Computational Study of Magnetic Particle Motion inside the Nasal Cavity under the Impact of an External Magnetic Field for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
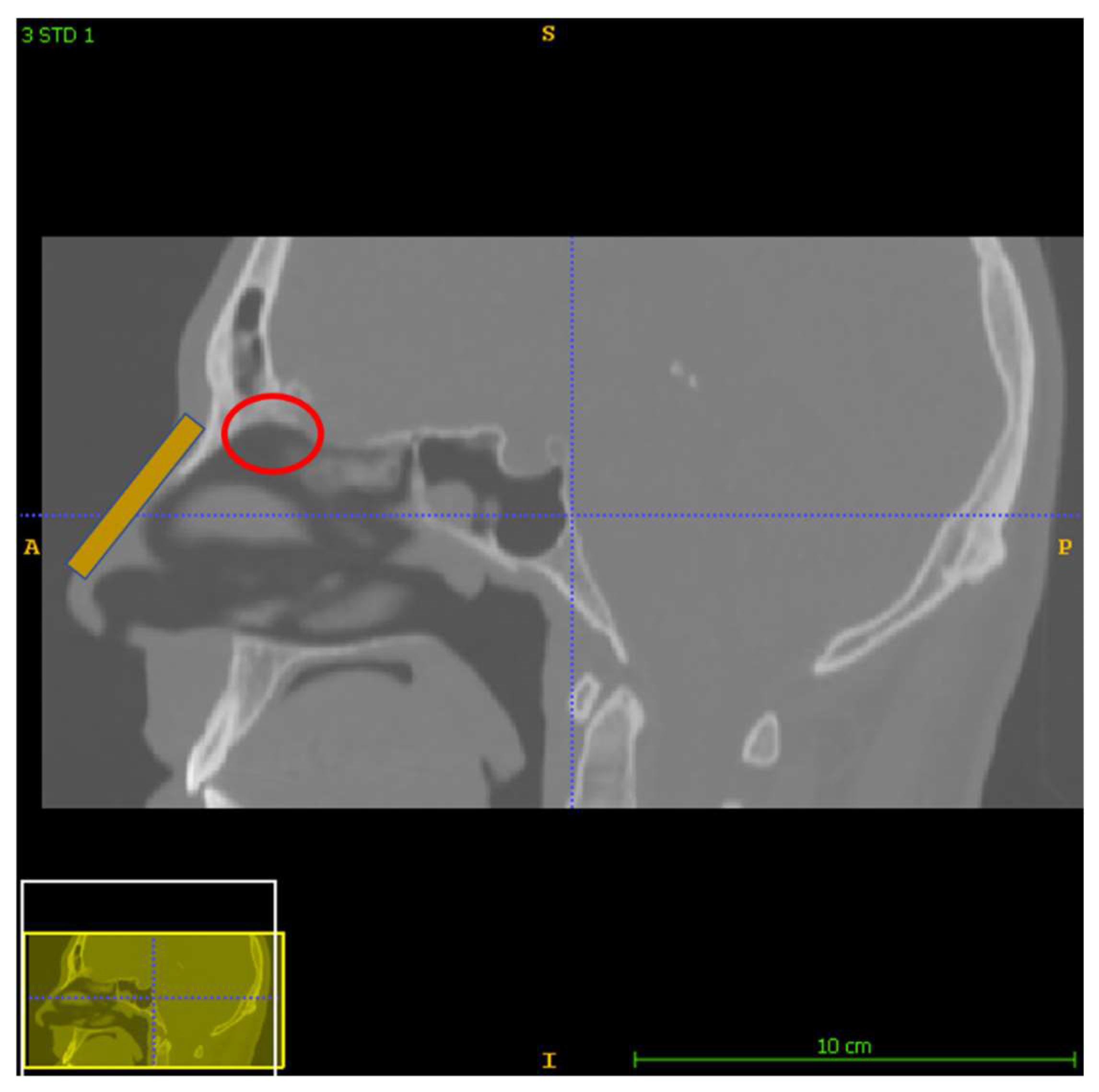
