Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review
Abstract
1. Introduction
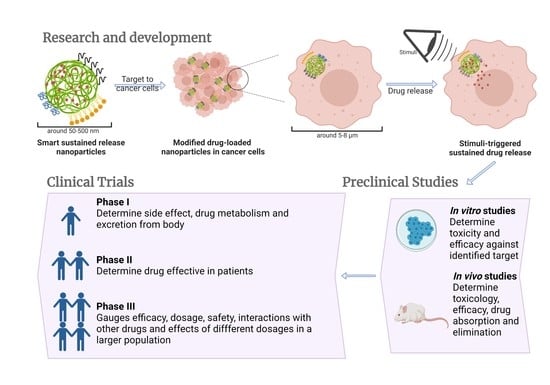
2. Smart Sustained Release Nanoparticles
2.1. Definition and Advantages of Smart Sustained Release Nanoparticles
| Smart Drug Delivery System | Sustained Drug Release System | |
|---|---|---|
| Definition | Release drugs in response to specific physiological triggers, at appropriate time and target site [9]. | Deliver drugs at a predetermined rate over an extended period of time [17]. |
| Physiological/clinical benefits |
|
2.2. Latest Formulation Strategies in the Smart Sustained Release Nanoparticles for Clinical Cancer Treatment
2.2.1. Smart Sustained Release Liposomes
2.2.2. Smart Sustained Release Dendrimers
2.2.3. Smart Sustained Release Micelles
2.2.4. Smart Sustained Release Polymeric Nanoparticles
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Doxil | PEGylated liposomal | Doxorubicin | Various cancer types | Approved by FDA (1995) | Passive-targeting formulation. Sustained drug release achieved by prolonged dissolution rate of drug crystals in the core of the liposome. | [41,42] |
| DaunoXome | Liposomal | Daunorubicin | HIV-associated Kaposi’s sarcoma | Approved by FDA (1996) | Daunorubicin in small unilamellar vesicles composed of DSPC and cholesterol in a 2:1 mole ratio with 45 nm average size. Drug release over a prolonged period (36 h or more). | [43,44,45] |
| Myocet | Liposomal | Doxorubicin | Metastatic breast cancer | Approved by EMA (2000) | Passive-targeting formulation. Loading technique involves a pH gradient and citrate complex leading to the high ratio of drug to lipid. | [46,47] |
| Lipo-dox | PEGylated liposomal | Doxorubicin | Metastatic breast cancer, ovarian cancer and AIDS-related Kaposi’s sarcoma | Approved by Department of Health of Taiwan (2002) | Lipid composition includes DSPC to reduce drug leakage during preparation and enhance liposomes stability. | [46] |
| Lipusu | Liposomal | Paclitaxel | Breast and non-small-cell lung cancer | Approved in China (2003) | Lipusu instead of conventional paclitaxel has been shown to have a markedly reduced toxicity while retaining equal efficacy in cancer models | [48] |
| CPX-351 | Liposomal | Cytarabine and daunorubicin (5:1 molar ratio) | Acute myeloid leukemia | Approved by FDA (2007) | Gel state at body temperature, providing stability and controlled drug release with limited systemic drug distribution. | [49] |
| Mepact | Liposomal | Mifamurtide | Osteosarcoma | Approved by EMA (2009) | Size < 100 nm with DOPS: POPC = 3:7 molar ratio, designed to target macrophages (phosphatidyl serine containing lipids provides signal to macrophages). The drug shows no cytotoxicity to normal or tumour cells in vitro. | [50] |
| Marqibo | Liposomal | Vincristine | Leukemia | Approved by FDA (2012) | SM and cholesterol as the liposomal carrier with the size around 100 nm. Increased extravasation into tumours and sustained drug release (approximately 18–39% release of encapsulated drug at 24 h at 37 °C). | [51,52] |
| Onivyde | Liposomal | Irinotecan | Metastatic pancreatic cancer | Approved by FDA (2015) | Sustained-release formulation could target tumour by EPR effect. Increased in vivo stability of drug, extended the circulation time. | [53] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| ThermoDox | Heat-sensitive liposomal | Doxorubicin | Hepatocellular carcinoma | Phase III | Thermo-sensitive lipids to high temperatures. Drug release is controlled by mild increases in temperature (39.5–43 °C). | [30,31] |
| S-CKD602 | PEGylated liposomal | CKD602 | Various cancer types | Phase II | Stealth liposome formulation, composed of phospholipids covalently linked to mPEG, leading to prolonged plasma exposure and superior tumour delivery. | [54,55] |
| CPX-1 | Liposomal | Irinotecan and floxuridine | Colorectal cancer | Phase II | Irinotecan and floxuridine in a fixed 1:1 molar ratio. CPX-1 overcomes the different PK of a single drug and can continue to maintain this ratio after intravenous injection. | [56,57,58] |
| LE-SN38 | Liposomal | SN-38 | Metastatic colorectal cancer | Phase II | Improved therapeutic index, efficacy and safety of insoluble SN-38. 50:40:10 molar ratio of DOPC, cholesterol and cardiolipin and a drug to lipid ratio of 1:18. Provide active drugs without conversion by using NeoLipid® patented technology. | [54,59,60] |
| INGN-401 | Liposomal | FUS1 | Lung cancer | Phase I | Targeted gene delivery of FUS1 tumour suppressor protein by “plasmid gene expression cassette”, which contains DNA encoding the FUS1 protein. The tightly wrap by cholesterol provides protection against the body’s defense mechanisms. | [61,62] |
| SPI-077 | PEGylated liposomal | Cisplatin | Head and neck cancer, lung cancer, ovarian cancer | Phase II | Long-circulating and sterically stabilized liposomes. Composed of neutral lipids with 110 nm size, and cisplatin to total lipid ratio is 1:70. | [63,64,65] |
| OSI-7904L | Liposomal | Thymidylate synthase inhibitor | Various cancer | Phase II | Manufactured by HSPC and cholesterol with OSI-7904 loaded in the aqueous cores, size 20–80 nm. Improved the efficacy and increased the half-life. | [64,66,67] |
| OSI-211 | Liposomal | Lurtotecan | Lung cancer, recurrent ovarian cancer | Phase II | Encapsulation of lurtetecan, an inhibitor of the mammalian topoisomerase I enzyme. Increased plasma residence time, improved biodistribution and therapeutic index of the drug. | [64,68,69,70] |
| SGT-53 | TfR-targeting liposomal | Wild-type p53 plasmid DNA | Solid tumours, glioblastoma, metastatic pancreatic cancer | Phase II | Active targeting formulation decorated with anti-TfR scFv as tumour targeting domain. Cationic liposomes internalized by receptor-mediated endocytosis. | [56,71,72] |
| MBP-426 | TfR-targeting Liposomal | Oxaliplatin | Gastric, oesophageal and gastro-oesophageal adenocarcinoma | Phase I/II | Active targeting formulation decorated with human transferrin ligand. pH-responsive liposomes due to the NGPE coating. The layer ensures rapidly disintegration of the particles under acidic conditions. | [56,73,74] |
| Anti-EGFR-IL-DOX | EGFR-targeting liposomal | Doxorubicin | Breast cancer | Phase II | Active targeting formulation decorated with Fab’ fragment of the anti-EGFR-antibody C225 to target EGFR expressing cells. | [56,75,76] |
| Atu027 | Liposomal | siRNA against protein kinase N3 | Advanced or metastatic pancreatic cancer | Phase I/II | Liposomes for RNAi therapy, delivering siRNA to silence the expression of protein kinase N3 in vascular endothelium. | [56,77,78] |
| DC-Chol-EGFR | Liposomal | EGFR antisense | Head and Neck cancer | Phase I | Cationic liposomes loading EGFR antisense sequence. | [79,80] |
| EndoTAG-1 | Liposomal | Paclitaxel | Pancreatic cancer, liver metastases and HER2-negative and triple-negative breast cancer | Phase III | Cationic liposomes to target angiogenic endothelial cells in solid tumours. | [56,69,81,82,83] |
| LErafAON | Liposomal | c-Raf ANO | Advanced solid tumour, advanced malignancy | Phase I | Cationic liposomes loading negatively charged c-raf-1 AON with the EE% > 85%. Average size of 400 nm. Pre-clinical analysis of LErafAON showed Raf-1 inhibition and tumour regression. | [80,84,85,86] |
| Lipoplatin | PEGylated liposomal | Cisplatin | Pancreatic cancer | Phase III | High EE% (95–97%), observed induction of tumor cell apoptosis with 200-fold higher concentration of cisplatin in tumours than free drug. Induced apoptosis to the endothelium of tumor vasculature, hence, portraying strong antiangiogenesis properties. | [54,69,87] |
| Lipovaxin-MM | DC-targeted liposomal | Melanoma antigens | Malignant melanoma | Phase I | Active targeting liposomes decorated with a multicomponent and multivalent DC targeting allogeneic melanoma. | [56,88,89] |
| MM-302 | HER2-targeted PEGylated liposomal | Doxorubicin | HER2-positive breast cancer | Phase II/III | Active targeting liposomes decorated with 45 single-chain anti-HER2 antibodies (scFv) targeting HER2-overexpressing tumour cells. | [56,90] |
| PNT2258 | Liposomal | DNA oligonucleotide against BCL-2 | Relapsed or refractory non-Hodgkin lymphoma and diffuse large B-cell lymphoma | Phase II | pH-responsive formulation, anionic at physiological pH. Average size of 130 nm. | [56,91,92] |
| Promitil | PEGylated liposomal | Mitomycin C | Advanced solid tumours | Phase I | Significantly lower toxicity profile in preclinical and phase 1 clinical investigations. Drug release is based on the cleavable dithiobenzyl bridge between Mitomycin C and glycerol lipids by reducing agents in tumours. | [93,94,95] |
| siRNA-EPHA2-DOPC | Liposomal | siRNA against EPHA2 | Advanced solid cancers | Phase I | Neutral liposomes loading siRNA to silence EPHA2 and to inhibit tumour cells growth. | [56,96,97] |
| Tecemotide | Liposomal | Mucin 1 antigen | NSCLC | Phase III | Lipopeptide, encapsulated with MPL and three different lipids in multilayer liposomes, designed to promote APCs uptake so that the peptide is processed via class I and class II HLA moleculesin and triggering cytotoxic T-lymphocytes-mediated mucin 1-specific cellular immune responses. | [56,98,99] |
| Aroplatin | Liposomal | Cisplatin analog | Various cancers | Phase II | NDDP loaded multi-layer liposomes, synthesized by mixing DMPC and DMPG lipids with acidified salt solution. Note: this is the first liposomal formulation entered into clinical study for delivery of cisplatin analogs. | [54,100,101] |
| LEP-ETU | Liposomal | Paclitaxel | Ovarian, breast and lung cancers | Phase II | 150 nm in size. Liposome carriers have 90:5:5 molar ratio of DOPC, cholesterol and cardiolipin. Drug to lipid molar ratio is 1:33. Maximum drug EE% is 85%. | [54,64,102,103] |
| Atragen | Liposomal | Tretinoin | Acute promyelocytic leukemia | Phase II | Liposomes composed of retinoic acid, DMPC and soybean oil, containing tretinoin as 2 mg/mL. Compared to free ATRA, this formulation can avoid liver microsomal clearance and show lower in vivo systemic toxicity. | [54,64,104] |
| Liposomal annamycin | Liposomal | Annamycin | Acute lymphocytic leukemia | Phase I/II | 7:3 molar ratio of DMPC:DMPG as the carriers loaded with Anamycin which could intercalate DNA and inhibit topoisomerase II, thereby inhibiting DNA replication and protein synthesis. | [54,105,106] |
| INX-0076 | Liposomal | Topotecan | Advanced solid tumours | Phase I | 45:55 molar ratio of cholesterol and ESM. INX-0076 is developed by sphingosomal platform, a novel platform for improved tumour targetability and the duration of exposure of loaded anticancer agents. | [54,107] |
| INX-0125 | Liposomal | Vinorelbine tartrate | Advanced solid tumours | Phase I | 45:55 molar ratio of cholesterol and ESM. Based on the sphingosomal platform as INX-0076. | [54] |
| LEM-ETU | Liposomal | Mitoxantrone | Various cancers | Phase I | 90:5:5 molar ratio of DOPC, cholesterol and cardiolipin. Cardiolipin, a negatively charged diphosphatidyl glycerol lipid, forms electrostatic interactions with the loaded drug leading to higher drug loading when compared to other liposome formulations. | [54] |
| Liposomal Grb-2 | Liposomal | Grb-2 | Various cancers | Phase I | Neutrally-charged DOPC formulation loading with an antisense oligonucleotide which is designed to inhibit the production of Grb-2. | [54,108,109,110] |
| Lipoxal | Liposomal | Oxaliplatin | Advanced gastrointestinal cancer | Phase I/II | Lipoxal had a half-life of 24–35 h in humans and MTD of 300 mg/m2. Reduced adverse reactions without reducing effectiveness, compared to oxaliplatin. | [111,112,113] |
| LiPlaCis | PEGylated liposomal | Cisplatin | Solid tumours | Phase I/II | The first controlled-release liposomal formulation encapsulated with cisplatin and modified with sPLA2, a tumour selective enzyme. LiPlaCis liposomes composed of DSPC/DSPG/DSPE-PEG2000 lipids. | [101,114,115,116] |
| DPX-0907 | Liposomal | Multi-tumour associated antigens | HLA-A2-positive advanced stage ovarian, breast and prostate cancer | Phase I | DPX-0907 contains a polynucleotide-based adjuvant, a universal T helper peptide and seven tumour-specific HLA-A2-restricted epitopes could show efficient induction of immune response to cancer peptides. | [56,117,118] |
| dHER2 + AS15 | Liposomal | Recombinant HER2, dHER2, antigen and AS15 adjuvant | Metastatic breast cancer | Phase I/II | Liposomal formulation containing three immune stimulating ingredients: dHER2 is a truncated form of the HER2 protein; AS15 is an immune adjuvant. | [56,119] |
