Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues
Abstract
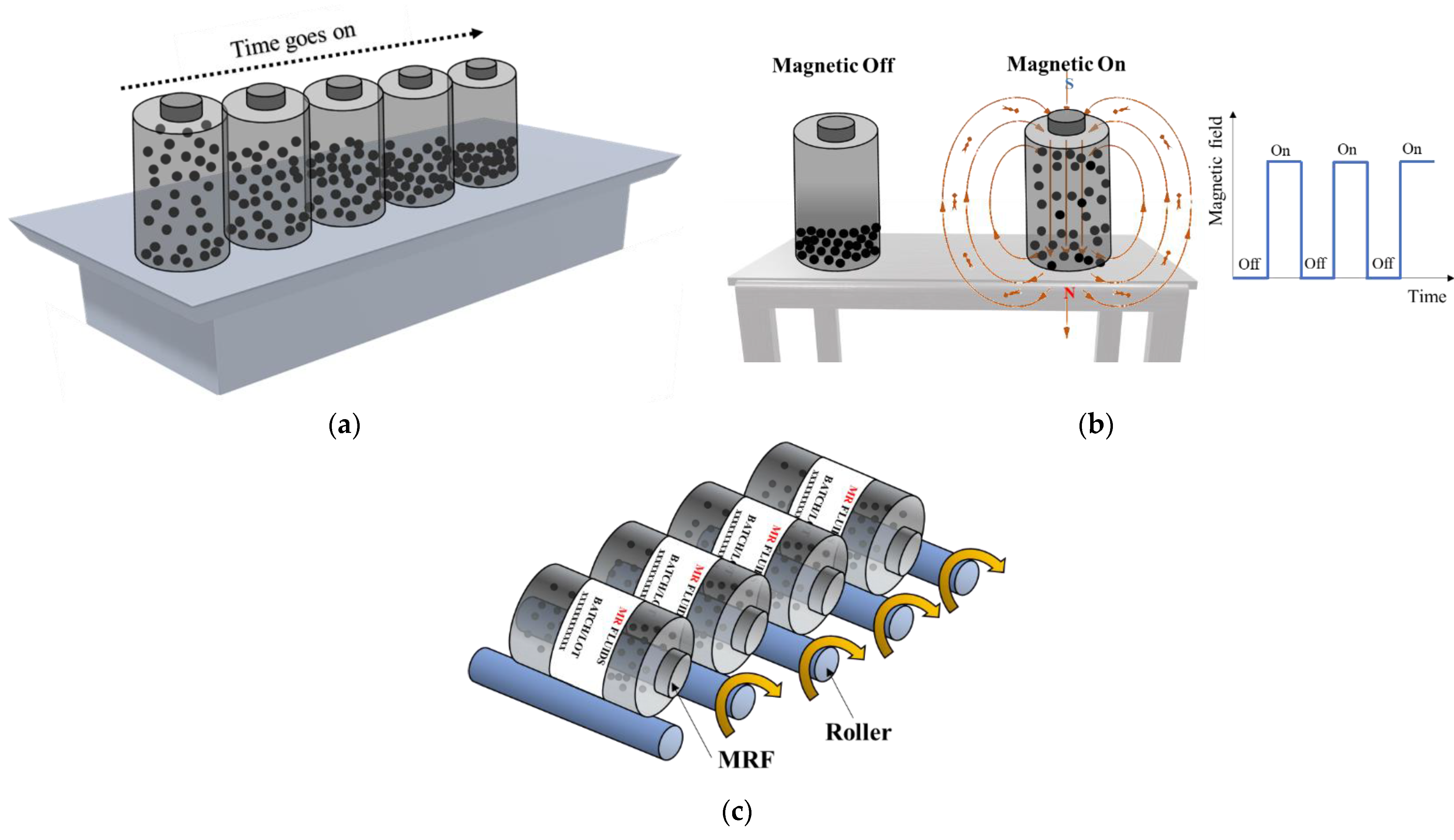
1. Introduction
2. Improvement of Sedimentation Stability
2.1. Particle Modification
2.2. Modification of Carrier Liquids
2.3. Use of Additives
2.4. Use of Combined Recipes
3. Challenging Works
3.1. Sedimentation Test
3.2. Sedimentation in Storage and Practical Use
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Utami, O.; Ubaidillah, U.; Mazlan, S.A.; Imaduddin, F.; Nordin, N.A.; Bahiuddin, I.; Aziz, S.A.A.; Mohamad, N.; Choi, S.-B. Material Characterization of a Magnetorheological Fluid Subjected to Long-Term Operation in Damper. Materials 2018, 11, 2195. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.A.N.; Mazlan, S.A.; Samin, P.M.; Idris, A.; Ubaidillah. Performance of bidisperse magnetorheological fluids utilizing superparamagnetic maghemite nanoparticles. AIP Conf. Proc. 2016, 1710, 030050. [Google Scholar] [CrossRef]
- Oh, J.-S.; Kim, K.-S.; Lee, Y.-S.; Choi, S.-B. Dynamic simulation of a full vehicle system featuring magnetorheological dampers with bypass holes. J. Intell. Mater. Syst. Struct. 2019, 31, 253–262. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoon, D.S.; Kim, G.W.; Choi, S.B.; Jeong, J.Y.; Kim, J.H.; Kim, S.J.; Kim, I.D. Road traveling test for vibration control of a wheel loader cabin installed with magnetorheological mounts. J. Intell. Mater. Syst. Struct. 2020, 32, 1336–1348. [Google Scholar] [CrossRef]
- Kang, B.H.; Hwang, J.H.; Choi, S.B. A New Design Model of an MR Shock Absorber for Aircraft Landing Gear Systems Considering Major and Minor Pressure Losses: Experimental Validation. Appl. Sci. 2021, 11, 7895. [Google Scholar] [CrossRef]
- Jeyasenthil, R.; Yoon, D.; Choi, S.; Kim, G. Robust semiactive control of a half-car vehicle suspension system with magnetorheological dampers: Quantitative feedback theory approach with dynamic decoupler. Int. J. Robust Nonlinear Control 2020, 31, 1418–1435. [Google Scholar] [CrossRef]
