Genosensing Applications of Glassy Carbon Electrodes Modified with Multi-Walled Carbon Nanotubes Non-Covalently Functionalized with Polyarginine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
- oligo21: 5′-TCA-ACA-TCA-GTCTGA-TAA-GCT-A-3′
- microRNA-21: 5′-UAG-CUU-AUC-AGA-CUG-AUGUUG-A-3′
- fully non-complementary sequence: 5′-GGG-GGG-GGGGGG-GGG-3′
- single-base mismatch: 5′-UAG-CUU-AUC-ACA-CUGAUG-UUG-A-3′
2.2. Apparatus
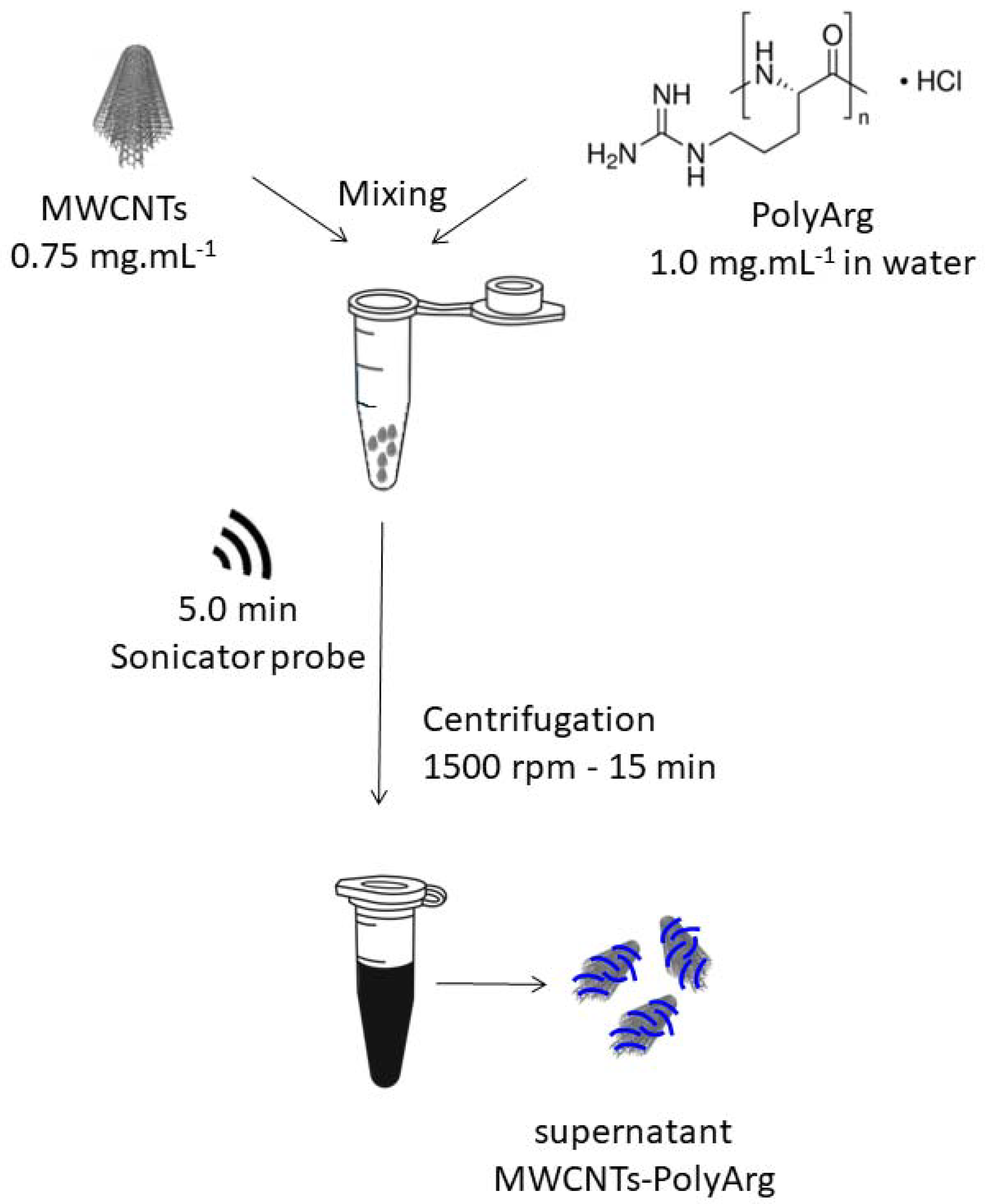
2.3. Preparation of MWCNTs Functionalized with PolyArg (MWCNTs-PolyArg)
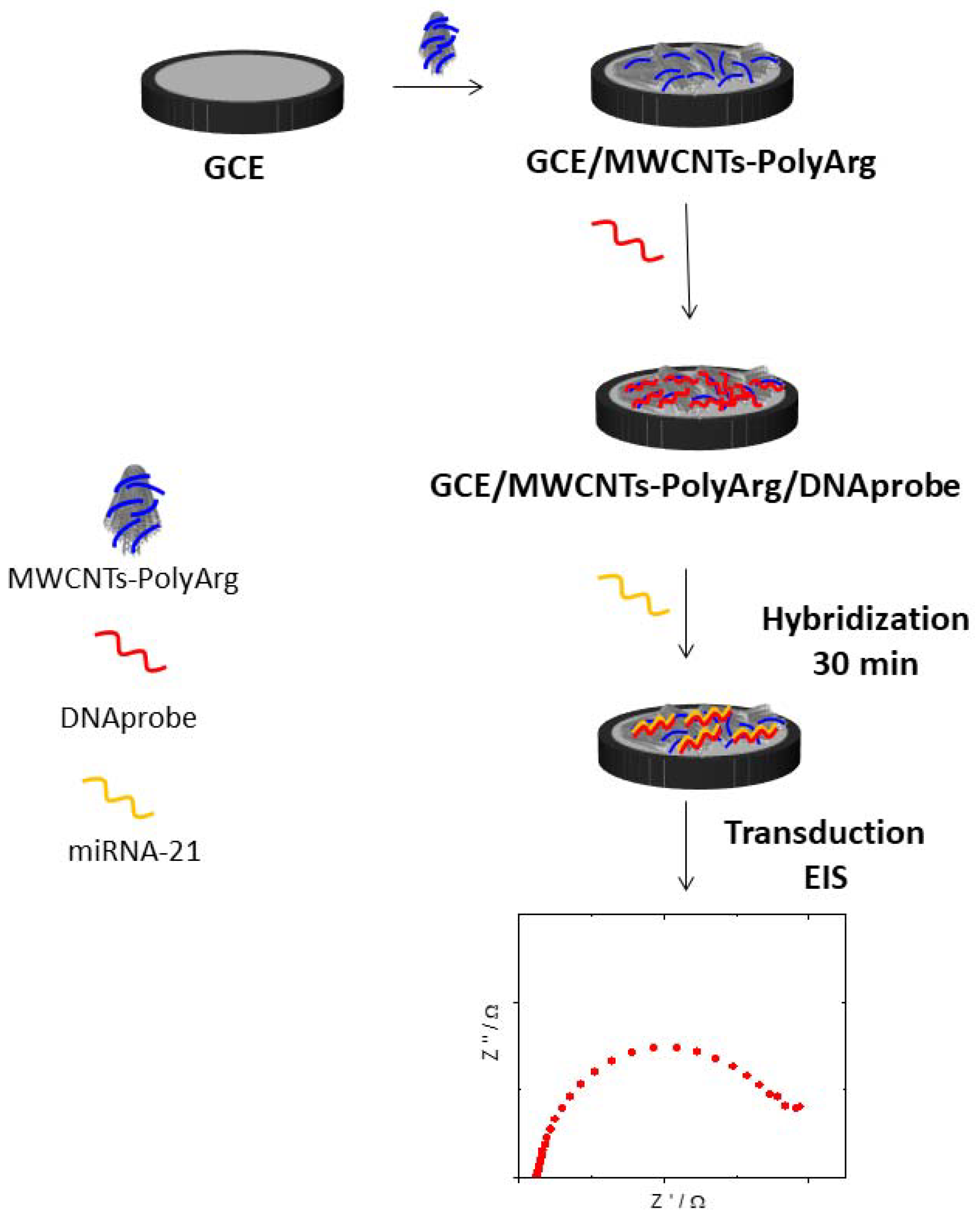
2.4. Preparation of GCE Modified with MWCNT-PolyArg (GCE/MWCNT-PolyArg)
2.5. Preparation of GCE/MWCNTs-PolyArg Modified with DNA
2.6. microRNA-21 Genosensor
3. Results
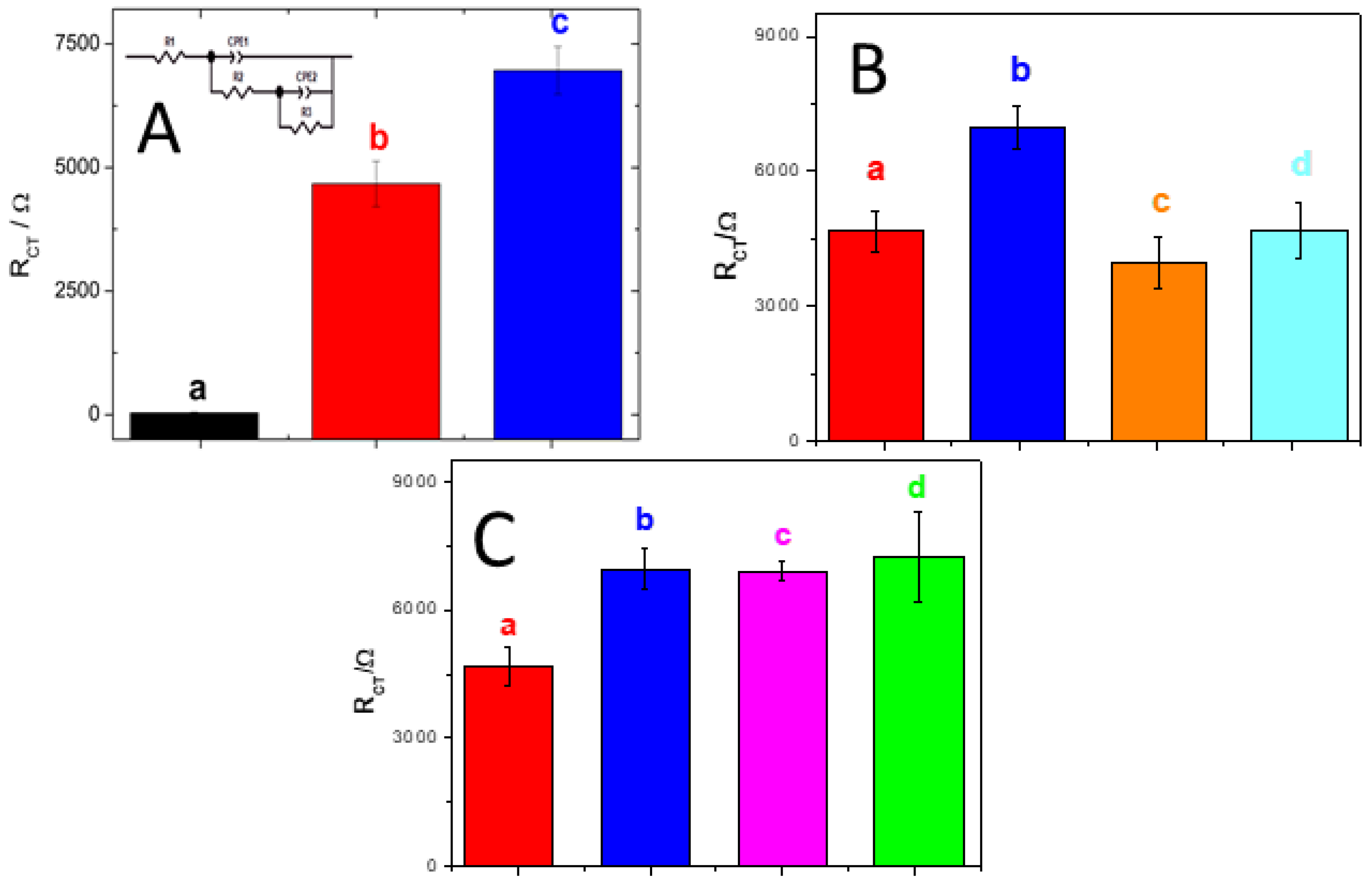
3.1. Adsorption and Electrooxidation of Different DNAs at GCE/MWCNT-PolyArg
3.2. GCE/MWCNT-PolyArg as a Platform to Build a miRNA-21 Genosensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostafa, I.; Tian, Y.; Anjum, S.; Hanif, S.; Hosseini, M.; Lou, B.; Xu, G. Comprehensive review on the electrochemical biosensors of different breast cancer biomarkers. Sens. Actuators B Chem. 2022, 365, 131944. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Application of electrochemical biosensors for the detection of microRNAs (miRNAs) related to cancer. Coord. Chem. Rev. 2022, 464, 214565. [Google Scholar] [CrossRef]
- Melinte, G.; Hosu, O.; Cristea, C.; Marrazza, G. DNA sensing technology a useful food scanning tool. TrAC—Trends Anal. Chem. 2022, 154, 116679. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.; Ozcelikay, G.; Budak, F.; Ozkan, S. Carbon Nanomaterials-Based Novel Hybrid Platforms for Electrochemical Sensor Applications in Drug Analysis. Crit. Rev. Anal. Chem. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhu, Y.; Zhang, Y.; Zhang, M.; Zhang, Y.; Qiao, L.; Yin, N.; Song, K.; Liu, M.; Wang, W. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem. J. 2021, 171, 106776. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Nanostructured material–based electrochemical sensing of oxidative DNA damage biomarkers 8-oxoguanine and 8-oxodeoxyguanosine: A comprehensive review. Microchim. Acta 2021, 188, 58. [Google Scholar] [CrossRef]