| MRX34 | Liposomal | miRNA-34a mimics | Primary liver cancer, solid tumours and haematological malignancies | Phase I | Composed of amphoteric lipids which confer positive charges to ensure an effective encapsulation of negatively charged miRNA-34a mimics. Liposomes have size of 110 nm and are anionic at neutral pH to minimize particle aggregation and electrostatic adhesion to the cell membrane of endothelial cells. | [56,120] |
| JVRS-100 | Liposomal | Plasmid DNA | Relapsed or refractory leukaemia | Phase I | Liposomes containing cationic lipid DOTIM and neutral lipid cholesterol on the membrane. JVRS-100 stimulate innate immune response to the presence of unmethylated CpG motif in the loaded plasmid. | [121,122] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Genexol-PM | Polymeric micelle | Paclitaxel | Breast cancer and small cell lung cancer | Approved in Korea (2007) | PEG-PLA block copolymers, with size of 20–50 nm. MTD 3 times higher when compared to paclitaxel. | [123] |
| Apealea | Polymeric micelles | Paclitaxel | Ovarian cancer | Approved by EMA (2018) | Cremophor®-free micellar formulation based on the patented excipient platform XR-17. Size 20–60 nm. Excipient ratio 1.3:1. | [124] |
| NC-6004 | Polymeric micelle | Cisplatin | Various cancers | Phase II/III | Mean diameter of around 30 nm and about 39 wt% drug loading. The free platinum is released in the presence of chloride ions. In 0.9% NaCl solution, only 19.6% and 47.8% platinum release at 24 h and 96 h at 37 °C, respectively. | [125] |
| NK-105 | Polymeric micelle | Paclitaxel | Metastatic or recurrent breast cancer | Phase III | A “core-shell-type” polymeric micelles made by block copolymers consisting of PEG and PASA. Size around 85 nm and 23 wt% drug loading. | [126] |
| NK-911 | Polymeric micelles | Doxorubicin | Metastatic pancreatic cancer | Phase II | Doxorubicin-conjugated PASA/PEG nanocarrier with size of 40 nm. Note: NK-911 is the first micellar formulation tested in humans. | [46] |
| NK-012 | Polymeric micelles | SN-38 | Advanced solid tumour | Phase II | PEG-PGlu (SN-38) amphiphilic block copolymer. SN-38 covalently linked to PGlu segment with average size of 20 nm. | [127,128] |
| SP1049C | Polymeric micelles | Doxorubicin | Advanced gastric cancer | Phase III | Pluronic L61 and Pluronic F127 block copolymers with doxorubicin physically loaded. Size around 22–27 nm. Pre-clinical analysis showed SP1049C therapy effectively suppresses the tumorigenicity and aggressiveness | [127,129,130] |
| NC-4016 | Polymeric micelles | Oxaluplatin | Advanced solid tumours | Phase I | Polymer-metal complexes of DACH-Pt and PEG-PGlu block copolymers. NC-4016 has around 15 h blood circulation half-life, with size around 40 nm and 32 wt% drug loading. | [127,131,132,133] |
| Lipotecan | Polymeric micelles | TLC388 (Camptothecin analog) | Various cancer | Phase I/II | TLC388 has a unique lactone ring modification. Other formulation properties not found. | [134,135] |
| NC-6300 | PEG-b-PAH polymeric micelles | Epirubicin | Solid tumours and soft tissue sarcoma | Phase I/II | PEG-polyaspartate block copolymer linked to Epirubicin by an acid-labile hydrazone bond, particle size of 60–70 nm. The block copolymers are partially substituted by hydrophobic benzyl groups to stabilize the micellar structure. | [136,137] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Eligard | PLGA PNPs | Leuprolide acetate | Prostate cancer | Approved by US FDA (2002) | Leuprolide acetate is administered via an implanted depot delivery system, which releases the drug in a controlled manner over defined intervals—1, 3, 4, or 6 months. Drug loading 4–6%. | [138,139,140] |
| BIND-014 | PSMA-targeting PEG-PLA PNPs | Docetaxel | NSCLC and mCRPC | Phase II | PEG-PLA copolymer nanoparticles physically loaded with docetaxel (drug loading around 10%) with a targeting small-molecule ligand specific for PSMA. Size around 100 nm. Note: BIND-014 is the first-in-man targeted and controlled-release nanoparticles for cancer therapy. | [141,142] |
| Transdrug | PEBCA PNPs | Doxorubicin | Hepatocellular carcinoma | Phase III | A molecular complex of doxorubicin adsorbed on PEBCA with size of 100–200 nm. 12-fold increase in drug exposure within the hepatic tumor tissue as compared to free doxorubicin. | [106,143,144] |
| DHAD-PBCA-NPs | PBCA PNPs | Mitoxantrone | Hepatocellular carcinoma | Phase II | Nanoparticles synthesized by PBCA, a biodegradable and bioavailable polymer, with a size of 55 nm and drug loading of 46.77%. | [145,146] |
| Docetaxel-PNP | PNPs | Docetaxel | Solid tumours | Phase I | PLA-COONa, and copolymer mPEG-PLA nanoparticles physically loaded with docetaxel. Other formulation properties not found. | [147,148] |
| SNS01-T | PNPs | siRNA against eIF5A and plasmid expressing eIF5A-K50R | Relapsed or refractory B cell malignancies | Phase I/II | Rod-shaped PEI nanoparticles loaded with both siRNA targeting eIF5A1 and an overexpression plasmid expressing the non-modifiable eIF5A-K50R mutant under the regulation of B-cell specific promoter. Average size of 72 nm. | [56,149,150] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| ALN-VSP | Lipid nanoparticle | siRNA | Liver cancer | Phase I | Combination of VEGF siRNA and KSP siRNA in a ratio of 1:1. Size around 80 nm with the neutral charged at physiologic pH. Note: the first lipid nanoparticle-formulated siRNA therapeutic to be tested in cancer patients. | [151,152] |
| DCR-MYC | Lipid nanoparticle | siRNA against MYC | Hepatocellular carcinoma | Phase I/II | DsiRNA encapsulated within an EnCoreTM lipid nanoparticle targeting c-Myc overexpressed cancerous cells. Note: the first siRNA therapeutic regimen targeting c-Myc that was evaluated clinically. | [153] |
| pbi-shRNA STMN1 LP | Lipid nanoparticle | shRNA against STMN1 | Advanced and/or metastatic cancer | Phase I | Cationic lipid particle loaded with a proprietary RNAi construct consisting of bifunctional shRNA against human STMN1. Other formulation properties not found. | [56,154,155] |
| TKM-080301 | Lipid nanoparticle | Anti-PLK1 siRNA | Various cancers | Phase I/II | SNALP loading siRNA targeting PLK1. Other properties not found. | [150,156,157] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Xyotax | PGA-Paclitaxel conjugate | Paclitaxel | Ovarian cancer | Phase III | PGA-Paclitaxel conjugation via an ester bond. Xyotax is highly water-soluble, with 37% drug loading. Paclitaxel is released by hydrolysis up to 14% in 24 h in physiological conditions, release is accelerated by lysosomal cathepsin B after endosomal uptake. | [74,158] |
| Prolindac | HPMA-DACH-platinum conjugate | DACH-platinum | Solid tumours | Phase II | DACH-platinum moiety conjugated with HPMA polymer via a pH-sensitive linker. Compared to unconjugated platinum drugs, AP5346 has a longer half-life and could release drug in acidic condition. Drug loading around 10 wt%. | [159,160] |
| EP0057 | PEG-Cyclodextrin-camptothecin conjugate | Camptothecin | Various tumours | Phase I/II | Cyclodextrin–PEG copolymer chemically conjugated to camptothecin. Drug loading around 10 wt%, and size of 20–60 nm. PEGylation increased residence time in the bloodstream and increased anti-tumour activity. | [74,161,162] |
| CRLX301 | PEG-Cyclodextrin-doxetaxel conjugate | Doxetaxel | Advanced solid tumours | Phase I/II | Cyclodextrin–PEG copolymer chemically conjugated to doxetaxel. Average size of 10–30 nm. Enhanced efficacy and improved pharmacokinetics, longer half-life and more than 20-fold higher drug concentration in tumour tissue, compared to doxetaxel. | [93,163,164] |
| PK1 (FCE28068) | HPMA-doxorubicin conjugate | Doxorubicin | Breast cancer, NSCLC, colorectal cancer | Phase III | HPMA copolymer covalently linked to doxorubicin via a peptidyl linker. Link is designed to be cleaved by lysosomal enzymes, with drug release after internalization. Polymer Mw 30 kDa. Total doxorubicin 6–8 wt%; free doxorubicin < 1% in respect of total. | [46,165] |
| PK2 (FCE28069) | HPMA-doxorubicin conjugate | Doxorubicin | Primary or metastatic liver cancer | Phase II | HPMA polymer conjugated to galactose residues and doxorubicin. Synthesized by a 27 kDa HPMA copolymer derivatized with 6.5% mol/wt, <2% free doxorubicin, and 2% mol/wt galactose. Note: PK2 has a similar structure to PK1, with the inclusion of galactosamine to specifically target hepatic cells. | [46,166] |
| PNU166945 | Polymer-drug conjugate | Paclitaxel | Solid tumours | Phase I | An HPMA copolymer-paclitaxel conjugate with the similar structure as PK1, but paclitaxel is conjugated to the terminal glycine by an ester bond. | [166] |
| MAG-CPT | MAG-camptothecin conjugate | Camptothecin | Various cancers | Phase I | MAG-campothecin conjugated via water soluble link. Average Mw of 18 kDa, and 10 wt% loading of camptothecin. | [135,167,168] |
| AP5280 | HPMA copolymer-platinum conjugate | Carboplatin platinate | Various cancers | Phase I/II | Platinum is linked to a HPMA backbone via a tetrapeptide spacer GFLG and an AMA chelating agent. Drug loading around 8.5 wt%. | [158,169,170] |
| CT-2106 | PGA-camptothecin conjugated | Camptothecin | Solid tumour, malignancies | Phase I/II | PGA conjugated to the hydroxyl group of camptothecin via a glycine linker. Solubility of camptothecin is increased, preventing opening of the lactone ring. Drug loading around 33–35 wt%. | [166,171,172] |
| Delimotecan | CMD-T2513 conjugated | T-2513 (camptothecin analogue) | Solid tumours | Phase I | T-2513 bound to CMD through a Gly-Gly-Gly linker, with a molecular weight of 130 kDa. Drug loading between 3–6 wt%. | [135,173] |
| Taxoprexin | Polymer-drug conjugate | Paclitaxel | Various cancer | Phase II/III | 2′-O-acyl conjugate of paclitaxel covalently bonded to the essential natural fatty acid DHA by an ester bond. Tumor AUCs for Taxoprexin are 61-fold higher at equitoxic doses and 8-fold higher at equimolar doses than paclitaxel. Other formulation properties not found. | [174,175] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Oncaspar | mPEG-protein conjugate | L-asparginase | Acute lymphoblastic leukemia | Approved by US FDA (1994) | Around 69–82 molecules of mPEG covalently conjugated to L-asparaginase. Increased half-life, sustained activity of L-asparaginase, reduced number of injections. | [123] |
| Neulasta | PEG-protein conjugate | Filgastrim | Chemotherapy induced neutropenia | Approved by US FDA (2002) | 20 kDa PEG molecule covalently conjugated to the α-amino group of the N-terminal methionine residue of Filgrastim, recombinant methionyl human G-CSF. Prolonged in vivo persistence. | [127,176] |
| SMANCS | Polymer-protein conjugate | Neocarzinostatin | Hepatocellular carcinoma | Approved in Japan (1994) | Passive-targeting formulation based on EPR effect. Neocarzinostatin conjugated to poly (styrene-comaleic acid) with the Mw of 16 kDa. In vivo t1/2 is 19 min. | [158,177] |
| Pegasys | PEG-protein nanoparticles | Interferon-α 2a | Various cancer | Phase I/II/III | Recombinant interferon α-2a (Mw > 19,000 Da) covalently conjugated to PEG chain (approximate Mw = 40,000 Da). Improved plasma half-life and uptake by liver, reduced dosing interval, but without sustained release pattern. | [103,178,179] |
| PegIntron | PEG-protein nanoparticles | Interferon-α 2b | Various cancer | Phase I/II/III | Recombinant Interferon-α 2b covalently conjugated to single straight-chain molecule of PEG with an average Mw of 12,000 Da. 10-fold increasing of plasma half-life from without compromising tertiary structure or spectrum of activity of IFN-α-2b. | [158,180,181,182] |
| ADI-PEG20 | PEG-protein nanoparticles | ADI | Various cancer | Phase I/II | PEG (Mw of 20,000) conjugated to ADI by a succinimidyl succinate linker. Prolonged half-life with around 50% of the specific enzyme activity. | [183,184] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Ontak | Protein nanoparticles | DAB389, truncated DT | cutaneous T-cell lymphoma | Approved by US FDA (1999) | DAB389, a truncated DT (the first 388 amino acid residues), as the toxin part. IL2 as the targeting part, could bind to high-affinity IL2 receptor expressed on the malignant cells and regulatory T cells. Mw = 58 kDa. | [185,186] |
| Abraxane | Protein nanoparticles | Paclitaxel | Various cancer | Approved by US FDA (2005) | Formed by lyophilized HSA and paclitaxel, 130 nm in diameter. Increased solubility of drug but without sustained release pattern. Drug loading 6.6 wt%. | [52,103,187] |
| ABI-008 | Protein nanoparticles | Docetaxel | Prostate cancer | Phase I/II | ABI-008, a solvent-free form of docetaxel, is based on NAB technology. Reduced side effects by eliminating polysorbate 80. | [188,189,190] |
| ABI-009 | Protein nanoparticles | Rapamycin | Various cancer | Phase I/II | Based on NAB technology. Rapamycin is a protein kinase inhibitor. Size around 100 nm. | [191] |