- Lenggana, B.W.; Ubaidillah, U.; Imaduddin, F.; Choi, S.-B.; Purwana, Y.M.; Harjana, H. Review of Magnetorheological Damping Systems on a Seismic Building. Appl. Sci. 2021, 11, 9339. [Google Scholar] [CrossRef]
- Do, X.P.; Choi, S.B. A state-of-the-art on smart materials actuators over the last decade: Control aspects for diverse applications. Smart Mater. Struct. 2022, 31, 053001. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lee, E.-S.; Choi, S.-B. A Cylindrical Grip Type of Tactile Device Using Magneto-Responsive Materials Integrated with Surgical Robot Console: Design and Analysis. Sensors 2022, 22, 1085. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, S.B. A New Tactile Transfer Cell Using Magnetorheological Materials for Robot-Assisted Minimally Invasive Surgery. Sensors 2021, 21, 3034. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hong, S.W.; Park, Y.J.; Kim, S.H.; Kim, G.W.; Choi, S.B. Tunable Young’s Moduli of Soft Composites Fabricated from Magnetorheological Materials Containing Microsized Iron Particles. Materials 2020, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.W.; Kang, S.R.; Hwang, Y.H.; Choi, S.B.; Lee, Y.S.; Han, M.S. Design and control of a parallel mechanism haptic master for robot surgery using magneto-rheological clutches and brakes. J. Intell. Mater. Syst. Struct. 2018, 29, 3829–3844. [Google Scholar] [CrossRef]
- Sohn, J.W.; Kim, G.W.; Choi, S.B. A State-of-the-Art Review on Robots and Medical Devices Using Smart Fluids and Shape Memory Alloys. Appl. Sci. 2018, 8, 1928. [Google Scholar] [CrossRef]
- Park, J.; Yoon, G.H.; Kang, J.W.; Choi, S.B. Design and control of a prosthetic leg for above-knee amputees operated in semi-active and active modes. Smart Mater. Struct. 2016, 25, 085009. [Google Scholar] [CrossRef]
- Cruze, D.C.; Hemalatha, G.; Jebadurai, S.V.S.; Sarala, L.; Tensing, D.; Christy, S.J.E. A Review on the Magnetorheological Fluid, Damper and Its Applications for Seismic Mitigation. Civ. Eng. J. 2018, 4, 3058. [Google Scholar] [CrossRef]
- Oh, J.S.; Sohn, J.W.; Choi, S.B. Applications of Magnetorheological Fluid Actuator to Multi-DOF Systems: State-of-the-Art from 2015 to 2021. Actuators 2022, 11, 44. [Google Scholar] [CrossRef]
