor Behavior. Curr. Biol. 2013, 23, 2430–2433. [Google Scholar] [CrossRef]
- Legon, W.; Rowlands, A.; Opitz, A.; Sato, T.F.; Tyler, W.J. Pulsed Ultrasound Differentially Stimulates Somatosensory Circuits in Humans as Indicated by EEG and fMRI. PLoS ONE 2012, 7, e51177. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Menz, M.D.; Oralkan, O.; Khuri-Yakub, P.T.; Baccus, S.A. Precise Neural Stimulation in the Retina Using Focused Ultrasound. J. Neurosci. 2013, 33, 4550–4560. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, L.; Qin, L.; Zhang, Y. Characterization of an Improved 1–3 Piezoelectric Composite by Simulation and Experi-ment. J. Appl. Biomater. Funct. Mater. 2017, 15 (Suppl. S1), e38–e44. [Google Scholar]
- Casper, A.; Liu, D.; Ebbini, E.S. Real-time control of multiple focus phased array heating patterns based on non-invasive ultrasound thermography. IEEE Trans. Biomed. Eng. 2012, 59, 95–105. [Google Scholar] [CrossRef]
- Ebbini, E.; Cain, C. Multiple-focus ultrasound phased-array pattern synthesis: Optimal driving-signal distributions for hyperthermia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 540–548. [Google Scholar] [CrossRef]
- Drinkwater, B.W.; Wilcox, P.D. Ultrasonic arrays for non-destructive evaluation: A review. NDT E Int. 2006, 39, 525–541. [Google Scholar]
- Hertzberg, Y.; Naor, O.; Volovick, A.; Shoham, S. Towards multifocal ultrasonic neural stimulation: Pattern generation algorithms. J. Neural Eng. 2010, 7, 056002. [Google Scholar] [CrossRef] [PubMed]
- Naor, O.; Hertzberg, Y.; Zemel, E.; Kimmel, E.; Shoham, S. Towards multifocal ultrasonic neural stimulation II: Design con-siderations for an acoustic retinal prosthesis. J. Neural Eng. 2012, 9, 026006. [Google Scholar] [CrossRef]
- Gao, M.; Yu, Y.; Zhao, H.; Li, G.; Jiang, H.; Wang, C.; Cai, F.; Chan, L.; Chiu, B.; Qian, W.; et al. A simulation study of ultrasonic retinal prosthesis with a novel contact-lens array for non-invasive retinal stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H. Basics of Biomedical Ultrasound for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Yu, Y.; Zhang, Z.; Cai, F.; Su, M.; Jiang, Q.; Zhou, Q.; Humayun, M.S.; Qiu, W.; Zheng, H. A Novel Racing Array Transducer for Noninvasive Ultrasonic Retinal Stimulation: A Simulation Study. Sensors 2019, 19, 1825. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.-A.; Huang, K.; Zhou, Q.; Humayun, M.S.; Yue, L. Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis. Micromachines 2020, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kumar, M.; Oralkan, O. An ultrasound-based non-invasive neural interface to the retina. In IEEE International Ultrasonics Symposium; IEEE: Piscataway, NJ, USA, 2014; pp. 2623–2626. [Google Scholar]
- Hajati, A.; Latev, D.; Gardner, D.; Hajati, A.; Imai, D.; Torrey, M.; Schoeppler, M. Three-dimensional microelectromechanical system piezoelectric ultrasound transducer. Appl. Phys. Lett. 2012, 101, 253101. [Google Scholar] [CrossRef]
- Muralt, P.; Ledermann, N.; Paborowski, J.; Barzegar, A.; Gentil, S.; Belgacem, B.; Petitgrand, S.; Bosseboeuf, A.; Setter, N. Piezoelectric micromachined ultrasonic transducers based on PZT thin films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2276–2288. [Google Scholar] [CrossRef]
- Melde, K.; Mark, A.; Qiu, T.; Fischer, P. Holograms for acoustics. Nature 2016, 537, 518–522. [Google Scholar] [CrossRef]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef]
- Ye, S.; Harasiewicz, K.; Pavlin, C.; Foster, F.S. Ultrasound characterization of ocular tissue in the frequency range from 50 MHz to 100 MHz. In Proceedings of the IEEE Ultrasonics Symposium Proceedings, Tucson, AZ, USA, 20–23 October 1992; IEEE: Piscataway, NJ, USA, 1994; Volume 2, pp. 1107–1112. [Google Scholar]
- De Korte, C.L.; Van der Steen, A.F.; Thijssen, J.M. Acoustic velocity and attenuation of eye tissues at 20 MHz. Ultrasound Med. Biol. 1994, 20, 471–480. [Google Scholar] [CrossRef]
- Southern California Trojans Logos History. Available online: https://sportslogohistory.com/southern-california-trojans-wordmark-logo (accessed on 17 August 2022).
- Duck, F.A. Medical and non-medical protection standards for ultrasound and infrasound. Prog. Biophys. Mol. Biol. 2007, 93, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, J.; Shrivastava, S.; Sallet, J.; Butler, C.R.; Cleveland, R.O. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol. 2019, 45, 1509–1536. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.; Huxley, A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]



| Designed Parameter | Value |
|---|---|
| Array Dimensions | OD = 11 mm, ID = 9 mm |
| Center Frequency | 20 MHz |
| Number of Element | 512, 10% sparse |
| Element Size | 0.15 × 0.15 mm2 |
| Element Pitch (1 λ) | 0.075 mm |
| Thickness | 1.4 mm |
| Stimulation Depth | 7 mm |
| Radial Curvature | 12 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Lu, G.; Kang, H.; Humayun, M.S.; Zhou, Q. Design and Simulation of a Ring Transducer Array for Ultrasound Retinal Stimulation. Micromachines 2022, 13, 1536. https://doi.org/10.3390/mi13091536
Xu C, Lu G, Kang H, Humayun MS, Zhou Q. Design and Simulation of a Ring Transducer Array for Ultrasound Retinal Stimulation. Micromachines. 2022; 13(9):1536. https://doi.org/10.3390/mi13091536
Chicago/Turabian StyleXu, Chenlin, Gengxi Lu, Haochen Kang, Mark S. Humayun, and Qifa Zhou. 2022. "Design and Simulation of a Ring Transducer Array for Ultrasound Retinal Stimulation" Micromachines 13, no. 9: 1536. https://doi.org/10.3390/mi13091536
APA StyleXu, C., Lu, G., Kang, H., Humayun, M. S., & Zhou, Q. (2022). Design and Simulation of a Ring Transducer Array for Ultrasound Retinal Stimulation. Micromachines, 13(9), 1536. https://doi.org/10.3390/mi13091536