| Rexin-G | Retroviral expression vectors | Phospholipid/microRNA-122 | Solid tumour | Approved by Philippine FDA (2007) | A nonreplicative-targeted retroviral vector which has a cytocidal cyclin G1 construct. Size around 100 nm. | [192,193] |
| HAS-MTX | Protein nanoparticles | Methotrexate | Transitional cell carcinoma | Phase II | Methotrexate convently conjugated with HSA (Mw = 67 kD), in1:1 molar ratio. Increased tumour uptake and subsequently release of drug in a time-dependent manner with half-life about two weeks. | [194,195] |
| Product Name | Formulation | Drug/Therapeutic Agent | Treatment | Status | Formulation Properties | Ref. |
|---|---|---|---|---|---|---|
| Nanocrystal | ||||||
| Nanotax | Nanocrystal | Paclitaxel | Peritoneal neoplasms | Phase I | Aqueous, stable nanocrystal suspension of paclitaxel. Naked, rod-shaped particles with 600–700 nm in size, based on SCF technology. A depot system, intraperitoneal administration provides the stable reservoir of paclitaxel, extended drug release, increased tumour exposure with reduced toxicity. | [196,197] |
| Panzem NCD | Nanocrystal | 2ME2 | Various cancer | Phase II | 2ME2 reconstituted as a NCD, improved PK properties and antitumour activity. Enhanced anticancer activity when plasma 2ME2 exposure is constant, for example, using implanted osmotic pump or multiple oral administrations every day. | [198,199] |
| Theralux | Nanocrystal | Thymectacin | NHL | Phase II | Formulated by a photosensitive drug and a device designed to eliminate cancer cells (used outside the body). Drug would undergo photodynamic activation when cancer cells are exposed to visible light using Theralux device, resulting in the death of the cancer cells, minimized side effects and toxicity. | [200,201] |
| Other Nanoparticles | ||||||
| NanoTherm | Iron oxide nanoparticles | NA | Glioblastoma | Approved in EU (2010) | Aminosilane-coated SPIONS for local hyperthermia to treat tumours. After injecting into tumours, an alternating magnetic field is applied to selectively heat the particles, leading to tumour microenvironment to be heated locally to 40–45 °C, resulting in cell death. Size around 15 nm. | [93,202,203] |
| NBTXR3 | HfO2 nanoparticles | SBRT | Various cancers | Phase II/III | 50 nm nanoparticle composed of crystalline HfO2 functionalised by negatively charged phosphate coating. NBTXR3 improves the efficacy of radiotherapy. | [204] |
| CYT-6091 | Colloidal gold nanoparticle | TNF | Advanced solid tumours | Phase I/II | Multivalent drug with 26 nm on size, designed to actively sequester TNF in solid tumours. TNF and thiol-derivatized PEG covalently linked to the surface of the colloidal gold nanoparticles without binding between PEG-THIOL and TNF. | [17,127,205] |
| AuroLase | Silica-gold nanoshells coated with PEG. | NA | Head and neck cancer, prostate neoplasms | Without FDA-defined phases (trials of devices or behavioral interventions) | Designed to thermally ablate solid tumours after stimulation with a near-infrared energy source. Silica core acts as a dielectric core, gold shell has thermal ablation capability after absorbing strongly near-infrared light, PEG layer provides stability. | [206,207] |
2.2.5. Other Smart Sustained Release Nanoparticles
2.2.6. Clinical Use of Nanoparticles as Cancer Therapeutics: A Perspective
3. Strategies for Achieving Sustained Drug Release from Polymeric (PLGA, PLA, PLGA/PLA) Nanoparticles
3.1. Drugs Loaded into Polymeric Matrix of Nanoparticles
3.2. Drug-Polymer Conjugated Nanoparticles
3.3. Surface Modification Using Hydrophilic Polymers
3.3.1. Use of Poly (Ethylene Glycol) (PEG)
3.3.2. Surface Absorption by Hydrophilic Cationic Polymers
3.4. Surface Modification Using Biomacromolecules
3.5. Hybrid Nanoparticles and the Core-Shell Structure
4. Physico-Chemical Properties and Formulation of PLGA/PLA Nanoparticles Impacting on Drug Release
4.1. Effect of Properties of Selected Polymer
4.1.1. Mw of Polymer
4.1.2. Composition, Crystallinity and Glass Transition Temperature (Tg) of Polymer
4.1.3. Polymer End-Group Capping
4.2. Effect of the Drug
4.2.1. Drug Characteristics
4.2.2. Drug Loading (DL)
4.3. Effect of Nanoparticle Properties
4.3.1. Size of Nanoparticles
4.3.2. Shape of Nanoparticles
4.3.3. Surface Charge of Nanoparticles
4.3.4. Fabrication Condition of Nanoparticles
5. External Stimuli for Triggered-Drug Release in Polymeric Nanoparticles
5.1. pH-Triggered Release
5.2. Thermo-Triggered Release
5.3. Light-Triggered Release
5.4. Magnetic Field-Triggered Release
5.5. Redox-Triggered Release
5.6. Enzyme-Triggered Release
6. Challenges of Sustained Release from Smart Nanoparticles
7. The Future of Sustained Release Smart Nanoparticles: Microfluidics?
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 21 September 2022).
- Shirsath, N.R.; Goswami, A.K. Nanocarriers Based Novel Drug Delivery as Effective Drug Delivery: A Review. Curr. Nanomater. 2019, 4, 71–83. [Google Scholar] [CrossRef]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Document Search—Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 1 April 2022).
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Marchal, S.; El Hor, A.; Millard, M.; Gillon, V.; Bezdetnaya, L. Anticancer Drug Delivery: An Update on Clinically Applied Nanotherapeutics. Drugs 2015, 75, 1601–1611. [Google Scholar] [CrossRef]
- Kalaydina, R.-V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery—Personal Remarks and Future Prospects. J. Pers. Med. 2021, 11, 229. [Google Scholar] [CrossRef]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H.; et al. Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef]
- Vyas, D.; Patel, M.; Wairkar, S. Strategies for active tumor targeting-an update. Eur. J. Pharmacol. 2022, 915, 174512. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Wan, X.; Yu, D.-G.; Wang, K.; Yang, Y.; Liu, Z.-P. Electrospun lipid-coated medicated nanocomposites for an improved drug sustained-release profile. Mater. Des. 2019, 162, 70–79. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Min Sen Oh, V.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. In Nanomaterials and Neoplasms; Jenny Stanford Publishing: New York, NY, USA, 2021; ISBN 978-0-429-02781-9. [Google Scholar]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, D.; Yin, Z.; Chi, X.; Wang, X.; Gao, J. Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: Magnetic control and pH-responsive release. J. Mater. Chem. 2012, 22, 15717–15725. [Google Scholar] [CrossRef]
- Omidi, Y. Smart Multifunctional Theranostics: Simultaneous Diagnosis and Therapy of Cancer. Bioimpacts 2011, 1, 145–147. [Google Scholar] [CrossRef]
- Kumar, K.P.S.; Bhowmik, D.; Chiranjib; Chandira, M.; Tripath, K.K. Innovations in Sustained Release Drug Delivery System and Its Market Opportunities. J. Chem. Pharm. Res. 2010, 2, 349–360. [Google Scholar]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 2010, 6, 4026–4044. [Google Scholar] [CrossRef]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Naziris, N.; Saitta, F.; Chrysostomou, V.; Libera, M.; Trzebicka, B.; Fessas, D.; Pispas, S.; Demetzos, C. pH-responsive chimeric liposomes: From nanotechnology to biological assessment. Int. J. Pharm. 2020, 574, 118849. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Zhang, L.; Liu, X.; Jiang, B.; Long, Z.; Jiang, Y. Cell Permeable NBD Peptide-Modified Liposomes by Hyaluronic Acid Coating for the Synergistic Targeted Therapy of Metastatic Inflammatory Breast Cancer. Mol. Pharm. 2019, 16, 1140–1155. [Google Scholar] [CrossRef]
- Dunne, M.; Epp-Ducharme, B.; Sofias, A.M.; Regenold, M.; Dubins, D.N.; Allen, C. Heat-activated drug delivery increases tumor accumulation of synergistic chemotherapies. J. Control. Release 2019, 308, 197–208. [Google Scholar] [CrossRef]
- Celsion. A Phase III, Randomized, Double Blind, Dummy-Controlled Study of ThermoDox® (Lyso-Thermosensitive Liposomal Doxorubicin-LTLD) in Hepatocellular Carcinoma (HCC) Using Standardized Radiofrequency Ablation (RFA) Treatment Time ≥ 45 Minutes for Solitary Lesions ≥ 3 cm to ≤ 7 cm; Clinical Trials: Washington, DC, USA, 2018. [Google Scholar]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, C.K.; Nguyen, D.H. Redox and pH Responsive Poly (Amidoamine) Dendrimer-Heparin Conjugates via Disulfide Linkages for Letrozole Delivery. Biomed Res. Int. 2017, 2017, 8589212. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Yu, C.; Wang, L.; Xu, Z.; Teng, W.; Wu, Z.; Xiong, D. Smart micelles self-assembled from four-arm star polymers as potential drug carriers for pH-triggered DOX release. J. Polym. Res. 2020, 27, 111. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Biswas, G.; Jena, B.C.; Sahoo, S.; Samanta, P.; Mandal, M.; Dhara, D. A copper-free click reaction for the synthesis of redox-responsive water-soluble core cross-linked nanoparticles for drug delivery in cancer therapy. Green Chem. 2019, 21, 5624–5638. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X.; Tian, K.; Zhou, T.; Li, J.; Zhang, R.; Liu, P. Novel fluorescent pH/reduction dual stimuli-responsive polymeric nanoparticles for intracellular triggered anticancer drug release. Chem. Eng. J. 2016, 295, 468–476. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Nan, L.; Peng, T.; Sun, L.; Zhou, J.; Xiao, Y.; Wang, J.; Sun, J.; Lu, W.; et al. Erythrocyte Membrane-Wrapped pH Sensitive Polymeric Nanoparticles for Non-Small Cell Lung Cancer Therapy. Bioconjug. Chem. 2017, 28, 2591–2598. [Google Scholar] [CrossRef]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Smart Drug Delivery Systems in the Treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef]
- O’Byrne, K.J.; Thomas, A.L.; Sharma, R.A.; DeCatris, M.; Shields, F.; Beare, S.; Steward, W.P. A phase I dose-escalating study of DaunoXome, liposomal daunorubicin, in metastatic breast cancer. Br. J. Cancer 2002, 87, 15–20. [Google Scholar] [CrossRef]
- Waterhouse, D.N.; Dos Santos, N.; Mayer, L.D.; Bally, M.B. Drug-drug interactions arising from the use of liposomal vincristine in combination with other anticancer drugs. Pharm. Res. 2001, 18, 1331–1335. [Google Scholar] [CrossRef]
- Forssen, E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.E.; Perkins, W.R.; Roberts, P.; Janoff, A.S. Liposome technology and the development of MyocetTM (liposomal doxorubicin citrate). Breast 2001, 10, 1–7. [Google Scholar] [CrossRef]
- Ye, L.; He, J.; Hu, Z.; Dong, Q.; Wang, H.; Fu, F.; Tian, J. Antitumor effect and toxicity of Lipusu in rat ovarian cancer xenografts. Food Chem. Toxicol. 2013, 52, 200–206. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Wu, H.; Ramanathan, R.K.; Zamboni, B.A.; Strychor, S.; Ramalingam, S.; Edwards, R.P.; Friedland, D.M.; Stoller, R.G.; Belani, C.P.; Maruca, L.J.; et al. Population pharmacokinetics of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. J. Clin. Pharmacol. 2012, 52, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Chi, K.; Miller, W.; Chia, S.; Hasanbasic, F.; Fisic, A.; Mayer, L.; Swenson, C.; Janoff, A.; Gelmon, K. Phase 1 study of CPX-1, a fixed ratio formulation of irinotecan (IRI) and floxuridine (FLOX), in patients with advanced solid tumors. J. Clin. Oncol. 2006, 24, 2014. [Google Scholar] [CrossRef]
- Jazz Pharmaceuticals. Multicenter, Open-Label, Phase 2 Study Of CPX-1 (Irinotecan HCl: Floxuridine) Liposome Injection In Patients With Advanced Colorectal Carcinoma; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Alliance for Clinical Trials in Oncology. Phase II Trial of LE SN38 in Patients With Metastatic Colorectal Cancer After Progression on Oxaliplatin; Clinical Trials: Washington, DC, USA, 2016. [Google Scholar]
- Liposome Encapsulated SN38 (LE-SN38) in Patients with Advanced Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00046540 (accessed on 21 September 2022).
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]
- Wolf, S.M.; Gupta, R.; Kohlhepp, P. Gene Therapy Oversight: Lessons for Nanobiotechnology. J. Law Med. Ethics 2009, 37, 659–684. [Google Scholar] [CrossRef] [PubMed]
- Insmed Incorporated. Phase Ib/IIa Study of SLIT Cisplatin by Inhalation in the Treatment of Patients Wtih Relapsed/Progressive Osteosarcoma Metastatic to the Lung; Clinical Trials: Washington, DC, USA, 2017. [Google Scholar]
- Von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Hang, Z.; Cooper, M.; Ziora, Z. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem. Compd. 2016, 4, 1. [Google Scholar] [CrossRef]
- Beutel, G.; Glen, H.; Schöffski, P.; Chick, J.; Gill, S.; Cassidy, J.; Twelves, C. Phase I study of OSI-7904L, a novel liposomal thymidylate synthase inhibitor in patients with refractory solid tumors. Clin. Cancer Res. 2005, 11, 5487–5495. [Google Scholar] [CrossRef]
- Definition of OSI-7904L-NCI Drug Dictionary-NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/liposome-encapsulated-osi-7904 (accessed on 21 September 2022).