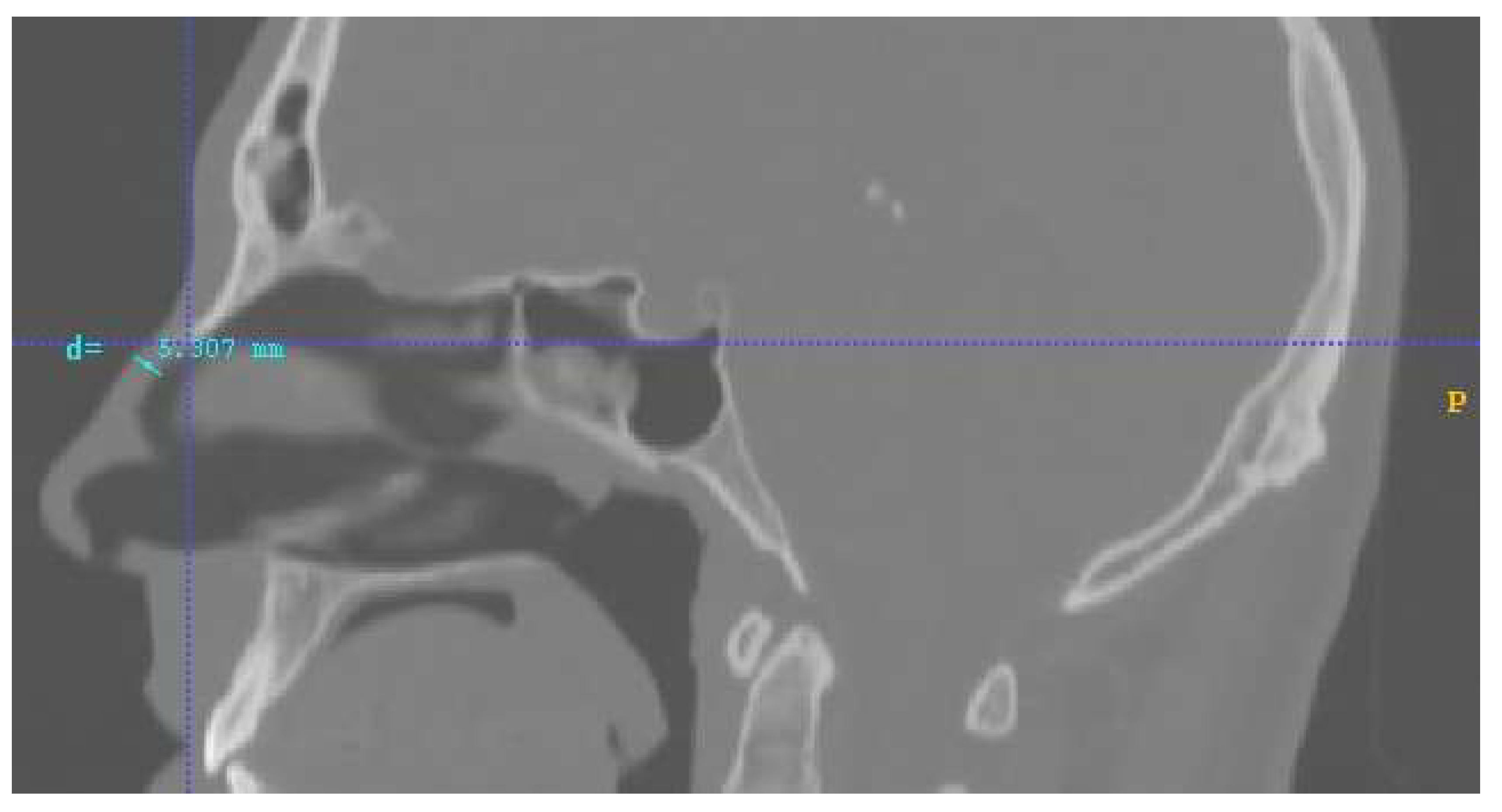
2.1. The Nose and Nasopharynx Model
2.2. Studied Protocols
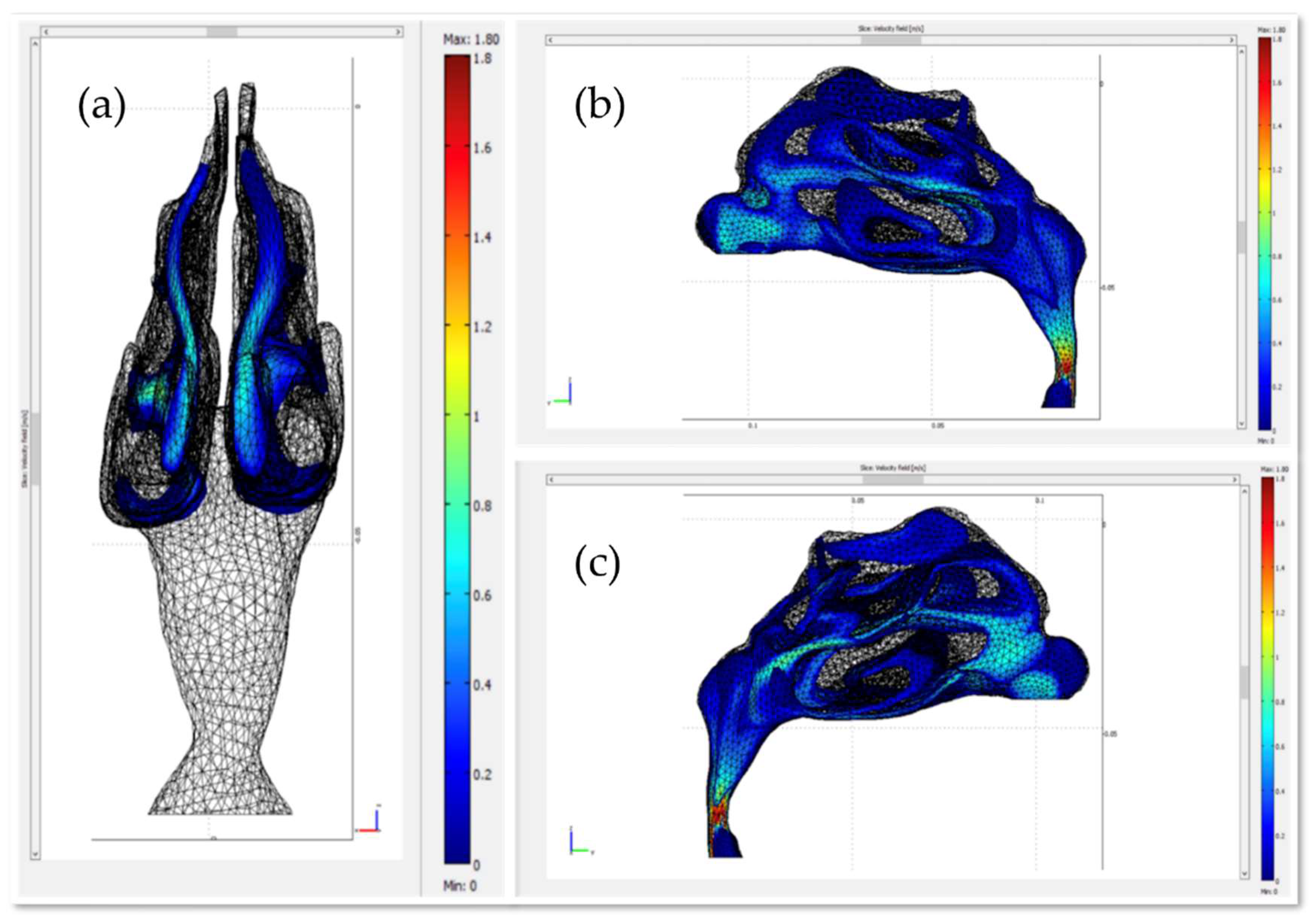
2.3. The Fluid Dynamics Approach
- the inhalation steady normal inflow velocity was 0.5 m/s at the entrance of the nostril [11]; The nostrils’ total area was 1.32∙10−4 m2;
- pressure at the bottom part of the nasopharynx was set to 0 atm;
- at the inner wall (mucosa), the flow velocity was set to 0 (no-slip boundary condition).