- Wang, Y.; Rinawati, M.; Zhan, J.; Lin, K.; Huang, C.; Chen, K.; Mizuguchi, H.; Jiang, J.; Hwang, B.; Yeh, M. Boron-Doped Graphene Quantum Dots Anchored to Carbon Nanotubes as Noble Metal-Free Electrocatalysts of Uric Acid for a Wearable Sweat Sensor. ACS Appl. Nano Mater. 2022, 5, 11100–11110. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Liu, Z.; Ling, G.; Zhang, P. Application of electrochemical sensors based on nanomaterials modifiers in the determination of antipsychotics. Colloids Surf. B Biointerfaces 2022, 214, 112442. [Google Scholar] [CrossRef]
- Eguílaz, M.; Dalmasso, P.R.; Rubianes, M.D.; Gutierrez, F.; Rodríguez, M.C.; Gallay, P.A.; López Mujica, M.; Ramírez, M.L.; Tettamanti, C.; Montemerlo, A.; et al. Recent advances in the development of electrochemical hydrogen peroxide carbon nanotube–based (bio)sensors. Curr. Opin. Electrochem. 2019, 14, 157–165. [Google Scholar] [CrossRef]
- Mujica, M.; Tamborelli, A.; Castellaro, A.; Barcudi, D.; Rubianes, M.; Rodríguez, M.; Saka, H.; Bocco, J.; Dalmasso, P.; Rivas, G. Impedimetric and amperometric genosensors for the highly sensitive quantification of SARS-CoV-2 nucleic acid using an avidin-functionalized multi-walled carbon nanotubes biocapture platform. Biosens. Bioelectron. X 2022, 12, 100222. [Google Scholar] [CrossRef]
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Encarnacion, L.; Morais, S. Laccase bioconjugate and multi-walled carbon nanotubes-based biosensor for bisphenol A analysis. Bioelectrochemistry 2022, 144, 108033. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.; Paukov, M.; Burdanova, M. Nanotube Functionalization: Investigation, Methods and Demonstrated Applications. Materials 2022, 15, 5386. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Ghaffarzadeh, M. Surface functionalization of carbon nanotubes via plasma discharge: A review. Inorg. Chem. Commun. 2022, 138, 109276. [Google Scholar] [CrossRef]
- Ferrier, D.; Honeychurch, K. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Rubianes, M.; Rivas, G. Dispersion of multi-wall carbon nanotubes in polyethylenimine: A new alternative for preparing electrochemical sensors. Electrochem. Commun. 2007, 9, 480–484. [Google Scholar] [CrossRef]
- Jalit, Y.; Rodríguez, M.; Rubianes, M.; Bollo, S.; Rivas, G. Glassy carbon electrodes modified with multiwall carbon nanotubes dispersed in polylysine. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1623–1631. [Google Scholar] [CrossRef]