- NCIC Clinical Trials Group. Randomized Phase II Study Of NX 211 Given By Two Different Intravenous Schedules In Advanced And/Or Recurrent Epithelial Ovarian Cancer; Clinical Trials: Washington, DC, USA, 2020. [Google Scholar]
- Lee, M.-K. Clinical usefulness of liposomal formulations in cancer therapy: Lessons from the experiences of doxorubicin. J. Pharm. Investig. 2019, 49, 203–214. [Google Scholar] [CrossRef]
- Tomkinson, B.; Bendele, R.; Giles, F.J.; Brown, E.; Gray, A.; Hart, K.; LeRay, J.D.; Meyer, D.; Pelanne, M.; Emerson, D.L. OSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALL. Leuk. Res. 2003, 27, 1039–1050. [Google Scholar] [CrossRef]
- SynerGene Therapeutics, Inc. Phase II Study of Combined Temozolomide and Targeted P53 Gene Therapy (SGT-53) for Treatment of Patients With Recurrent Glioblastoma; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Kim, S.-S.; Rait, A.; Kim, E.; Pirollo, K.F.; Chang, E.H. A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine 2015, 11, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Mebiopharm Co., Ltd. A Phase Ib/II Study of MBP-426 in Patients with Second Line Gastric, Gastro Esophageal, or Esophageal Adenocarcinoma; Clinical Trials: Washington, DC, USA, 2014. [Google Scholar]
- Krishnan, V.; Rajasekaran, A.K. Clinical nanomedicine: A solution to the chemotherapy conundrum in pediatric leukemia therapy. Clin. Pharmacol. Ther. 2014, 95, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Swiss Group for Clinical Cancer Research. Anti-EGFR-immunoliposomes Loaded With Doxorubicin in Patients With Advanced Triple Negative EGFR Positive Breast Cancer—A Multicenter Single Arm Phase II Trial; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Wicki, A.; Ritschard, R.; Loesch, U.; Deuster, S.; Rochlitz, C.; Mamot, C. Large-scale manufacturing of GMP-compliant anti-EGFR targeted nanocarriers: Production of doxorubicin-loaded anti-EGFR-immunoliposomes for a first-in-man clinical trial. Int. J. Pharm. 2015, 484, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Silence Therapeutics GmbH. A Phase Ib/Iia Study of Combination Therapy with Gemcitabine and Atu027 In Subjects with Locally Advanced or Metastatic Pancreatic Adenocarcinoma; Clinical Trials: Washington, DC, USA, 2016. [Google Scholar]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef]
- University of Pittsburgh. Phase I Trial of Intratumoral EGFR Antisense DNA and DC-Chol Liposomes in Advanced Oral Squamous Cell Carcinoma; Clinical Trials: Washington, DC, USA, 2016. [Google Scholar]
- Lee, J.-H.; Lee, M.-J. Liposome-Mediated Cancer Gene Therapy: Clinical Trials and their Lessons to Stem Cell Therapy. Bull. Korean Chem. Soc. 2012, 33, 433–442. [Google Scholar] [CrossRef][Green Version]
- SynCore Biotechnology Co., Ltd. An Open-label, Randomized, Controlled Phase III Trial Evaluating the Efficacy and Safety of EndoTAG®-1 in Combination with Paclitaxel and Gemcitabine Compared With Paclitaxel and Gemcitabine as First-line Therapy in Patients With Visceral Metastatic Triple-negative Breast Cancer; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- Eichhorn, M.E.; Ischenko, I.; Luedemann, S.; Strieth, S.; Papyan, A.; Werner, A.; Bohnenkamp, H.; Guenzi, E.; Preissler, G.; Michaelis, U.; et al. Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int. J. Cancer 2010, 126, 1235–1245. [Google Scholar] [CrossRef]
- Chen, L.-T.; Hitre, E.; Lee, W.-J.; Bai, L.-Y.; Papaï, Z.; Kang, S.Y.; Dvorkin, M.; Choi, H.J.; Oh, S.C.; Artru, P.; et al. 834TiP—A randomized controlled, open label, adaptive phase III Trial to evaluate safety and efficacy of endoTAG-1 plus gemcitabine versus gemcitabine alone in patients with measurable locally advanced and/or metastatic adenocarcinoma of the pancreas failed on FOLFIRINOX treatment. Ann. Oncol. 2019, 30, v321. [Google Scholar] [CrossRef]
- INSYS Therapeutics Inc. Phase I Study of an Easy-to-Use Intravenous Formulation of Liposome Entrapped C-raf Antisense Oligonucleotide (LErafAON-ETU) Administered on a Weekly Schedule in Patients With Advanced Cancer; Clinical Trials: Washington, DC, USA, 2011. [Google Scholar]
- Rudin, C.M.; Marshall, J.L.; Huang, C.H.; Kindler, H.L.; Zhang, C.; Kumar, D.; Gokhale, P.C.; Steinberg, J.; Wanaski, S.; Kasid, U.N.; et al. Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: A phase I study. Clin. Cancer Res. 2004, 10, 7244–7251. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Wei, Y.-Q.; Gao, G.-P.; Wei, X.-W. Clinical Evaluations of Toxicity and Efficacy of Nanoparticle-Mediated Gene Therapy. Hum. Gene Ther. 2018, 29, 1227–1234. [Google Scholar] [CrossRef]
- Boulikas, T. Molecular mechanisms of cisplatin and its liposomally encapsulated form, Lipoplatin. Cancer Ther. 2007, 5, 349–376. [Google Scholar]
- Lipotek Pty Ltd. A Phase I Open-label Study of the Safety and Immunogenicity of Escalating Doses of Lipovaxin-MM, a Novel Melanoma Immunotherapeutic, in Patients With Metastatic Melanoma; Clinical Trials: Washington, DC, USA, 2012. [Google Scholar]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.C.; Altin, J.; Parish, C.R.; et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Espelin, C.; Leonard, S.; Geretti, E.; Wickham, T.; Hendriks, B. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016, 76, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Sierra Oncology, Inc. PNT2258-02: A Pilot Phase II Study of PNT2258 for Treatment of Relapsed or Refractory Non-Hodgkin’s Lymphoma; Clinical Trials: Washington, DC, USA, 2020. [Google Scholar]
- Tolcher, A.; Rodrigueza, W.; Rasco, D.; Patnaik, A.; Papadopoulos, K.; Amaya, A.; Moore, T.; Gaylor, S.; Bisgaier, C.; Mina, P.; et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 73, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Intravenously Administered Pegylated Liposomal Mitomycin-C Lipid-based Prodrug (PROMITIL) in Cancer Patients with Solid Tumors. No Study Results Posted. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01705002 (accessed on 21 September 2022).
- Tian, X.; Warner, S.B.; Wagner, K.T.; Caster, J.M.; Zhang, T.; Ohana, P.; Gabizon, A.A.; Wang, A.Z. Preclinical Evaluation of Promitil, a Radiation-Responsive Liposomal Formulation of Mitomycin C Prodrug, in Chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 547–555. [Google Scholar] [CrossRef]
- Anderson, M.D. Cancer Center. EphA2 Gene Targeting Using Neutral Liposomal Small Interfering RNA Delivery: A Phase I Clinical Trial; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- Gomes-da-Silva, L.C.; Simões, S.; Moreira, J.N. Challenging the future of siRNA therapeutics against cancer: The crucial role of nanotechnology. Cell Mol. Life Sci. 2014, 71, 1417–1438. [Google Scholar] [CrossRef]
- Wurz, G.T.; Kao, C.-J.; Wolf, M.; DeGregorio, M.W. Tecemotide: An antigen-specific cancer immunotherapy. Null 2014, 10, 3383–3393. [Google Scholar] [CrossRef]
- EMD Serono. A Multi-Center Phase III Randomized, Double-Blind Placebo-Controlled Study of the Cancer Vaccine Stimuvax® (L-BLP25 or BLP25 Liposome Vaccine) in Non-Small Cell Lung Cancer (NSCLC) Subjects with Unresectable Stage III Disease; Clinical Trials: Washington, DC, USA, 2015. [Google Scholar]
- NYU Langone Health. Phase II Clinical Study of a Liposome Entrapped Cisplatin Analog (L-NDDP) Administered Intrapleurally in Patients With Malignant Pleural Mesothelioma; Clinical Trials: Washington, DC, USA, 2011. [Google Scholar]
- Liu, D.; He, C.; Wang, A.Z.; Lin, W. Application of liposomal technologies for delivery of platinum analogs in oncology. Int. J. Nanomed. 2013, 8, 3309–3319. [Google Scholar] [CrossRef]
- INSYS Therapeutics Inc. A Multicenter, Open-Label, Phase II Study of LEP-ETU for Efficacy and Safety in Patients With Metastatic Breast Cancer; Clinical Trials: Washington, DC, USA, 2012. [Google Scholar]
- Tan, Y.F.; Lao, L.L.; Xiong, G.M.; Venkatraman, S. Controlled-release nanotherapeutics: State of translation. J. Control. Release 2018, 284, 39–48. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef]
- Moleculin Biotech, Inc. Phase 1/2 Study of Liposomal Annamycin for the Treatment of Subjects with Acute Myeloid Leukemia (AML) that Is Refractory to or Relapsed after Induction Therapy; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. WIREs Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Cheng, M.-Y. Clinically-Proven Liposome-Based Drug Delivery: Formulation, Characterization and Therapeutic Efficacy. J. Nanomed. Biother. Discov. 2012, 1, 195. [Google Scholar] [CrossRef]
- Bio-Path Holdings, Inc. A Phase Ib/IIa Single-Arm, Open-Label Clinical Trial to Evaluate the Safety, Pharmacokinetics, and Efficacy of BP1001 (a Liposomal Grb2 Antisense Oligonucleotide) in Combination with Dasatinib in Patients with Philadelphia Chromosome Positive (Ph+) Chronic Myelogenous Leukemia (CML) Including Chronic Phase Patients Who Have Failed Initial Tyrosine Kinase Inhibitor (TKI) Therapy, Accelerated or Blast Phase, Ph+ Acute Myeloid Leukemia (AML) or High-risk Ph+ Myelodysplastic Syndrome (MDS); Clinical Trials: Washington, DC, USA, 2020. [Google Scholar]
- Tari, A.M.; Gutiérrez-Puente, Y.; Monaco, G.; Stephens, C.; Sun, T.; Rosenblum, M.; Belmont, J.; Arlinghaus, R.; Lopez-Berestein, G. Liposome-incorporated Grb2 antisense oligodeoxynucleotide increases the survival of mice bearing bcr-abl-positive leukemia xenografts. Int. J. Oncol. 2007, 31, 1243–1250. [Google Scholar] [PubMed]
- Ohanian, M.; Kantarjian, H.M.; Ravandi, F.; Borthakur, G.; Garcia-Manero, G.; Andreeff, M.; Jabbour, E.; Konopleva, M.; Lim, M.; Pierce, S.; et al. Safety, Pharmacokinetics, and Efficacy of BP-100-1.01 (Liposomal Grb-2 Antisense Oligonucleotide) in Patients with Refractory or Relapsed Acute Myeloid Leukemia (AML), Philadelphia Chromosome Positive Chronic Myelogenous Leukemia (CML), Acute Lymphoblastic Leukemia (ALL), and Myelodysplastic Syndrome (MDS). Blood 2015, 126, 3801. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Tippayamontri, T.; Kotb, R.; Sanche, L.; Paquette, B. New therapeutic possibilities of combined treatment of radiotherapy with oxaliplatin and its liposomal formulation, LipoxalTM, in rectal cancer using xenograft in nude mice. Anticancer Res. 2014, 34, 5303–5312. [Google Scholar] [PubMed]
- Stathopoulos, G.P.; Boulikas, T.; Kourvetaris, A.; Stathopoulos, J. Liposomal oxaliplatin in the treatment of advanced cancer: A phase I study. Anticancer Res. 2006, 26, 1489–1493. [Google Scholar] [PubMed]
- Allarity Therapeutics. Phase I/II Study to Evaluate the Safety and Tolerability of LiPlaCis (Liposomal Cisplatin Formulation) in Patients With Advanced or Refractory Tumours; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- Pourhassan, H.; Clergeaud, G.; Hansen, A.E.; Østrem, R.G.; Fliedner, F.P.; Melander, F.; Nielsen, O.L.; O’Sullivan, C.K.; Kjær, A.; Andresen, T.L. Revisiting the use of sPLA2-sensitive liposomes in cancer therapy. J. Control. Release 2017, 261, 163–173. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.J.A.; Slingerland, M.; Loos, W.J.; Wiemer, E.A.C.; Burger, H.; Mathijssen, R.H.J.; Kroep, J.R.; den Hollander, M.A.G.; van der Biessen, D.; Lam, M.-H.; et al. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer 2010, 46, 3016–3021. [Google Scholar] [CrossRef]
- ImmunoVaccine Technologies, Inc. (IMV Inc.). A Phase I Study of Two Different Doses of the Subcutaneous Administration of an Immunotherapeutic Vaccine, DPX-0907 in Advanced Stage Patients with Ovarian, Breast or Prostate Cancer; Clinical Trials: Washington, DC, USA, 2015. [Google Scholar]
- Karkada, M.; Berinstein, N.L.; Mansour, M. Therapeutic vaccines and cancer: Focus on DPX-0907. Biologics 2014, 8, 27–38. [Google Scholar] [CrossRef]
- Morse, M. A Monocentric, Open-label Phase I/II Study to Assess dHER2+AS15 Cancer Immunotherapeutic Given in Combination With Lapatinib to Patients With ErbB2 Overexpressing Metastatic Breast Cancer Refractory to Trastuzumab; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Trial of the Immunostimulant JVRS-100 for the Treatment of Patients With Relapsed or Refractory Leukemia; Clinical Trials: Washington, DC, USA, 2019.