2.4. The Magnetostatic Approach
2.5. The Particle Tracing Approach
- speed of fluid;
- particle speed (initially, );
- radius of magnetic microparticle;
- μ: dynamic viscosity of the fluid; and
- is the magnetic force acting on the magnetic particles under the effect of an external magnetic field, defined aswhere
- magnetic permeability of free space;
- : volume of the particle;
- : magnetic susceptibility; and
- : intensity of magnetic field.
2.6. Numerical Solutions
3. Results
The Particle-Tracing Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in Neurodegenerative Diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-Mediated Transport of Nanoparticle-Bound Drugs Across the Blood-Brain Barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–Brain Barrier Delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; Mayer, U.; Van Tellingen, O.; Beijnen, J.H. The Functional Role of P-Glycoprotein in the Blood–Brain Barrier. J. Pharm. Sci. 1997, 86, 881–884. [Google Scholar] [CrossRef]
- Alexander, A.; Agrawal, M.; Uddin, A.; Siddique, S.; Shehata, A.M.; Shaker, M.A.; Ata Ur Rahman, S.; Abdul, M.I.M.; Shaker, M.A. Recent Expansions of Novel Strategies towards the Drug Targeting into the Brain. Int. J. Nanomed. 2019, 14, 5895–5909. [Google Scholar] [CrossRef]
- Illum, L. Nasal Drug Delivery: New Developments and Strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Chow, H.S.; Chen, Z.; Matsuura, G.T. Direct Transport of Cocaine from the Nasal Cavity to the Brain Following Intranasal Cocaine Administration in Rats. J. Pharm. Sci. 1999, 88, 754–758. [Google Scholar] [CrossRef]
- Illum, L. Transport of Drugs from the Nasal Cavity to the Central Nervous System. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Z.; Si, X.A. Improving Intranasal Delivery of Neurological Nanomedicine to the Olfactory Region Using Magnetophoretic Guidance of Microsphere Carriers. Int. J. Nanomed. 2015, 10, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Zhang, Z.; Si, X.A.; Yang, J.; Deng, W. Optimization of Magnetophoretic-Guided Drug Delivery to the Olfactory Region in a Human Nose Model. Biomech. Model. Mechanobiol. 2016, 15, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Remmo, A.; Löwa, N.; Kosch, O.; Eberbeck, D.; Ludwig, A.; Kampen, L.; Grüttner, C.; Wiekhorst, F. Cell Tracking by Magnetic Particle Imaging: Methodology for Labeling THP-1 Monocytes with Magnetic Nanoparticles for Cellular Imaging. Cells 2022, 11, 2892. [Google Scholar] [CrossRef]
- Paredes-Orta, C.; Mendiola-Santibañez, J.D.; Ibrahimi, D.; Rodríguez-Reséndiz, J.; Díaz-Florez, G.; Olvera-Olvera, C.A. Hyperconnected Openings Codified in a Max Tree Structure: An Application for Skull-Stripping in Brain MRI T1. Sensors 2022, 22, 1378. [Google Scholar] [CrossRef]
- Voronin, D.V.; Sindeeva, O.A.; Kurochkin, M.A.; Mayorova, O.; Fedosov, I.V.; Semyachkina-Glushkovskaya, O.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. In Vitro and in Vivo Visualization and Trapping of Fluorescent Magnetic Microcapsules in a Bloodstream. ACS Appl. Mater. Interfaces 2017, 9, 6885–6893. [Google Scholar] [CrossRef]
- Demina, P.A.; Abalymov, A.A.; Voronin, D.V.; Sadovnikov, A.V.; Lomova, M.V. Highly-Magnetic Mineral Protein–Tannin Vehicles with Anti-Breast Cancer Activity. Mater. Chem. Front. 2021, 5, 2007–2018. [Google Scholar] [CrossRef]
- Nanobiomaterials: Nanostructured Materials for Biomedical Applications; Narayan, R., Ed.; Woodhead publishing series in biomaterials; WP, Woodhead Publishing, an Imprint of Elsevier: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2017; ISBN 978-0-08-100716-7. [Google Scholar]
- Flores-Rojas, G.G.; López-Saucedo, F.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Magnetic Nanoparticles for Medical Applications: Updated Review. Macromol 2022, 2, 374–390. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Fusion 360; Autodesk: San Rafael, CA, USA, 2019.