- Dalmasso, P.; Pedano, M.; Rivas, G. Dispersion of multi-wall carbon nanotubes in polyhistidine: Characterization and analytical applications. Anal. Chim. Acta 2012, 710, 58–64. [Google Scholar] [CrossRef]
- Gutierrez, F.; Rubianes, M.; Rivas, G. Dispersion of multi-wall carbon nanotubes in glucose oxidase: Characterization and analytical applications for glucose biosensing. Sens. Actuators B Chem. 2012, 161, 191–197. [Google Scholar] [CrossRef]
- Eguílaz, M.; Gutiérrez, A.; Rivas, G. Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: Enhanced direct electron transfer and analytical applications. Sens. Actuators B Chem. 2016, 225, 74–80. [Google Scholar] [CrossRef]
- Gutierrez, F.; Rubianes, M.; Rivas, G. New bioanalytical platform based on the use of avidin for the successful exfoliation of multi-walled carbon nanotubes and the robust anchoring of biomolecules. Application for hydrogen peroxide biosensing. Anal. Chim. Acta 2019, 1065, 12–20. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef] [PubMed]
- Mujica, M.; Rubianes, M.; Rivas, G. A multipurpose biocapture nanoplatform based on multiwalled-carbon nanotubes non-covalently functionalized with avidin: Analytical applications for the non-amplified and label-free impedimetric quantification of BRCA1. Sens. Actuators B Chem. 2022, 357, 131304. [Google Scholar] [CrossRef]
- Ortiz, E.; Gallay, P.; Galicia, L.; Eguílaz, M.; Rivas, G. Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with Concanavalin A: A new building-block with supramolecular recognition properties for the development of electrochemical biosensors. Sens. Actuators B Chem. 2019, 292, 254–262. [Google Scholar] [CrossRef]
- Primo, E.; Cañete-Rosales, P.; Bollo, S.; Rubianes, M.; Rivas, G. Dispersion of bamboo type multi-wall carbon nanotubes in calf-thymus double stranded DNA. Colloids Surf. B Biointerfaces 2013, 108, 329–336. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Gutierrez, F.; Eguílaz, M.; Parrado, C.; Rivas, G. Non-covalent Functionalization of Multi-wall Carbon Nanotubes with Polyarginine: Characterization and Analytical Applications for Uric Acid Quantification. Electroanalysis 2018, 30, 1416–1424. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Multi-walled Carbon Nanotubes Non-covalently Functionalized with Polyarginine: A New Alternative for the Construction of Reagentless NAD+/Dehydrogenase-based Ethanol Biosensor. Electroanalysis 2019, 31, 805–812. [Google Scholar] [CrossRef]