- Fairman, J.; Liu, K.H.; Menne, S. Prevention of liver tumor formation in woodchucks with established hepatocellular carcinoma by treatment with cationic liposome-DNA complexes. BMC Cancer 2017, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJ Pharm. Pharm. Sci. 2014, 1, 13. [Google Scholar] [CrossRef]
- Oasmia Pharmaceutical. An Appealing Metamorphosis. Available online: https://www.edisongroup.com/publication/an-appealing-metamorphosis/ (accessed on 22 September 2022).
- Binkhathlan, Z.; Lavasanifar, A. Chapter 15—Effects of block copolymer micelles on the pharmacokinetics of encapsulated drugs. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: New York, NY, USA, 2019; pp. 507–546. ISBN 978-0-12-816200-2. [Google Scholar]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Ma, P.; Xiao, H.; Li, C.; Dai, Y.; Cheng, Z.; Hou, Z.; Lin, J. Inorganic Nanocarriers for Platinum Drug Delivery. Mater. Today 2015, 18, 554–564. [Google Scholar] [CrossRef]
- NanoCarrier Co., Ltd. A Phase 1 Dose-Escalation and Pharmacokinetic Study of NC-4016 in Patients With Advanced Solid Tumors or Lymphoma; Clinical Trials: Washington, DC, USA, 2018. [Google Scholar]
- Ueno, T.; Endo, K.; Hori, K.; Ozaki, N.; Tsuji, A.; Kondo, S.; Wakisaka, N.; Murono, S.; Kataoka, K.; Kato, Y.; et al. Assessment of antitumor activity and acute peripheral neuropathy of 1,2-diaminocyclohexane platinum (II)-incorporating micelles (NC-4016). Int. J. Nanomed. 2014, 2014, 3005–3012. [Google Scholar] [CrossRef]
- Ghamande, S.; Lin, C.-C.; Cho, D.C.; Shapiro, G.I.; Kwak, E.L.; Silverman, M.H.; Tseng, Y.; Kuo, M.-W.; Mach, W.B.; Hsu, S.-C.; et al. A phase 1 open-label, sequential dose-escalation study investigating the safety, tolerability, and pharmacokinetics of intravenous TLC388 administered to patients with advanced solid tumors. Investig. New Drugs 2014, 32, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Liu, X.; Zhang, W.; Xiong, X.; Han, Z.; Liang, X. Camptothecin-based nanodrug delivery systems. Cancer Biol. Med. 2017, 14, 363–370. [Google Scholar] [CrossRef] [PubMed]
- NanoCarrier Co., Ltd. A Phase 1b/2 Dose-Escalation and Expansion Trial of NC-6300 (Nanoparticle Epirubicin) in Patients With Advanced Solid Tumors or Advanced, Metastatic, or Unresectable Soft Tissue Sarcoma; Clinical Trials: Washington, DC, USA, 2020. [Google Scholar]
- Harada, M.; Bobe, I.; Saito, H.; Shibata, N.; Tanaka, R.; Hayashi, T.; Kato, Y. Improved anti-tumor activity of stabilized anthracycline polymeric micelle formulation, NC-6300. Cancer Sci. 2011, 102, 192–199. [Google Scholar] [CrossRef]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Packhaeuser, C.B.; Schnieders, J.; Oster, C.G.; Kissel, T. In situ forming parenteral drug delivery systems: An overview. Eur. J. Pharm. Biopharm. 2004, 58, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-assembled targeted nanoparticles: Evolution of technologies and bench to bedside translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef]
- Onxeo. Multicentre, Randomised, Controlled, Open-label Study Comparing the Efficacy and Safety of Doxorubicin TransdrugTM to Best Standard of Care in Patients With Advanced Hepatocellular Carcinoma. ReLive Study; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Merle, P.; Camus, P.; Abergel, A.; Pageaux, G.; Masliah, C.; Bronowicki, J.; Zarski, J.; Pelletier, G.; Bouattour, M.; Farloux, L.; et al. Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO Open 2017, 2, e000238. [Google Scholar] [CrossRef]
- Telrandhe, R. Nanotechnology for Cancer Therapy: Recent Developments. Eur. J. Pharm. Med. Res. 2016, 3, 284–294. [Google Scholar]
- Zhou, Q.; Sun, X.; Zeng, L.; Liu, J.; Zhang, Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Samyang Biopharmaceuticals Corporation. A Trial to Determine the Maximum Tolerated Dose and Evaluate the Safety and Pharmacokinetics of Docetaxel-PNP, Polymeric Nanoparticle Formulation of Docetaxel, in Subjects With Advanced Solid Malignancies; Clinical Trials: Washington, DC, USA, 2017. [Google Scholar]
- Song, S.Y.; Kim, K.-P.; Jeong, S.-Y.; Park, J.; Park, J.; Jung, J.; Chung, H.K.; Lee, S.-W.; Seo, M.H.; Lee, J.-S.; et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: Phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016, 7, 77348–77357. [Google Scholar] [CrossRef] [PubMed]
- Senesco Technologies, Inc. Phase 1/2 Open-Label, Multiple-Dose, Dose-Escalation Study to Evaluate the Safety and Tolerability of SNS01-T Administered by Intravenous Infusion in Patients With Relapsed or Refractory B Cell Malignancies; Clinical Trials: Washington, DC, USA, 2014. [Google Scholar]
- Francis, S.M.; Taylor, C.A.; Tang, T.; Liu, Z.; Zheng, Q.; Dondero, R.; Thompson, J.E. SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide. Mol. Ther. 2014, 22, 1643–1652. [Google Scholar] [CrossRef]
- Reghupaty, S.C.; Sarkar, D. Current Status of Gene Therapy in Hepatocellular Carcinoma. Cancers 2019, 11, E1265. [Google Scholar] [CrossRef]
- Barros, S.A.; Gollob, J.A. Safety profile of RNAi nanomedicines. Adv. Drug. Deliv. Rev. 2012, 64, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Gazzali, A.M.; Bakhtiar, A. Clinical Advances of siRNA-Based Nanotherapeutics for Cancer Treatment. Pharmaceutics 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Gradalis, Inc. Phase I Trial of Intratumoral Bi-functional shRNA Stathmin 1-knockdown Lipoplex in Patients With Advanced and/or Metastatic Cancer; Clinical Trials: Washington, DC, USA, 2018. [Google Scholar]
- Rao, D.D.; Wang, Z.; Senzer, N.; Nemunaitis, J. RNA interference and personalized cancer therapy. Discov. Med. 2013, 15, 101–110. [Google Scholar]
- Seyfoori, A.; Sarfarazijami, S.; Seyyed Ebrahimi, S.A. pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1437–1443. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Siddikuzzaman; Palanimuthu, D.; Somasundaram, K.; Samuelson, A.G. Biotin Decorated Gold Nanoparticles for Targeted Delivery of a Smart-Linked Anticancer Active Copper Complex: In Vitro and In Vivo Studies. Bioconjug. Chem. 2016, 27, 2874–2885. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Campone, M.; Rademaker-Lakhai, J.M.; Bennouna, J.; Howell, S.B.; Nowotnik, D.P.; Beijnen, J.H.; Schellens, J.H.M. Phase I and pharmacokinetic trial of AP5346, a DACH-platinum-polymer conjugate, administered weekly for three out of every 4 weeks to advanced solid tumor patients. Cancer Chemother. Pharmacol. 2007, 60, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Nowotnik, D.P.; Cvitkovic, E. ProLindac (AP5346): A review of the development of an HPMA DACH platinum Polymer Therapeutic. Adv. Drug Deliv. Rev. 2009, 61, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Polymeric nanotherapeutics: Clinical development and advances in stealth functionalization strategies. Nanoscale 2014, 6, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Madan, M.D.; National Cancer Institute. A Single Arm Phase II Study Combining CRLX101, a Nanoparticle Camptothecin, With Enzalutamide in Patients With Progressive Metastatic Castration Resistant Prostate Cancer Following Prior Enzalutamide Treatment; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- Zamboni, W.C.; Markman, B.; de Souza, P.; Dees, E.C.; Gangadhar, T.; Eliasof, S.; Murphy, C.; Senderowicz, A.; Wang, H. Abstract 2047: Pharmacokinetics (PK) of CRLX301, a novel nanoparticle-drug conjugate (NDC) containing the payload docetaxel, in patients with refractory solid tumors. Cancer Res. 2016, 76, 2047. [Google Scholar] [CrossRef]
- Lazarus, D.; Kabir, S.; Eliasof, S. Abstract 5643: CRLX301, a novel tumor-targeted taxane nanopharmaceutical. Cancer Res. 2012, 72, 5643. [Google Scholar] [CrossRef]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-0-387-77668-2. [Google Scholar]
- Parveen, S.; Arjmand, F.; Tabassum, S. Clinical developments of antitumor polymer therapeutics. RSC Adv. 2019, 9, 24699–24721. [Google Scholar] [CrossRef]
- Bissett, D.; Cassidy, J.; de Bono, J.S.; Muirhead, F.; Main, M.; Robson, L.; Fraier, D.; Magnè, M.L.; Pellizzoni, C.; Porro, M.G.; et al. Phase I and pharmacokinetic (PK) study of MAG-CPT (PNU 166148): A polymeric derivative of camptothecin (CPT). Br. J. Cancer 2004, 91, 50–55. [Google Scholar] [CrossRef]
- University of Glasgow. A Phase I Study to Evaluate MAG-CPT (PNU 166148) Given as One Single Intravenous Administration Every 4 Weeks in Patients With Advanced Solid Tumors; Clinical Trials: Washington, DC, USA, 2013. [Google Scholar]
- Rademaker-Lakhai, J.M.; Terret, C.; Howell, S.B.; Baud, C.M.; De Boer, R.F.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M.; Droz, J.-P. A Phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin. Cancer Res. 2004, 10, 3386–3395. [Google Scholar] [CrossRef]
- Bouma, M.; Nuijen, B.; Stewart, D.R.; Rice, J.R.; Jansen, B.A.; Reedijk, J.; Bult, A.; Beijnen, J.H. Stability and compatibility of the investigational polymer-conjugated platinum anticancer agent AP 5280 in infusion systems and its hemolytic potential. Anticancer Drugs 2002, 13, 915–924. [Google Scholar] [CrossRef]
- Homsi, J.; Simon, G.R.; Garrett, C.R.; Springett, G.; De Conti, R.; Chiappori, A.A.; Munster, P.N.; Burton, M.K.; Stromatt, S.; Allievi, C.; et al. Phase I trial of poly-L-glutamate camptothecin (CT-2106) administered weekly in patients with advanced solid malignancies. Clin. Cancer Res. 2007, 13, 5855–5861. [Google Scholar] [CrossRef]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Sakakibara, H.; Yano, T.; Suzuki, T.; Okuno, S. Determinants for the drug release from T-0128, camptothecin analogue-carboxymethyl dextran conjugate. J. Control. Release 2000, 69, 399–412. [Google Scholar] [CrossRef]
- Meng, Z.; Lv, Q.; Lu, J.; Yao, H.; Lv, X.; Jiang, F.; Lu, A.; Zhang, G. Prodrug Strategies for Paclitaxel. Int. J. Mol. Sci. 2016, 17, 796. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Ellis, P.; Dunlop, D.; Ranson, M.; Danson, S.; Schacter, L.; Talbot, D. DHA-paclitaxel (Taxoprexin) as first-line treatment in patients with stage IIIB or IV non-small cell lung cancer: Report of a phase II open-label multicenter trial. J. Thorac. Oncol. 2006, 1, 984–990. [Google Scholar] [CrossRef]
- Piedmonte, D.M.; Treuheit, M.J. Formulation of Neulasta (pegfilgrastim). Adv. Drug Deliv. Rev. 2008, 60, 50–58. [Google Scholar] [CrossRef]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185. [Google Scholar] [CrossRef]
- Larsen, T.S. Danish Study of Low-dose Interferon Alpha Versus Hydroxyurea in the Treatment of Philadelphia Chromosome Negative (Ph-)Chronic Myeloid Neoplasms; Clinical Trials: Washington, DC, USA, 2022. [Google Scholar]
- University of Michigan Rogel Cancer Center. Targeting Cross-presentation With Peginterferon Alfa-2a to Enhance Anti-leukemic Responses After Allogeneic Transplantation in High Risk Acute Myeloid Leukemia; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Kroep, J.R. Chemo-Immunotherapy, Gemcitabine With Pegylated Interferon Alpha-2b (Peg-Intron) With and Without p53 Synthetic Long Peptide (p53 SLP) Vaccine, for Patients With Platinum Resistant Ovarian Cancer CHIP Trial; Clinical Trials: Washington, DC, USA, 2014. [Google Scholar]
- Wang, C. Induction of Graft Versus Tumor Effect of Pegylated Interferon Alpha-2b for Patients With Relapsed Hematological Malignancies After Allogeneic Stem Cell Transplantation; Clinical Trials: Washington, DC, USA, 2021. [Google Scholar]
- Bukowski, R.M.; Tendler, C.; Cutler, D.; Rose, E.; Laughlin, M.M.; Statkevich, P. Treating cancer with PEG Intron: Pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer 2002, 95, 389–396. [Google Scholar] [CrossRef]
- Ludwig Institute for Cancer Research. Phase 1/2 Study of ADI-SS PEG 20,000mw in Patients With Advanced Melanoma; Clinical Trials: Washington, DC, USA, 2017. [Google Scholar]
- Feun, L.; Savaraj, N. Pegylated arginine deiminase: A novel anticancer enzyme agent. Expert Opin. Investig. Drugs 2006, 15, 815–822. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jun, S.-Y.; Kim, Y.-S. Critical Issues in the Development of Immunotoxins for Anticancer Therapy. J. Pharm. Sci. 2020, 109, 104–115. [Google Scholar] [CrossRef]
- Baldo, B.A. Chimeric fusion proteins used for therapy: Indications, mechanisms, and safety. Drug Saf. 2015, 38, 455–479. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Zheng, X.; Xia, R.; Liu, S.; Xie, Z.; Jing, X. Albumin-bound paclitaxel dimeric prodrug nanoparticles with tumor redox heterogeneity-triggered drug release for synergistic photothermal/chemotherapy. Nano Res. 2019, 12, 877–887. [Google Scholar] [CrossRef]
- Celgene. A Phase I/II Trial of ABI-008 (Nab-docetaxel) in Patients With Hormone-refractory Prostate Cancer; Clinical Trials: Washington, DC, USA, 2019. [Google Scholar]