- COMSOL Multiphysics®. COMSOL AB: Stockholm, Sweden. Available online: www.comsol.com (accessed on 19 February 2019).
- Cowan, J.M.; Burris, J.M.; Hughes, J.R.; Cunningham, M.P. The Relationship of Normal Body Temperature, End-Expired Breath Temperature, and BAC/BrAC Ratio in 98 Physically Fit Human Test Subjects. J. Anal. Toxicol. 2010, 34, 238–242. [Google Scholar] [CrossRef]
- Temam, R. Navier-Stokes Equations: Theory and Numerical Analysis; AMS Chelsea Pub: Providence, Rhode Island, 2001; ISBN 978-0-8218-2737-6. [Google Scholar]
- Jafari, S.; Mair, L.O.; Weinberg, I.N.; Baker-McKee, J.; Hale, O.; Watson-Daniels, J.; English, B.; Stepanov, P.Y.; Ropp, C.; Atoyebi, O.F.; et al. Magnetic Drilling Enhances Intra-Nasal Transport of Particles into Rodent Brain. J. Magn. Magn. Mater. 2019, 469, 302–305. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of Cerebrospinal Fluid Is Predominantly through Lymphatic Vessels and Is Reduced in Aged Mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of Nose-to-Brain Delivery of Nanoemulsions: Cargoes but Not Vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Laminar Airflow and Nanoparticle or Vapor Deposition in a Human Nasal Cavity Model. J. Biomech. Eng. 2006, 128, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. Regional Deposition of Nasal Sprays in Adults: A Wide Ranging Computational Study. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2968. [Google Scholar] [CrossRef]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. An Idealized Geometry That Mimics Average Nasal Spray Deposition in Adults: A Computational Study. Comput. Biol. Med. 2019, 107, 206–217. [Google Scholar] [CrossRef] [PubMed]

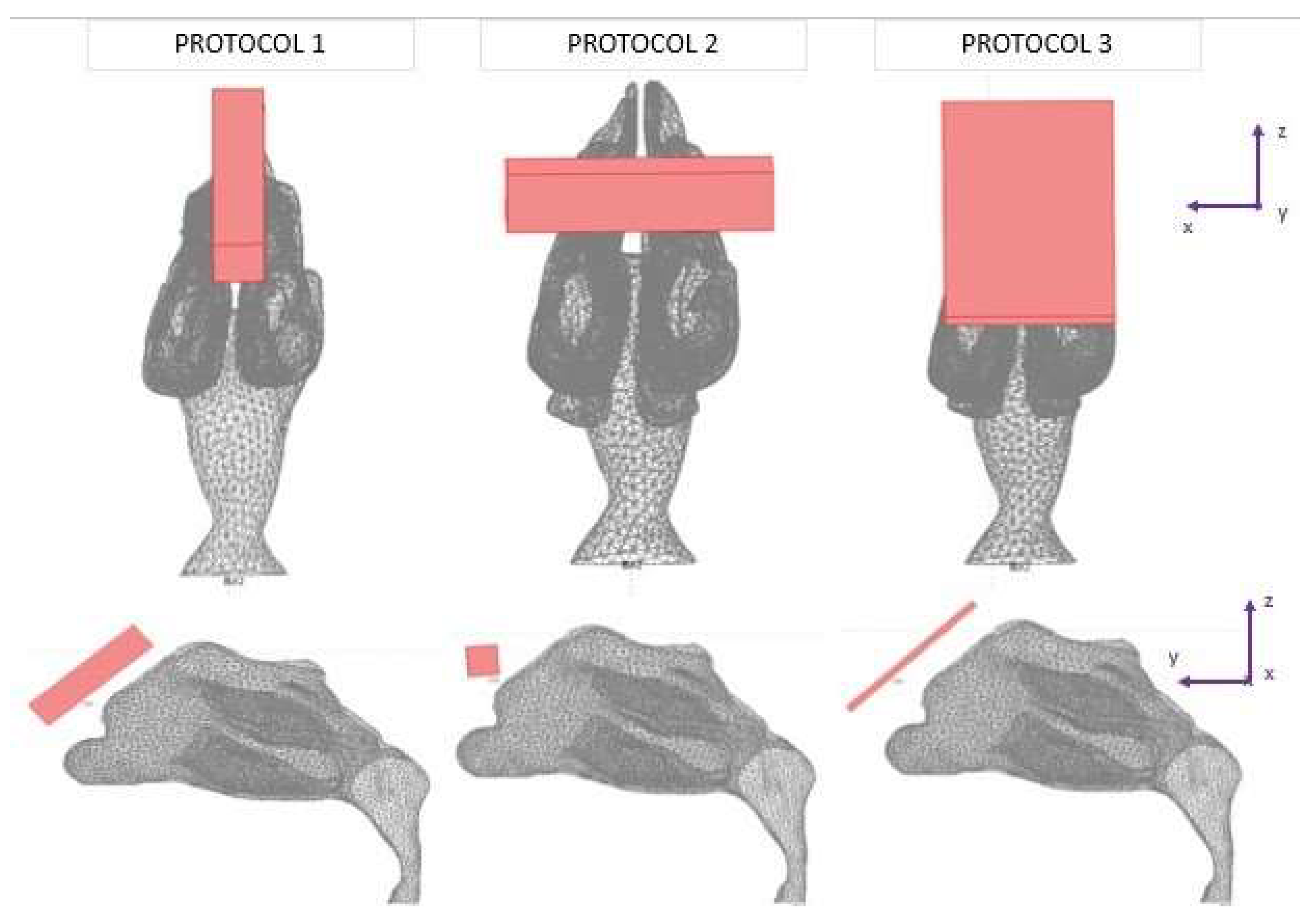


| Protocols | Trajectory of Microparticles under the Impact of an External Magnetic Field | Release Distance from Nasal Wall |
|---|---|---|
| Protocol 1 | 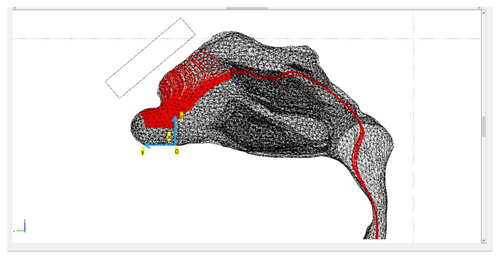 | nostril’s length |
| Protocol 2 | 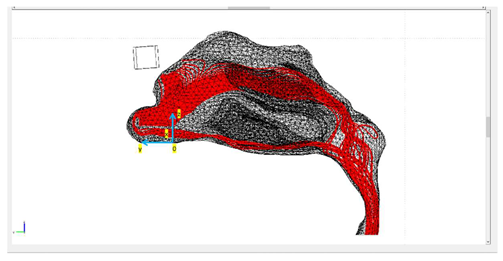 | nostril’s length |
| Protocol 3 | 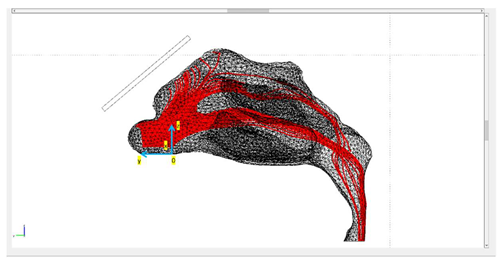 | nostril’s length |
| Protocol | Gradient of the Magnetic Flux Density B Created by the Permanent Magnets |
|---|---|
| 1 |  |
| 2 | 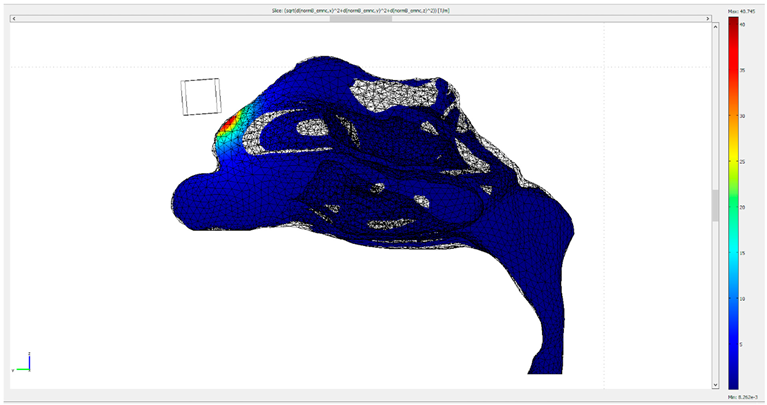 |
| 3 | 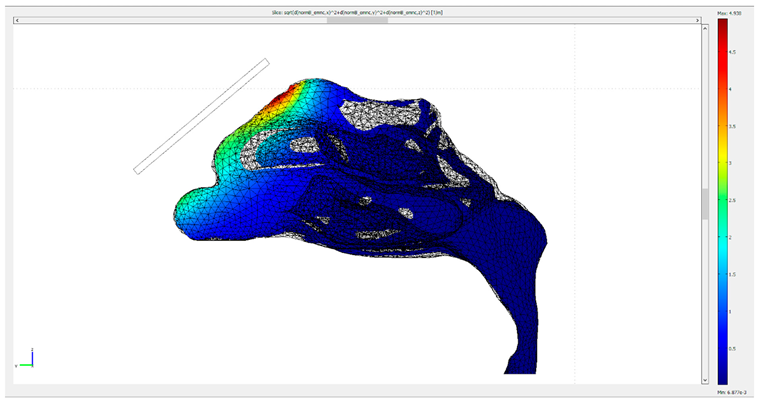 |
| Reported Studies in the Literature | Main Contributions of the Present Work |
|---|---|
| The protocol for similar magnetization value ( A/m) achieved a delivery efficiency of ~1% [12]. | Protocol 3 is twice as efficient |
| The delivery efficiency for a particle size on the nanometer scale was approximately 0.5% [28]. | Protocol 3 has approximately four times better delivery than non-magnetic guided protocols based only on aerodynamic control. |