- Muhammad, A.; Yao, X.-L.; Deng, Z.-C. Review of magnetorheological (MR) fluids and its applications in vibration control. J. Mar. Sci. Appl. 2006, 5, 17–29. [Google Scholar] [CrossRef]
- Baranwal, D.; Deshmukh, T.S. MR-Fluid Technology and Its Application—A Review. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 563–569. [Google Scholar]
- Liu, G.; Gao, F.; Wang, D.; Liao, W.-H. Medical applications of magnetorheological fluid: A systematic review. Smart Mater. Struct. 2022, 31, 043002. [Google Scholar] [CrossRef]
- Chin, B.D.; Park, J.H.; Kwon, M.H.; Park, O.O. Rheological properties and dispersion stability of magnetorheological (MR) suspensions. Rheol. Acta 2001, 40, 211–219. [Google Scholar] [CrossRef]
- Lim, S.T.; Cho, M.S.; Jang, I.B.; Choi, H.J. Magnetorheological characterization of carbonyl iron based suspension stabilized by fumed silica. J. Magn. Magn. Mater. 2004, 282, 170–173. [Google Scholar] [CrossRef]
- Mrlík, M.; Ilčíková, M.; Pavlínek, V.; Mosnacek, J.; Peer, P.; Filip, P. Improved thermooxidation and sedimentation stability of covalently-coated carbonyl iron particles with cholesteryl groups and their influence on magnetorheology. J. Colloid Interface Sci. 2013, 396, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Phu, D.X.; Seong, M.S.; Upadhyay, R.V.; Choi, S.B. A low sedimentation magnetorheological fluid based on plate-like iron particles, and verification using a damper test. Smart Mater. Struct. 2014, 23, 027001. [Google Scholar] [CrossRef]
- Shah, K.; Choi, S.-B. The influence of particle size on the rheological properties of plate-like iron particle based magnetorheological fluids. Smart Mater. Struct. 2014, 24, 015004. [Google Scholar] [CrossRef]