- Tan, Y.L.; Ho, H.K. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov. Today 2018, 23, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Hou, S.; Schmid, A.; Desai, N. Abstract 348: ABI-009 (nab-Sirolimus) improves tumor accumulation and antitumor activity over oral mTOR inhibitors. Cancer Res. 2019, 79, 348. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Gupta, S.; Li, C. Interventional Nanotheranostics of Pancreatic Ductal Adenocarcinoma. Theranostics 2016, 6, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.M.; Hall, F.L. Rexin-G, a targeted genetic medicine for cancer. Expert Opin. Biol. Ther. 2010, 10, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Bolling, C.; Graefe, T.; Lübbing, C.; Jankevicius, F.; Uktveris, S.; Cesas, A.; Meyer-Moldenhauer, W.-H.; Starkmann, H.; Weigel, M.; Burk, K.; et al. Phase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinoma. Investig. New Drugs 2006, 24, 521–527. [Google Scholar] [CrossRef]
- Vis, A.N.; van der Gaast, A.; van Rhijn, B.W.G.; Catsburg, T.K.; Schmidt, C.; Mickisch, G.H.J. A phase II trial of methotrexate-human serum albumin (MTX-HSA) in patients with metastatic renal cell carcinoma who progressed under immunotherapy. Cancer Chemother. Pharmacol. 2002, 49, 342–345. [Google Scholar] [CrossRef]
- Kollipara, S.; Bende, G.; Movva, S.; Saha, R. Application of rotatable central composite design in the preparation and optimization of poly(lactic-co-glycolic acid) nanoparticles for controlled delivery of paclitaxel. Drug Dev. Ind. Pharm. 2010, 36, 1377–1387. [Google Scholar] [CrossRef]
- Williamson, S.K.; Johnson, G.A.; Maulhardt, H.A.; Moore, K.M.; McMeekin, D.S.; Schulz, T.K.; Reed, G.A.; Roby, K.F.; Mackay, C.B.; Smith, H.J.; et al. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax®) in patients with peritoneal malignancies. Cancer Chemother. Pharmacol. 2015, 75, 1075–1087. [Google Scholar] [CrossRef]
- Harrison, M.R.; Hahn, N.M.; Pili, R.; Oh, W.K.; Hammers, H.; Sweeney, C.; Kim, K.; Perlman, S.; Arnott, J.; Sidor, C.; et al. A Phase II Study of 2-Methoxyestradiol (2ME2) NanoCrystal® Dispersion (NCD) in Patients with Taxane-Refractory, Metastatic Castrate-Resistant Prostate Cancer (CRPC). Investig. New Drugs 2011, 29, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2014, 32, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Kiadis Pharma. Phase I, Dose-ranging, Open-label, Study of a Single Administration of T-cells Add-back Depleted of Host Alloreactive Cells Using TheraluxTM Therapy, Following Haploidentical Peripheral Blood Stem Cell Transplantation Submitted to CD34+ Cell Selection, in Patients With Severe Hematologic Malignancies; Clinical Trials: Washington, DC, USA, 2013. [Google Scholar]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Paciotti, G.; Kingston, D.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Nanospectra Biosciences, Inc. A Pilot Study of AuroLase(tm) Therapy in Patients With Refractory and/or Recurrent Tumors of the Head and Neck; Clinical Trials: Washington, DC, USA, 2016. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Li, Z.; Fan, J.; Tong, C.; Zhou, H.; Wang, W.; Li, B.; Liu, B.; Wang, W. A smart drug-delivery nanosystem based on carboxylated graphene quantum dots for tumor-targeted chemotherapy. Nanomedicine 2019, 14, 2011–2025. [Google Scholar] [CrossRef]
- Varukattu, N.B.; Vivek, R.; Rejeeth, C.; Thangam, R.; Ponraj, T.; Sharma, A.; Kannan, S. Nanostructured pH-responsive biocompatible chitosan coated copper oxide nanoparticles: A polymeric smart intracellular delivery system for doxorubicin in breast cancer cells. Arab. J. Chem. 2020, 13, 2276–2286. [Google Scholar] [CrossRef]
- Venditto, V.J.; Szoka, F.C. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Kwon, S.-H.; Choi, J.H.; Lee, A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Ali, S.; Tariq, I.; Ali, M.Y.; Pinnapreddy, S.R.; Preis, E.; Wölk, C.; Harvey, R.D.; Hause, G.; Brüßler, J.; et al. Ultrasound-Responsive Smart Drug Delivery System of Lipid Coated Mesoporous Silica Nanoparticles. Pharmaceutics 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of Gold Nanoparticle-based Drug Delivery System in Cancer Therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Gotov, O.; Battogtokh, G.; Ko, Y.T. Docetaxel-Loaded Hyaluronic Acid-Cathepsin B-Cleavable-Peptide-Gold Nanoparticles for the Treatment of Cancer. Mol. Pharm. 2018, 15, 4668–4676. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Golbabaie, A.; Kasaeian, A. Carbon nanotubes: A review of novel strategies for cancer diagnosis and treatment. Mater. Sci. Eng. C 2017, 76, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]
- Pathak, N.; Pathak, P. Nanoparticles and target Drug delivery for cancer treatment: A Comprehensive review. Int. J. Drug Regul. Aff. 2019, 7, 53–58. [Google Scholar] [CrossRef]
- Thassu, D.; Deleers, M.; Pathak, Y.V. (Eds.) Nanoparticulate Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-16461-3. [Google Scholar]
- Farshad, M. 1—Plastic pipe systems. In Plastic Pipe Systems; Farshad, M., Ed.; Elsevier Science: Oxford, UK, 2006; pp. 1–27. ISBN 978-1-85617-496-1. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2000, 41, 992–993. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mollo, A.R.; Corrigan, O.I. Effect of poly-hydroxy aliphatic ester polymer type on amoxycillin release from cylindrical compacts. Int. J. Pharm. 2003, 268, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sah, E.; Sah, H. Recent Trends in Preparation of Poly(lactide- co -glycolide) Nanoparticles by Mixing Polymeric Organic Solution with Antisolvent. J. Nanomater. 2015, 2015, 794601. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of poly(dl-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Nava-Arzaluz, M.G.; Piñón-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223. [Google Scholar] [CrossRef]
- Reis, C.P.; Martinho, N.; Rosado, C.; Fernandes, A.S.; Roberto, A. Design of polymeric nanoparticles and its applications as drug delivery systems for acne treatment. Drug Dev. Ind. Pharm. 2014, 40, 409–417. [Google Scholar] [CrossRef]
- Naik, J.; Kulkarni, R.; Lokhande, A.; Mishra, S. Development of sustained Release Micro/Nano particles Using Different solvent Emulsification Technique: A Review. Int. J. Pharma Biosci. 2012, 3, 573–590. [Google Scholar]
- Maji, R.; Dey, N.S.; Satapathy, B.S.; Mukherjee, B.; Mondal, S. Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. Int. J. Nanomed. 2014, 9, 3107–3118. [Google Scholar] [CrossRef]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef]
- Guo, M.; Rong, W.-T.; Hou, J.; Wang, D.-F.; Lu, Y.; Wang, Y.; Yu, S.-Q.; Xu, Q. Mechanisms of chitosan-coated poly(lactic-co-glycolic acid) nanoparticles for improving oral absorption of 7-ethyl-10-hydroxycamptothecin. Nanotechnology 2013, 24, 245101. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Revina, A.M.; Khandelwal, V.K.M.; Balakumar, K.; Prakash, J.; Franklin, G.; Sheeba, C.J. Delivery as nanoparticles reduces imatinib mesylate-induced cardiotoxicity and improves anticancer activity. Int. J. Nanomed. 2015, 10, 3163–3170. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ahmad, I.; Fouly, H.; Dr, F.-A. Poly(lactic-co-glycolic acid) Nanoparticles Loaded with Callistemon citrinus Phenolics Exhibited Anticancer Properties against Three Breast Cancer Cell Lines. J. Food Qual. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J. Ring-opening polymerization-mediated controlled formulation of polylactide-drug nanoparticles. J. Am. Chem. Soc. 2009, 131, 4744–4754. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef]
- Yoo, H.S.; Oh, J.E.; Lee, K.H.; Park, T.G. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm. Res. 1999, 16, 1114–1118. [Google Scholar] [CrossRef]
- Kratz, F.; Beyer, U.; Schütte, M.T. Drug-polymer conjugates containing acid-cleavable bonds. Crit. Rev. Ther. Drug Carrier Syst. 1999, 16, 245–288. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Belén, L.H.; de Rangel-Yagui, C.O.; Beltrán Lissabet, J.F.; Effer, B.; Lee-Estevez, M.; Pessoa, A.; Castillo, R.L.; Farías, J.G. From Synthesis to Characterization of Site-Selective PEGylated Proteins. Front. Pharmacol. 2019, 10, 1450. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef]
- Pamujula, S.; Hazari, S.; Bolden, G.; Graves, R.A.; Chinta, D.D.; Dash, S.; Kishore, V.; Mandal, T.K. Cellular delivery of PEGylated PLGA nanoparticles. J. Pharm. Pharmacol. 2012, 64, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Mima, Y.; Hashimoto, Y.; Shimizu, T.; Kiwada, H.; Ishida, T. Anti-PEG IgM Is a Major Contributor to the Accelerated Blood Clearance of Polyethylene Glycol-Conjugated Protein. Mol. Pharm. 2015, 12, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Mishra, P.; Jain, N.K. Long circulating PEGylated poly(D,L-lactide-co-glycolide) nanoparticulate delivery of Docetaxel to solid tumors. J. Drug Target. 2008, 16, 424–435. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, J.; Wang, Y.; Wang, X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci. Rep. 2017, 7, 18014. [Google Scholar] [CrossRef]
- Verma, M.S.; Liu, S.; Chen, Y.Y.; Meerasa, A.; Gu, F.X. Size-tunable nanoparticles composed of dextran-b-poly(D,L-lactide) for drug delivery applications. Nano Res. 2012, 5, 49–61. [Google Scholar] [CrossRef]
- Surassmo, S.; Saengkrit, N.; Ruktanonchai, U.R.; Suktham, K.; Woramongkolchai, N.; Wutikhun, T.; Puttipipatkhachorn, S. Surface modification of PLGA nanoparticles by carbopol to enhance mucoadhesion and cell internalization. Colloids Surf. B Biointerfaces 2015, 130, 229–236. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; Okour, A.R.; Dave, R.H. Surface modification of PLGA nanoparticles using chitosan: Effect of molecular weight, concentration, and degree of deacetylation. Adv. Polym. Technol. 2018, 37, 3066–3075. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Lim, L.-Y. Paclitaxel-loaded PLGA nanoparticles: Potentiation of anticancer activity by surface conjugation with wheat germ agglutinin. J. Control. Release 2005, 108, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri, S.; Darvishi, B.; Kamalinia, G.; Amini, M.; Fallah, M.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. Daru 2013, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chui, W.-K.; Ho, P.C. Design of a multifunctional PLGA nanoparticulate drug delivery system: Evaluation of its physicochemical properties and anticancer activity to malignant cancer cells. Pharm. Res. 2009, 26, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J.-H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef]
- Jia, Y.; Yuan, M.; Yuan, H.; Huang, X.; Sui, X.; Cui, X.; Tang, F.; Peng, J.; Chen, J.; Lu, S.; et al. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int. J. Nanomed. 2012, 7, 1697–1708. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.; Gu, F.; Rhee, J.-W.; Wang, A.; Radovic-Moreno, A.; Alexis, F.; Langer, R.; Farokhzad, O. Self-Assembled Lipid−Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Su, W.; Wang, S.; Liao, Z.; Niu, R.; Chang, J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials 2010, 31, 8741–8748. [Google Scholar] [CrossRef]
- Lee, S.-M.; Park, H.; Yoo, K.-H. Synergistic cancer therapeutic effects of locally delivered drug and heat using multifunctional nanoparticles. Adv. Mater. 2010, 22, 4049–4053. [Google Scholar] [CrossRef] [PubMed]
- Nehilla, B.J.; Allen, P.G.; Desai, T.A. Surfactant-free, drug-quantum-dot coloaded poly(lactide-co-glycolide) nanoparticles: Towards multifunctional nanoparticles. ACS Nano 2008, 2, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.E.; Lee, S.H.; Yu, J.H.; Lee, J.H.; Park, T.G.; Hyeon, T. Designed Fabrication of a Multifunctional Polymer Nanomedical Platform for Simultaneous Cancer- Targeted Imaging and Magnetically Guided Drug Delivery. Adv. Mater. 2008, 20, 478–483. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Su, C.-H.; Wu, P.-C.; Yeh, C.-S. Multifunctional polymeric nanoparticles for combined chemotherapeutic and near-infrared photothermal cancer therapy in vitro and in vivo. Chem. Commun. (Camb) 2010, 46, 3167–3169. [Google Scholar] [CrossRef]
- McKeen, L. Introduction to Plastics and Polymers Compositions. In The Effect of UV Light and Weather on Plastics and Elastomers; William Andrew Publishing: New York, NY, USA, 2013; pp. 1–16. ISBN 978-1-4557-2851-0. [Google Scholar]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Polylactic-Co-Glycolic Acid (PLGA)/Konstantinos Avgoustakis. In Encyclopedia of Biomaterials and Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-15406-5.