- Jalit, Y.; Moreno, M.; Gutierrez, F.; Sánchez Arribas, A.; Chicharro, M.; Bermejo, E.; Zapardiel, A.; Parrado, C.; Rivas, G.; Rodríguez, M. Adsorption and electrooxidation of nucleic acids at glassy carbon electrodes modified with multi-wall carbon nanotubes dispersed in polylysine. Electroanalysis 2013, 25, 1116–1121. [Google Scholar] [CrossRef]
- Pedano, M.; Rivas, G. Immobilization of DNA on glassy carbon electrodes for the development of affinity biosensors. Biosens. Bioelectron. 2003, 18, 269–277. [Google Scholar] [CrossRef]
- Cai, X.; Rivas, G.; Farias, P.A.M.; Shiraishi, H.; Wang, J.; Palecek, E. Evaluation of Different Carbon Electrodes for Adsorptive Stripping Analysis of Nucleic Acids. Electroanalysis 1996, 8, 753–758. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Wang, J.; Jonsson, C.; Paleček, E. Trace Measurements of RNA by Potentiometric Stripping Analysis at Carbon Paste Electrodes. Anal. Chem. 1995, 67, 4065–4070. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Jonsson, C.; Balakrishnan, M. Adsorptive Stripping Potentiometry of DNA at Electrochemically Pretreated Carbon Paste Electrodes. Electroanalysis 1996, 8, 20–24. [Google Scholar] [CrossRef]
- Wang, J.; Rivas, G.; Fernandes, J.R.; Jiang, M.; Lopez Paz, J.L.; Waymire, R.; Nielsen, T.W.; Getts, R.C. Adsorption and Detection of DNA Dendrimers at Carbon Electrodes. Electroanalysis 1998, 10, 553–556. [Google Scholar] [CrossRef]
- Kaplan, M.; Kilic, T.; Guler, G.; Mandli, J.; Amine, A.; Ozsoz, M. A novel method for sensitive microRNA detection: Electropolymerization based doping. Biosens. Bioelectron. 2017, 92, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cao, W.; Liu, W.; Lu, Z.; Zhu, D.; Chao, J.; Weng, L.; Wang, L.; Fan, C.; Wang, L. Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens. Bioelectron. 2017, 94, 552–559. [Google Scholar] [CrossRef]
- Meng, T.; Zhao, D.; Ye, H.; Feng, Y.; Wang, H.; Zhang, Y. Construction of an ultrasensitive electrochemical sensing platform for microRNA-21 based on interface impedance spectroscopy. J. Colloid Interface Sci. 2020, 578, 164–170. [Google Scholar] [CrossRef]