- Jönkkäri, I.; Isakov, M.; Syrjälä, S. Sedimentation stability and rheological properties of ionic liquid–based bidisperse magnetorheological fluids. J. Intell. Mater. Syst. Struct. 2014, 26, 2256–2265. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Li, M.; Cui, G.; Che, H.; Mu, J.; Zhang, X.; Tong, Y.; Dong, X. Magnesium ferrite nanocrystal clusters for magnetorheological fluid with enhanced sedimentation stability. Solid State Sci. 2017, 63, 70–75. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kumagae, T.; Abe, I.; Inoue, A. Particle Sedimentation in Magnetorheological Fluid and Its Effect. In Proceedings of the 2017 IEEE Xplore, Sheraton Arabella Park Hotel, Munich, Germany, 3–7 July 2017. [Google Scholar] [CrossRef]
- Rahim, M.S.A.; Ismail, I.; Choi, S.B.; Azmi, W.H.; Aqida, S.N. Thermal conductivity enhancement and sedimentation reduction of magnetorheological fluids with nano-sized Cu and Al additives. Smart Mater. Struct. 2017, 26, 115009. [Google Scholar] [CrossRef]
- Vėžys, J.; Mažeika, D.; Kandrotaitė-Janutienė, R.; Dragašius, E.; Kilikevičius, A.; Korobko, E.V. Sedimentation Influence on Magnetorheological Brake Torque Moment. Strength Mater. 2018, 50, 357–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, Z. The study of magnetorheological fluids sedimentation behaviors based on volume fraction of magnetic particles and the mass fraction of surfactants. Mater. Res. Express 2019, 6, 126127. [Google Scholar] [CrossRef]
- Dassisti, M.; Brunetti, G.; Rizzuti, A.; Mastrorilli, P. Three-Disperse Magnetorheological Fluids Based on Ferrofluids: Induced Modification of Sedimentation by Addition of Nanoparticles. Key Eng. Mater. 2020, 865, 73–78. [Google Scholar] [CrossRef]
- Choi, J.; Han, S.; Kim, J.; Seo, Y. Strong and Stable Magnetorheological Fluids Based on Flaky Sendust-Co0.4Fe0.4Ni0.2 Nanocomposite Particles. ACS Appl. Mater. Interfaces 2021, 13, 26581–26589. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Nam, K.T.; Kim, S.; Seo, Y. Synergistic Effects of Nonmagnetic Carbon Nanotubes on the Performance and Stability of Magnetorheological Fluids Containing Carbon Nanotube-Co0.4Fe0.4Ni0.2 Nanocomposite Particles. Nano Lett. 2021, 21, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, H.; Hu, Z.; Ding, D. Viscosity and sedimentation behaviors of the magnetorheological suspensions with oleic acid/dimer acid as surfactants. J. Magn. Magn. Mater. 2016, 417, 214–221. [Google Scholar] [CrossRef]