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar] [CrossRef]
- Galant, O.; Bae, S.; Silberstein, M.N.; Diesendruck, C.E. Highly Stretchable Polymers: Mechanical Properties Improvement by Balancing Intra- and Intermolecular Interactions. Adv. Funct. Mater. 2020, 30, 1901806. [Google Scholar] [CrossRef]
- Ansary, R.H.; Awang, M.B.; Rahman, M.M. Biodegradable Poly(D,L-lactic-co-glycolic acid)-Based Micro/Nanoparticles for Sustained Release of Protein Drugs—A Review. Trop. J. Pharm. Res. 2014, 13, 1179. [Google Scholar] [CrossRef]
- Luan, X.; Bodmeier, R. Influence of the poly(lactide-co-glycolide) type on the leuprolide release from in situ forming microparticle systems. J. Control. Release 2006, 110, 266–272. [Google Scholar] [CrossRef]
- Frank, A.; Rath, S.K.; Venkatraman, S.S. Controlled release from bioerodible polymers: Effect of drug type and polymer composition. J. Control. Release 2005, 102, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.J.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.-X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Kim, J.M.; Seo, K.S.; Jeong, Y.K.; Hai, B.L.; Kim, Y.S.; Khang, G. Co-effect of aqueous solubility of drugs and glycolide monomer on in vitro release rates from poly(D,L-lactide-co-glycolide) discs and polymer degradation. J. Biomater. Sci. Polym. Ed. 2005, 16, 991–1007. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.-S. PLGA/TPGS nanoparticles for controlled release of paclitaxel: Effects of the emulsifier and drug loading ratio. Pharm. Res. 2003, 20, 1864–1872. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef]
- Liechty, W.B.; Peppas, N.A. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012, 80, 241–246. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Rao, J. Shedding Light on Tumors Using Nanoparticles. ACS Nano 2008, 2, 1984–1986. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Corrigan, O.I.; Ramtoola, Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials 2000, 21, 1659–1668. [Google Scholar] [CrossRef]
- Yin Win, K.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.-C.; Allémann, E.; De Jaeghere, F.; Doelker, E.; Gurny, R. Biodegradable nanoparticles—From sustained release formulations to improved site specific drug delivery. J. Control. Release 1996, 39, 339–350. [Google Scholar] [CrossRef]
- Yadav, K.S.; Chuttani, K.; Mishra, A.K.; Sawant, K.K. Effect of Size on the Biodistribution and Blood Clearance of Etoposide-Loaded PLGA Nanoparticles. PDA J. Pharm. Sci. Technol. 2011, 65, 131–139. [Google Scholar]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.Y.; Mei, X.G. Preparation and in vitro evaluation of thienorphine-loaded PLGA nanoparticles. Drug Deliv. 2016, 23, 787–793. [Google Scholar] [CrossRef][Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G.J.; Greeshma, M.M.; Menon, D. Impact of poly(lactic-co-glycolic acid) nanoparticle surface charge on protein, cellular and haematological interactions. Colloids Surf. B Biointerfaces 2015, 136, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, Y.; Hu, W.; Jiang, H.; Zhang, J.; Zhang, L. Effect of preparation conditions on the size and encapsulation properties of mPEG-PLGA nanoparticles simultaneously loaded with vincristine sulfate and curcumin. Pharm. Dev. Technol. 2013, 18, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, C. Tuning the Size of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles Fabricated by Nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Ibraheem, S.; Abdul Mahdi, S.; Albukhaty, S.; Haider, A.J.; Kadhim, A.A.; Kadhim, K.A.; Kadhim, H.A.; Al-Karagoly, H. Smart Nanoformulation Based on Polymeric Magnetic Nanoparticles and Vincristine Drug: A Novel Therapy for Apoptotic Gene Expression in Tumors. Life 2021, 11, 71. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical Cancer Nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59. [Google Scholar] [CrossRef]
- Clawson, C.; Ton, L.; Aryal, S.; Fu, V.; Esener, S.; Zhang, L. Synthesis and Characterization of Lipid–Polymer Hybrid Nanoparticles with pH-Triggered Poly(ethylene glycol) Shedding. Langmuir 2011, 27, 10556–10561. [Google Scholar] [CrossRef]
- Wadajkar, A.; Bhavsar, Z.; Ko, C.-Y.; Koppolu, B.P.; Cui, W.; Tang, L.; Nguyen, K. Multifunctional Particles for Melanoma-Targeted Drug Delivery. Acta Biomater. 2012, 8, 2996–3004. [Google Scholar] [CrossRef]
- Yu, L.; Ci, T.; Zhou, S.; Zeng, W.; Ding, J. The thermogelling PLGA–PEG–PLGA block copolymer as a sustained release matrix of doxorubicin. Biomater. Sci. 2013, 1, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.; Kang, J.; Oh, S.J.; Ko, H.-J.; Son, J.-H.; Lee, K.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Smart drug-loaded polymer gold nanoshells for systemic and localized therapy of human epithelial cancer. Adv. Mater. 2009, 21, 4339–4342. [Google Scholar] [CrossRef]