- La, M.; Zhang, Y.; Gao, Y.; Li, M.; Liu, L.; Chang, Y. Impedimetric detection of microRNAs by the signal amplification of streptavidin induced in situ formation of biotin phenylalanine nanoparticle networks. J. Electrochem. Soc. 2020, 167, 117505. [Google Scholar] [CrossRef]
- Mandli, J.; Amine, A. Impedimetric genosensor for miRNA-34a detection in cell lysates using polypyrrole. J. Solid State Electrochem. 2018, 22, 1007–1014. [Google Scholar] [CrossRef]
- Zouari, M.; Campuzano, S.; Pingarrón, J.; Raouafi, N. Amperometric biosensing of miRNA-21 in serum and cancer cells at nanostructured platforms using anti-DNA–RNA hybrid antibodies. ACS Omega 2018, 3, 8923–8931. [Google Scholar] [CrossRef]
- Vargas, E.; Povedano, E.; Montiel, V.; Torrente-Rodríguez, R.; Zouari, M.; Montoya, J.; Raouafi, N.; Campuzano, S.; Pingarrón, J. Single-step incubation determination of miRNAs in cancer cells using an amperometric biosensor based on competitive hybridization onto magnetic beads. Sensors 2018, 18, 863. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Li, J.; Jin, X.; Liu, Y.; Huang, S. Ultrasensitive electrochemical microRNA-21 biosensor coupling with carboxylate-reduced graphene oxide-based signal-enhancing and duplex-specific nuclease-assisted target recycling. Sens. Actuators B Chem. 2019, 297, 126740. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Chen, R.; Wang, X.; Wang, Y.; Wei, J.; Zhang, K.; Zhang, C. Electrochemical biosensing of circulating microRNA-21 in cerebrospinal fluid of medulloblastoma patients through target-induced redox signal amplification. Microchim. Acta 2022, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Huang, W.; Qin, C.; Yu, A.; Lai, G. Exonuclease-assisted target recycling for ultrasensitive electrochemical detection of microRNA at vertically aligned carbon nanotubes. Nanoscale 2019, 11, 11262–11269. [Google Scholar] [CrossRef] [PubMed]
- Mahmudunnabi, R.; Umer, M.; Seo, K.; Park, D.; Chung, J.; Shiddiky, M.; Shim, Y. Exosomal microRNAs array sensor with a bioconjugate composed of p53 protein and hydrazine for the specific lung cancer detection. Biosens. Bioelectron. 2022, 207, 114149. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Feng, J.; Tao, D.; Yan, H.; Ding, J.; Jaffrezic-Renault, N.; Guo, Z. A novel conductive nanocomposite-based biosensor for ultrasensitive detection of microRNA-21 in serum, using methylene blue as mediator. Bioelectrochemistry 2022, 148, 108256. [Google Scholar] [CrossRef] [PubMed]