- Aguilera Portillo, M.; Iglesias, G.R. Magnetic Nanoparticles as a Redispersing Additive in Magnetorheological Fluid. J. Nanomater. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Bossis, G.; Volkova, O.; Lacis, S.; Meunier, A. Magnetorheology. In Ferrofluids; Springer: Berlin/Heidelberg, Germany, 2002; pp. 202–230. [Google Scholar] [CrossRef]
- López-López, M.T.; De Vicente, J.; Bossis, G.; González-Caballero, F.; Durán, J.D.G. Preparation of stable magnetorheological fluids based on extremely bimodal iron–magnetite suspensions. J. Mater. Res. 2005, 20, 874–881. [Google Scholar] [CrossRef]
- Vannarth, R.R.; Babu, K.M.; Jebraj, P.M. Study on sedimentation stability of mahua and simarouba oil based magnetorheological fluids. Acta Mech. Malays. 2019, 2, 39–44. [Google Scholar] [CrossRef]
- Jinaga, R.; Jagadeesha, T.; Kolekar, S.; Choi, S.-B. The Synthesis of Organic Oils Blended Magnetorheological Fluids with the Field-Dependent Material Characterization. Int. J. Mol. Sci. 2019, 20, 5766. [Google Scholar] [CrossRef]
- Harsh, R.; Sankar, D.; Luitel, A.K.K.; Nerusu, P. Change in Sedimentation Rate of MR Fluid with Different Base Fluids. J. Eng. Technol. Innov. Res. 2021, 8, 463–468. [Google Scholar]
- Su, Z.; Luo, Y.; Wang, Y.; Luo, J.; Ji, D. Study on sedimentation stability of magnetorheological fluids based on different lubricant formulations. Mater. Res. Express 2020, 7, 085702. [Google Scholar] [CrossRef]
- López-López, M.T.; Zugaldía, A.; González-Caballero, F.; Durán, J.D.G. Sedimentation and redispersion phenomena in iron-based magnetorheological fluids. J. Rheol. 2006, 50, 543–560. [Google Scholar] [CrossRef]