| Specific release point of MNPs [29,30] | Calculation of the olfactory deposition efficacy for microparticles released across a line |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradakis, N.; Maniotis, N.; Samaras, T. Computational Study of Magnetic Particle Motion inside the Nasal Cavity under the Impact of an External Magnetic Field for Biomedical Applications. Micromachines 2022, 13, 1816. https://doi.org/10.3390/mi13111816
Pradakis N, Maniotis N, Samaras T. Computational Study of Magnetic Particle Motion inside the Nasal Cavity under the Impact of an External Magnetic Field for Biomedical Applications. Micromachines. 2022; 13(11):1816. https://doi.org/10.3390/mi13111816
Chicago/Turabian StylePradakis, Nikolaos, Nikolaos Maniotis, and Theodoros Samaras. 2022. "Computational Study of Magnetic Particle Motion inside the Nasal Cavity under the Impact of an External Magnetic Field for Biomedical Applications" Micromachines 13, no. 11: 1816. https://doi.org/10.3390/mi13111816
APA StylePradakis, N., Maniotis, N., & Samaras, T. (2022). Computational Study of Magnetic Particle Motion inside the Nasal Cavity under the Impact of an External Magnetic Field for Biomedical Applications. Micromachines, 13(11), 1816. https://doi.org/10.3390/mi13111816