- Mujica, M.; Zhang, Y.; Bédioui, F.; Gutiérrez, F.; Rivas, G. Label-free graphene oxide–based SPR genosensor for the quantification of microRNA21. Anal. Bioanal. Chem. 2020, 412, 3539–3546. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Yang, X.; Wang, K.; Zhu, J.; He, L.; Li, Q. Surface plasmon resonance biosensor for enzyme-free amplified microRNA detection based on gold nanoparticles and DNA supersandwich. Sens. Actuators B Chem. 2016, 223, 613–620. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, H.; Ru, C.; Zhu, F.; Zou, H.; Gao, P.; Huang, Z.; Wang, J. Plasmonic biosensor for the highly sensitive detection of microRNA-21 via the chemical etching of gold nanorods under a dark-field microscope. Biosens. Bioelectron. 2022, 201, 113942. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Yang, X.; Wang, K.; Zhang, H.; Nie, W. High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide-gold nanoparticles composites. Talanta 2017, 174, 521–526. [Google Scholar] [CrossRef]


| Electrochemical Impedance Spectroscopy—EIS | |||||
|---|---|---|---|---|---|
| Target | Platform-Analytical Signal | LOD | Linear Range R2 | Real Sample | Ref |
| miRNA-21 | Polypyrrole modified pencil graphite electrode. Change in Rct or meldola blue signal. | 12.25 nM | -/0.977 | MCF-7 and HUH-7 | [33] |
| miRNA-21 | AuNPs-decorated MoS2 nanosheet (AuNPs@MoS2) as electrode modifier and signal-amplifier element. | 7.08 fM | 10 fM–1.0 nM/0.99 | Human serum | [34] |
| miRNA-21 | Hybridization chain reaction (HCR) amplification-based impedimetric biosensor. Two sequences are used to trigger HCR amplification (H1 and H2). | 4.63 fM | 10 fM–50 pM/0.998 | Human serum | [35] |
| miRNA-21 | Impedimetric detection of the miRNAs by the signal amplification of insulating biomaterials. Biotinylated miRNA with the same sequence as that of target miRNA was captured by the sensor. | 0.1 fM | 0.1–250 fM/- | Cell lysates | [36] |
| miRNA-34a | Electrochemical entrapment of the probe (antimiRNA-34a) into polypyrrole (PPy) performed by electropolymerization. | 0.2 μg.mL−1 | 5–80 μg.mL−1/0.986 | MCF-7 | [37] |