- Viota, J.L.; Delgado, A.; Arias, J.; Durán, J. Study of the magnetorheological response of aqueous magnetite suspensions stabilized by acrylic acid polymers. J. Colloid Interface Sci. 2008, 324, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lita, M.; Han, A.; Susan-Resiga, D. Characterization of sedimentation and high magnetic field flow behavior of some magnetorheological fluids. J. Physics: Conf. Ser. 2009, 149, 012071. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Jing, Q. Effect of seven different additives on the properties of MR fluids. J. Physics: Conf. Ser. 2009, 149, 012086. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Lysenko, S.N. Magnetic fluids stabilized by polypropylene glycol. J. Magn. Magn. Mater. 2011, 323, 1198–1202. [Google Scholar] [CrossRef]
- Fang, F.F.; Liu, Y.D.; Choi, H.J. Carbon nanotube coated magnetic carbonyl iron microspheres prepared by solvent casting method and their magneto-responsive characteristics. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 412, 47–56. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, F.; Wu, X.; Jian, J.W. A Novel Preparation Process for Magnetorheological Fluid with High Sedimentation Stability. Mater. Manuf. Processes 2016, 31, 2030–2036. [Google Scholar] [CrossRef]
- Xu, Z.D.; Chen, B.B. Experimental and Numerical Study on Magnetorheological Fluids Based on Mixing Coated Magnetic Particles. J. Mater. Civ. Eng. 2016, 28, 04015198. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Ruan, X.; Zhao, J.; Gong, X. The Influence of Additives on the Rheological and Sedimentary Properties of Magnetorheological Fluid. Front. Mater. 2021, 7, 631069. [Google Scholar] [CrossRef]
- Roupec, J.; Michal, L.; Strecker, Z.; Kubík, M.; Macháček, O.; Choi, H.J. Influence of clay-based additive on sedimentation stability of magnetorheological fluid. Smart Mater. Struct. 2021, 30, 027001. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Wang, Y.; Luo, J.; Chen, Y. Research on characterization method and influencing factors of sedimentation stability of magnetorheological fluid. Korea-Australia Rheol. J. 2021, 33, 309–320. [Google Scholar] [CrossRef]
- Narwade, P.; Deshmukh, R.; Nagarkar, M. A Review on Sedimentation of Magnetorheological (MR) fluid. Solid State Technol. 2020, 63, 2318–3150. [Google Scholar]