| miRNA-21 | GCE/MWCNTs-PolyArg/DNAp. Rct of the redox probe hydroquinone/benzoquinone as analytical signal. | 3 fM | 1.0 × 10−14–1.0 × 10−12 M 0.992 | Serum and urine | This Work |
| Amperometry—Differential Pulse Voltammetry (DPV) | |||||
| miRNA-21 | Thiolated DNA capture probe immobilized at gold nanoparticles–nanostructured electrode surface. Analytical signal obtained by amperometry using a specific antibody, horseradish peroxidase/H2O2/hydroquinone. | 29 fM | 0.096−25 pM/- | Human serum | [38] |
| miRNA-21 | Competitive DNA-target miRNA hybridization on the surface of magnetic microbeads. Amperometric transduction at screen-printed carbon electrodes. | 0.2 nM | 0.7–10.0 nM/0.999 | MCF-7 and MCF-10A | [39] |
| miRNA-21 | Capture DNA (cDNA) self-assembled on the surface of gold electrode. Analytical signal obtained from the DPV current due to the accumulation of methylene blue. | 0.01 fM | 0.05–5 fM/0.995 | Human serum | [40] |
| miRNA-21 | Covalent assembling of the capture DNA at the gold nanoparticle-coated glassy carbon electrode. Analytical signal due to DPV current of the accumulated methylene blue at the hybrid obtained by sandwich hybridization with a long guanine-rich sequence. | 56 fM | 0.5–80 pM/0.991 | Medulloblastoma cell extracts and clinical CSF | [41] |
| miRNA-21 | Carboxylated single-walled carbon nanotubes immobilized at an aryldiazonium salt-modified electrode, as a platform to attach a ferrocene-labeled single-stranded DNA by non-covalent adsorption. Electrochemical signal due to the release of this labeled DNA. | 3.5 fM | 0.01 pM–100 pM/0.996 | - | [42] |
| miRNA-21/155/A-205/let-7b | miRNA captured from lysed exosomes in specially designed capture probe modified magnetic beads, followed by T4 DNA polymerase-mediated and in situ formation of chimeric 5′ -miRNA-DNA-3′ (target). | 92 aM | 100 aM–10 pM | Human serum | [43] |
| miRNA-21 | A nanocomposite containing thionine, reduced graphene oxide, ordered mesoporous carbon, and gold nanoparticles was used to increase the specific surface area of a glassy carbon electrode and amplify the DPV signal. | 0.046 fM | 0.1 fM–1.0 pM | Human serum | [44] |
| Surface Plasmon Resonance (SPR) | |||||
| miRNA-21 | Platform obtained by self-assembling of two poly(diallyldimethylammonium chloride) (PDDA) bilayers and graphene oxide at a gold surface modified with 3-mercaptopropane sulfonate (MPS), followed by the covalent attachment of the DNA probe. | 0.3 fM | 1.0 × 10−15–1.0 × 10−6 M | Urine | [45] |