- Ashtiani, M.; Hashemabadi, S.; Ghaffari, A. A review on the magnetorheological fluid preparation and stabilization. J. Magn. Magn. Mater. 2015, 374, 716–730. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Chen, G. Static yield stress of ferrofluid-based magnetorheological fluids. Rheol. Acta 2009, 48, 457–466. [Google Scholar] [CrossRef]
- Susan-Resiga, D.; Vékás, L. Ferrofluid based composite fluids: Magnetorheological properties correlated by Mason and Casson numbers. J. Rheol. 2017, 61, 401–408. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Magnetorheological particle clouds. J. Magn. Magn. Mater. 2019, 479, 301–306. [Google Scholar] [CrossRef]
- Zhou, F.; Luo, Y.; Ren, H. Study of sedimentation stability of magnetorheological Fluid. Adv. Mater. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Singh, H.; Gill, H.S.; Sehgal, S.S. Synthesis and Sedimentation Analysis of Magneto Rheological Fluids. Indian J. Sci. Technol. 2016, 9, 210. [Google Scholar] [CrossRef]
- Morillas, J.R.; De Vicente, J. Magnetorheology: A review. Soft Matter 2020, 16, 9614–9642. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.; Han, M.; Li, A.; Tian, Z.; Wu, X. Preparation of a novel magnetorheological fluid for high temperatures. Soft Matter 2021, 17, 10350–10358. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Koh, A.S. Performance and Stability of Magnetorheological Fluids—A Detailed Review of the State of the Art. Adv. Eng. Mater. 2021, 23, 2001458. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, H.; Liao, C.; Wang, N.; Choi, S.B. Hydrodynamic Behaviors of Settled Magnetorheological Fluid Redispersion under Active Dispersing Mechanism: Simulation and Experiment. Smart Mater. Struct. 2022, 31, 9. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Ruiz-Morón, L.F.; Monesma, J.I.; Durán, J.; Delgado, A. An experimental method for the measurement of the stability of concentrated magnetic fluids. J. Colloid Interface Sci. 2007, 311, 475–480. [Google Scholar] [CrossRef]
- Lijesh, K.P.; Muzakkir, S.M.; Hirani, H. Rheological measurement of redispersibility and settling to analyze the effect of surfactants on MR particles. Tribol.-Mater. Surfaces Interfaces 2016, 10, 53–62. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, X.; Liu, G.; Ma, W.; Wereley, N.M. Measuring the sedimentation rate in a magnetorheological fluid column via thermal conductivity monitoring. Smart Mater. Struct. 2016, 25, 055007. [Google Scholar] [CrossRef]
- Choi, Y.-T.; Xie, L.; Wereley, N.M. Testing and analysis of magnetorheological fluid sedimentation in a column using a vertical axis inductance monitoring system. Smart Mater. Struct. 2016, 25, 04LT01. [Google Scholar] [CrossRef]
- Wen, M.; Chambers, J.; Sherman, S.G.; Wereley, N.M. Monitoring sedimentation of magnetorheological fluids using a vertical axis monitoring system with a low aspect ratio sensor coil. Smart Mater. Struct. 2019, 28, 025039. [Google Scholar] [CrossRef]

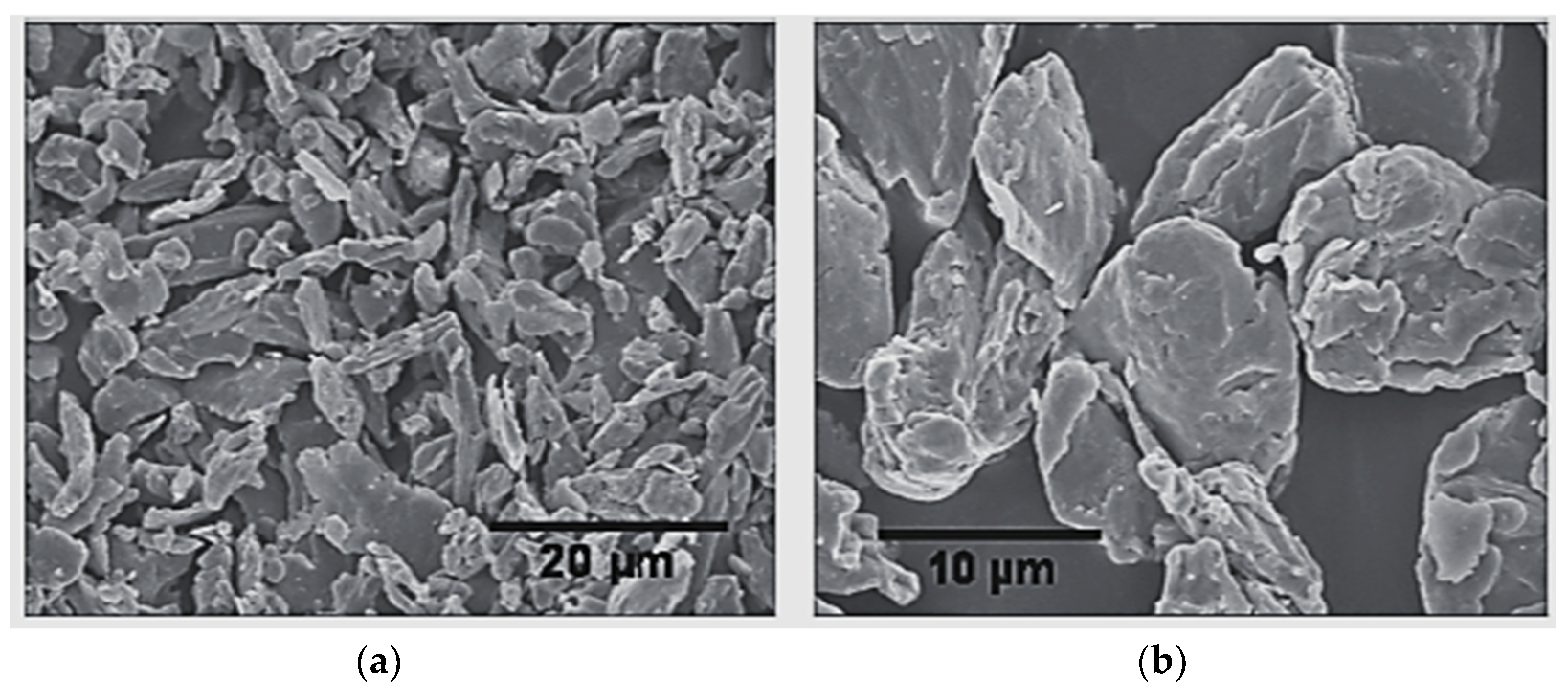
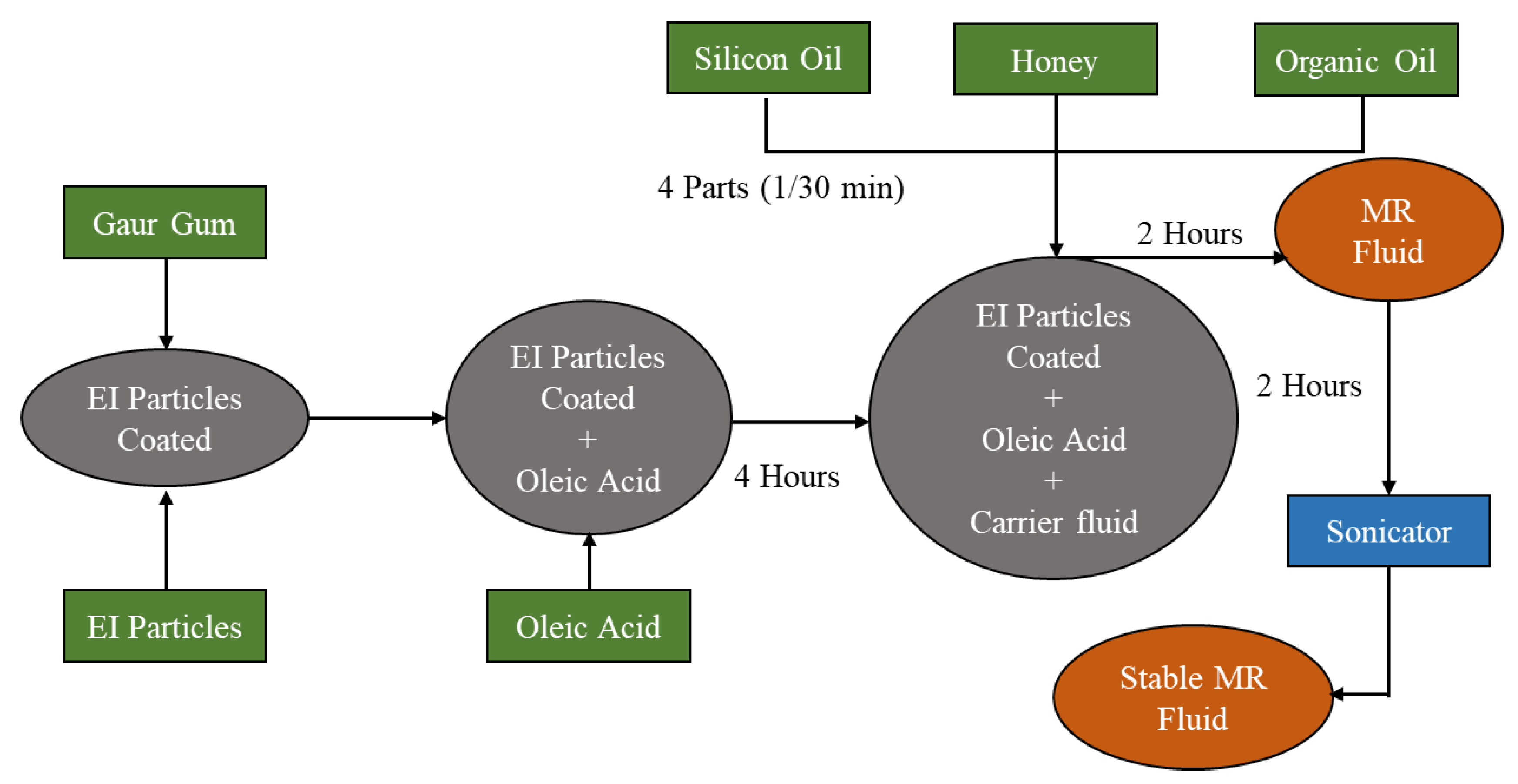
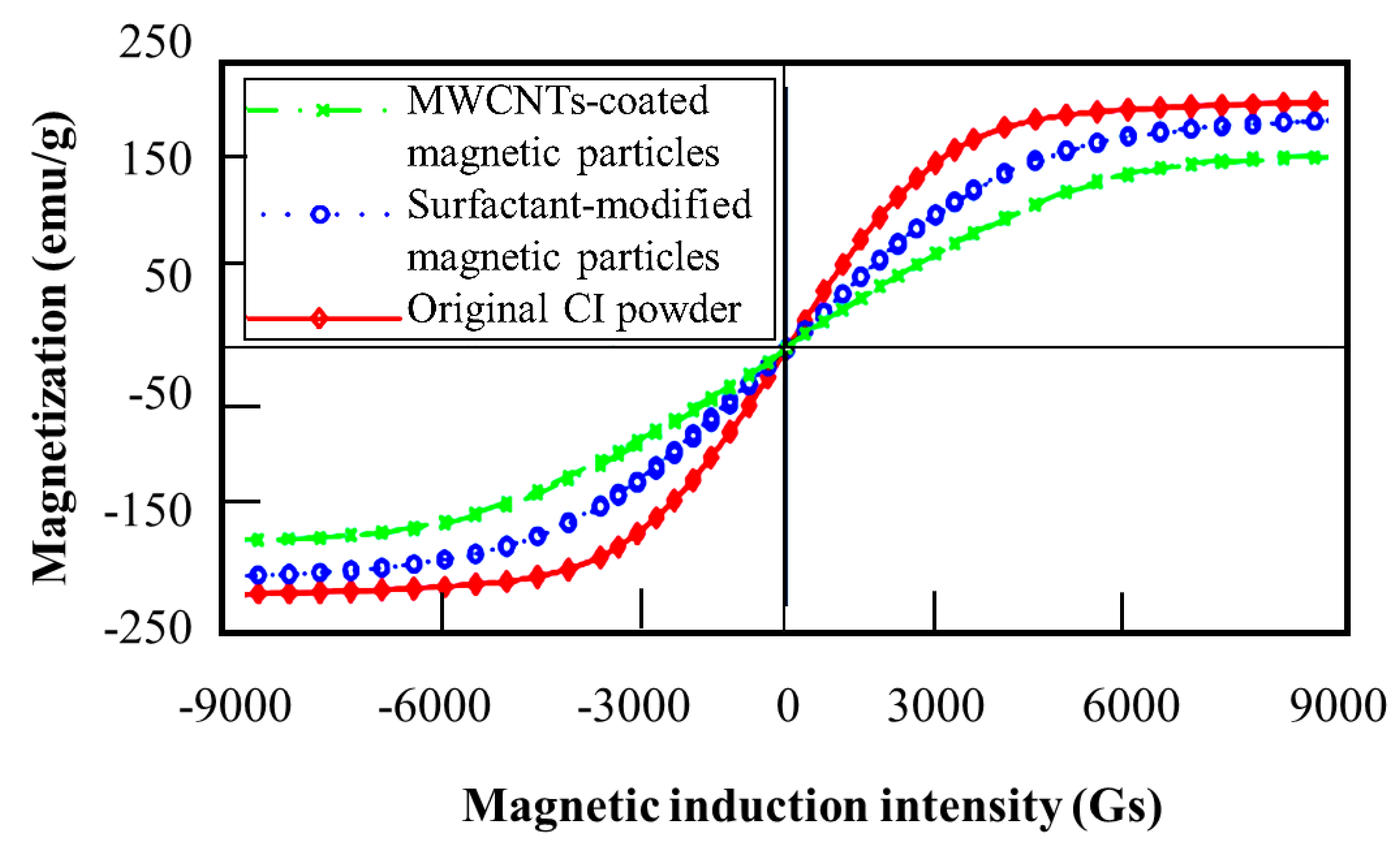
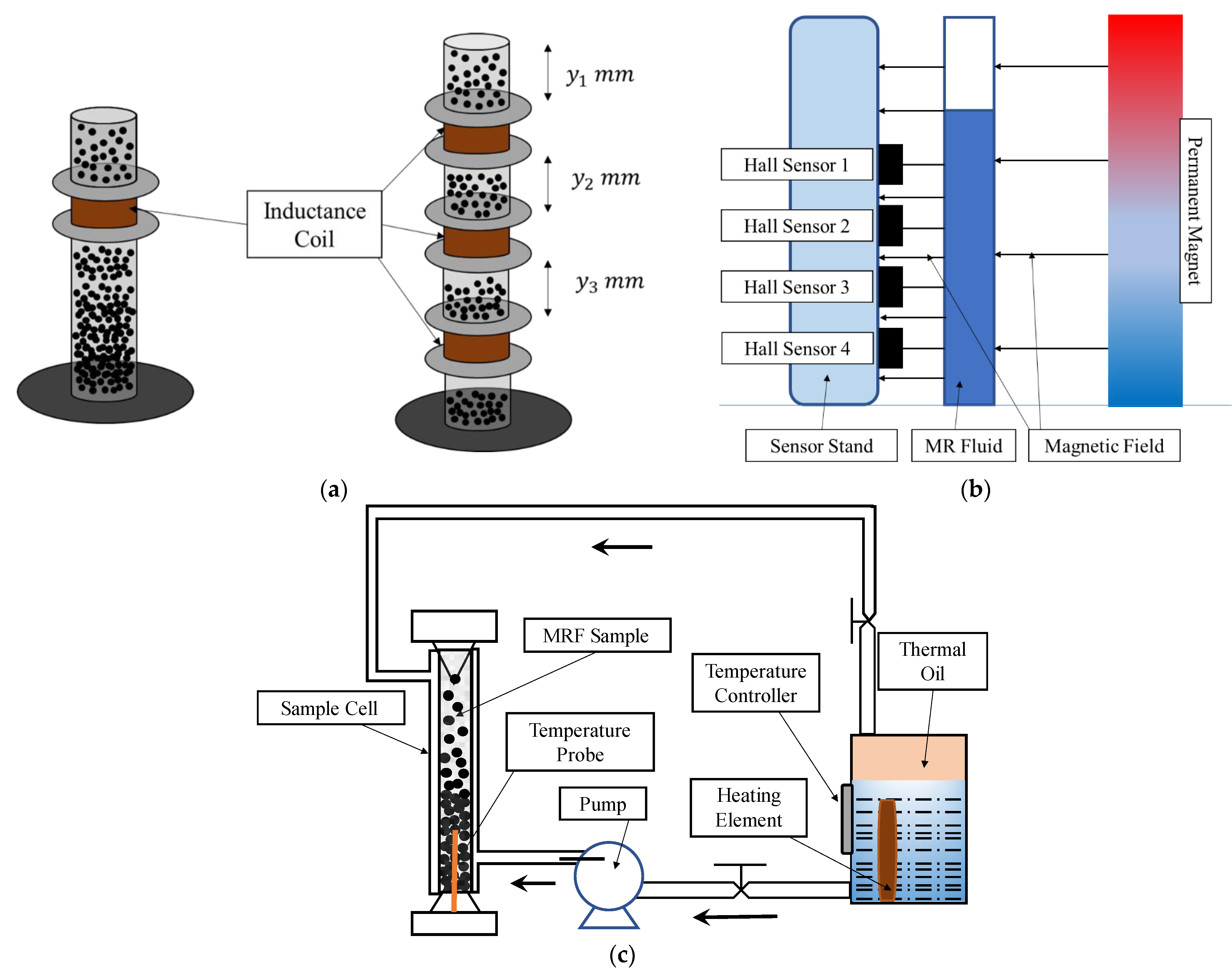

| Typical Particles | Recipes |
|---|---|
|
|
| Typical Base Oils | Recipes |
|---|---|
|
|
| Typical Additives | Recipes |
|---|---|
|
|
| References | Recipes |
|---|---|
| M. Ashtiani et al., 2014 [54] |
|
| F. Zhou et al., 2015 [58] |
|
| H. Singh et al., 2016 [59] |
|
| J.R. Morillas and J. Vicente, 2020 [60] |
|
| S. Thiagarajan and A. S. Koh, 2021 [62] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-B. Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues. Micromachines 2022, 13, 1904. https://doi.org/10.3390/mi13111904
Choi S-B. Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues. Micromachines. 2022; 13(11):1904. https://doi.org/10.3390/mi13111904
Chicago/Turabian StyleChoi, Seung-Bok. 2022. "Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues" Micromachines 13, no. 11: 1904. https://doi.org/10.3390/mi13111904
APA StyleChoi, S.-B. (2022). Sedimentation Stability of Magnetorheological Fluids: The State of the Art and Challenging Issues. Micromachines, 13(11), 1904. https://doi.org/10.3390/mi13111904