| miRNA-21/miRNA-155 | Sensor based on two-dimensional nanomaterial of antimonene for the specific label-free detection of miRNA-21 and miRNA-155. | 10 aM | 10−17 to 10−11 M | - | [46] |
| miRNA | Enzyme-free amplified biosensor based on gold nanoparticles coupled with DNA supersandwich. The DNA-linked gold nanoparticles as the primary amplification element hybridizes with the capture DNA on the Au film and initiates the subsequent secondary amplification. In the presence of target, stem-loop structure of capture DNA on the Au film surface was unfolded and DNA-linked gold nanoparticles were bound to Au film by hybridization with terminus of capture DNA. | 8 fM | - | Human serum | [47] |
| miRNA | Based on the produced-I2 triggered chemical etching of gold nanorods to a smaller size, resulting in a significant blue shift and high decrease of the localized surface plasmon resonance (LSPR) scattering | 71.22 fM | 0.1–10.000 pM/0.995 | Human serum | [48] |
| miRNA-141 | Based on two layers of graphene oxide–gold nanoparticles (GO–AuNPs). | 0.1 fM | - | Human serum | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallay, P.; López Mujica, M.; Bollo, S.; Rivas, G. Genosensing Applications of Glassy Carbon Electrodes Modified with Multi-Walled Carbon Nanotubes Non-Covalently Functionalized with Polyarginine. Micromachines 2022, 13, 1978. https://doi.org/10.3390/mi13111978
Gallay P, López Mujica M, Bollo S, Rivas G. Genosensing Applications of Glassy Carbon Electrodes Modified with Multi-Walled Carbon Nanotubes Non-Covalently Functionalized with Polyarginine. Micromachines. 2022; 13(11):1978. https://doi.org/10.3390/mi13111978
Chicago/Turabian StyleGallay, Pablo, Michael López Mujica, Soledad Bollo, and Gustavo Rivas. 2022. "Genosensing Applications of Glassy Carbon Electrodes Modified with Multi-Walled Carbon Nanotubes Non-Covalently Functionalized with Polyarginine" Micromachines 13, no. 11: 1978. https://doi.org/10.3390/mi13111978
APA StyleGallay, P., López Mujica, M., Bollo, S., & Rivas, G. (2022). Genosensing Applications of Glassy Carbon Electrodes Modified with Multi-Walled Carbon Nanotubes Non-Covalently Functionalized with Polyarginine. Micromachines, 13(11), 1978. https://doi.org/10.3390/mi13111978