- Park, H.; Yang, J.; Lee, J.; Haam, S.; Choi, I.-H.; Yoo, K.-H. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano 2009, 3, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, J.; Chan, M.; Almutairi, A. Light-Triggered Intramolecular Cyclization in Poly(lactic-co-glycolic acid)-Based Polymers for Controlled Degradation. Macromolecules 2015, 48, 3166–3172. [Google Scholar] [CrossRef]
- Konan-Kouakou, Y.N.; Boch, R.; Gurny, R.; Allémann, E. In vitro and in vivo activities of verteporfin-loaded nanoparticles. J. Control. Release 2005, 103, 83–91. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Conte, C.; Mastrotto, F.; Taresco, V.; Tchoryk, A.; Quaglia, F.; Stolnik, S.; Alexander, C. Enhanced uptake in 2D- and 3D- lung cancer cell models of redox responsive PEGylated nanoparticles with sensitivity to reducing extra- and intracellular environments. J. Control. Release 2018, 277, 126–141. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Li, Y.; Tian, Y.; Sun, H.; Ammar, O.; Tu, J.; Wang, B.; Sun, C. Co-delivery of siRNA and paclitaxel into cancer cells by hyaluronic acid modified redox-sensitive disulfide-crosslinked PLGA–PEI nanoparticles. RSC Adv. 2015, 5, 46464–46479. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, R.; Billecke, N.; Schwendy, M.; Pütz, S.; Bonn, M.; Parekh, S.H.; Klapper, M.; Müllen, K. Polylactide-block-Polypeptide-block-Polylactide Copolymer Nanoparticles with Tunable Cleavage and Controlled Drug Release. Adv. Funct. Mater. 2014, 24, 4026–4033. [Google Scholar] [CrossRef]
- Mi, Y.; Wolfram, J.; Mu, C.; Liu, X.; Blanco, E.; Shen, H.; Ferrari, M. Enzyme-responsive multistage vector for drug delivery to tumor tissue. Pharmacol. Res. 2016, 113, 92–99. [Google Scholar] [CrossRef]
- Du, J.; O’Reilly, R.K. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009, 5, 3544–3561. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Kim, B.Y.S.; Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2018, 2, 797–809. [Google Scholar] [CrossRef]
- Lee, D.R.; Park, J.S.; Bae, I.H.; Lee, Y.; Kim, B.M. Liquid crystal nanoparticle formulation as an oral drug delivery system for liver-specific distribution. Int. J. Nanomed. 2016, 11, 853–871. [Google Scholar] [CrossRef]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control Release 2011, 151, 131–138. [Google Scholar] [CrossRef]
- Pardeshi, N.N.; Qi, W.; Dahl, K.; Caplan, L.; Carpenter, J.F. Microparticles and Nanoparticles Delivered in Intravenous Saline and in an Intravenous Solution of a Therapeutic Antibody Product. J. Pharm. Sci. 2017, 106, 511–520. [Google Scholar] [CrossRef]
- Karpenko, L.I.; Rudometov, A.P.; Sharabrin, S.V.; Shcherbakov, D.N.; Borgoyakova, M.B.; Bazhan, S.I.; Volosnikova, E.A.; Rudometova, N.B.; Orlova, L.A.; Pyshnaya, I.A.; et al. Delivery of mRNA Vaccine against SARS-CoV-2 Using a Polyglucin:Spermidine Conjugate. Vaccines 2021, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, C.; Yang, S.; Xiao, W.; Zheng, Q.; Song, X. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J. Control. Release 2021, 335, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef]
- Shokoohinia, P.; Hajialyani, M.; Sadrjavadi, K.; Akbari, M.; Rahimi, M.; Khaledian, S.; Fattahi, A. Microfluidic-assisted preparation of PLGA nanoparticles for drug delivery purposes: Experimental study and computational fluid dynamic simulation. Res. Pharm. Sci. 2019, 14, 459–470. [Google Scholar] [CrossRef]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio. Mater. 2020, 3, 107–120. [Google Scholar] [CrossRef]
- Gkionis, L.; Aojula, H.; Harris, L.K.; Tirella, A. Microfluidic-assisted fabrication of phosphatidylcholine-based liposomes for controlled drug delivery of chemotherapeutics. Int. J. Pharm. 2021, 604, 120711. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Li, W.; Correia, A.; Khan, M.M.; Rahim, M.A.; Santos, H.A. Microfluidic fabrication and characterization of Sorafenib-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery. Int. J. Pharm. 2020, 581, 119275. [Google Scholar] [CrossRef]
- Ghasemi Toudeshkchouei, M.; Zahedi, P.; Shavandi, A. Microfluidic-Assisted Preparation of 5-Fluorouracil-Loaded PLGA Nanoparticles as a Potential System for Colorectal Cancer Therapy. Materials 2020, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
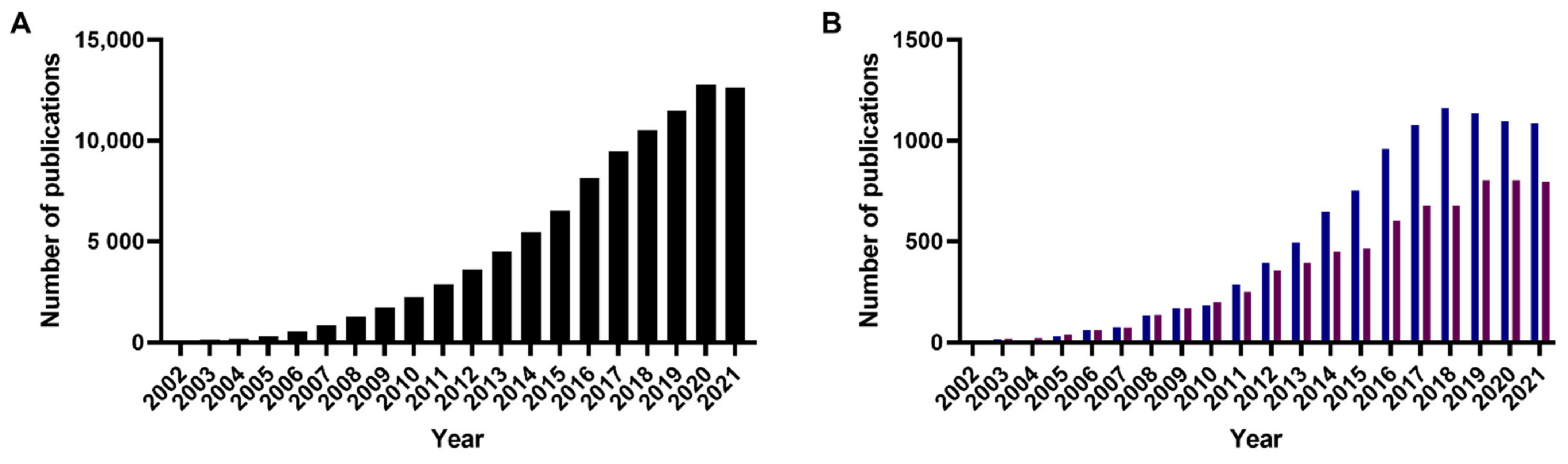
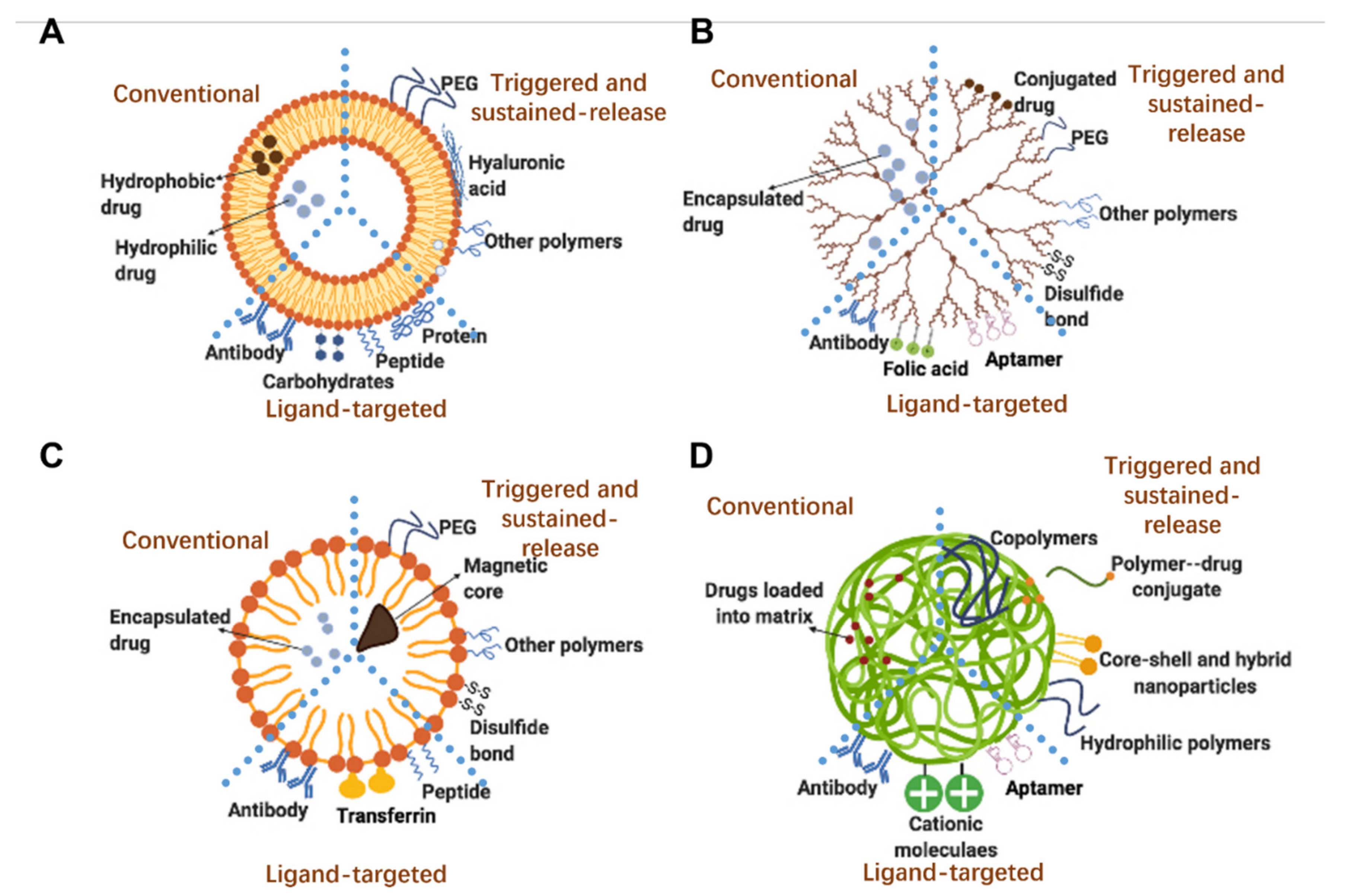
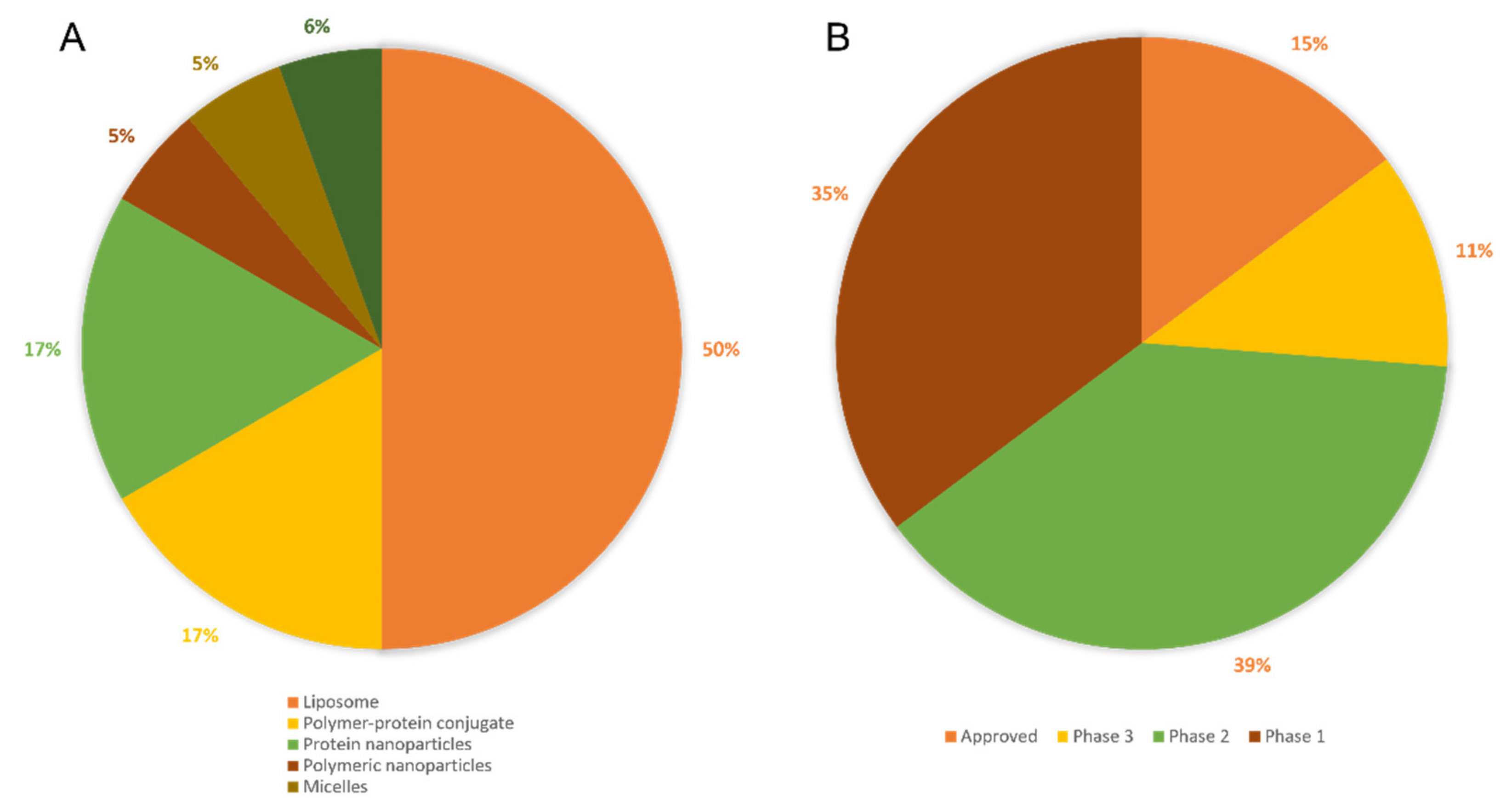
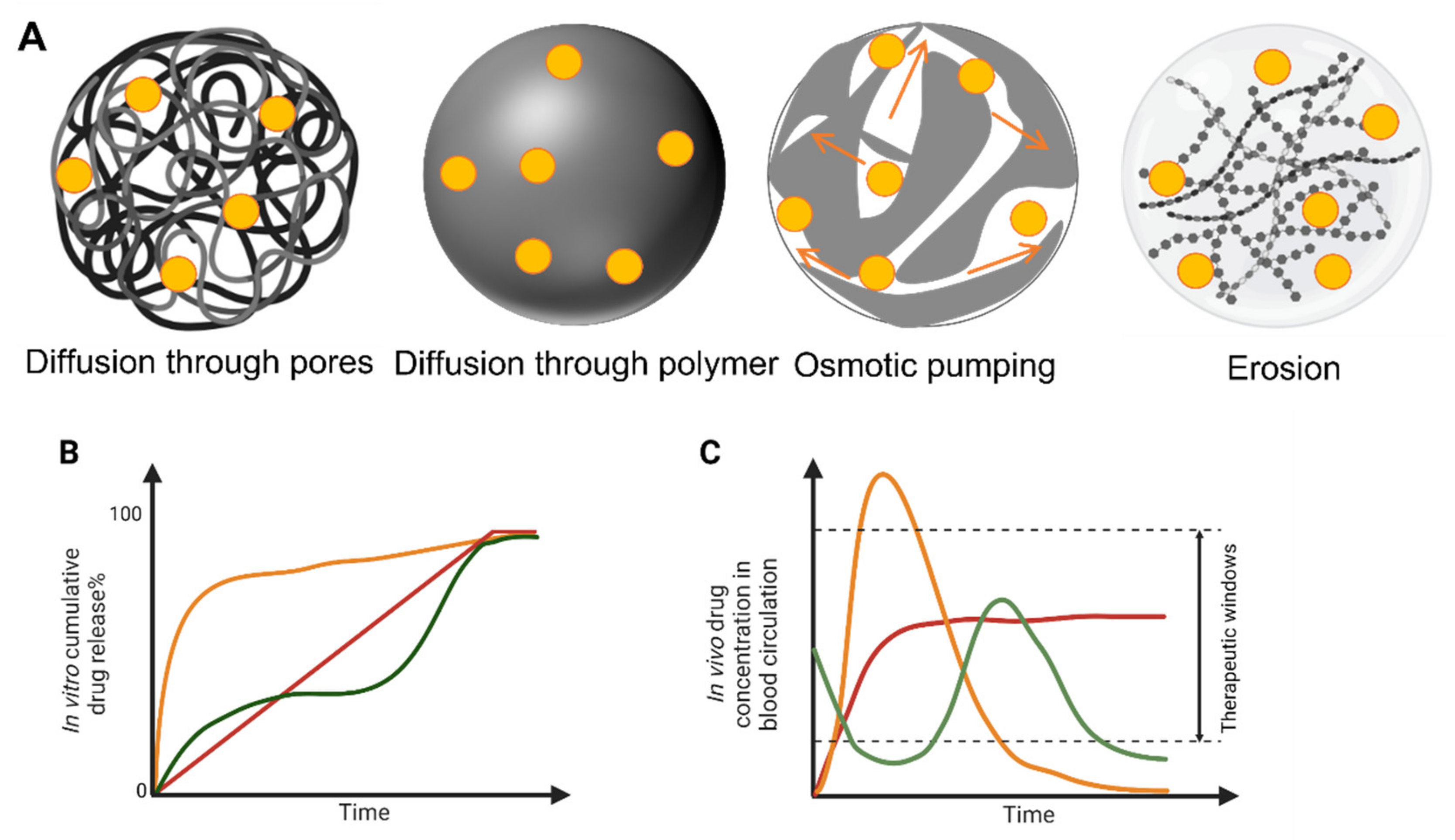
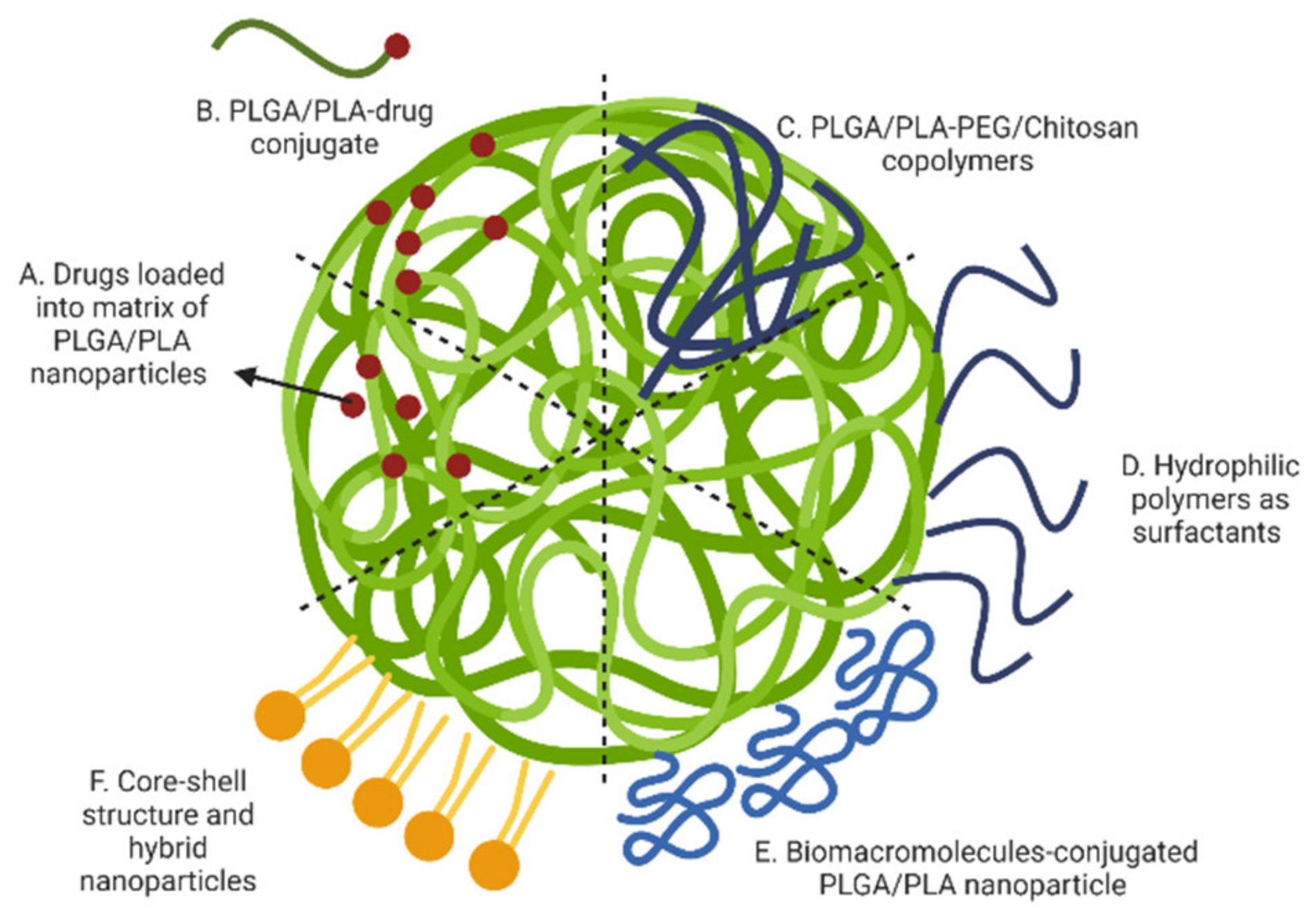
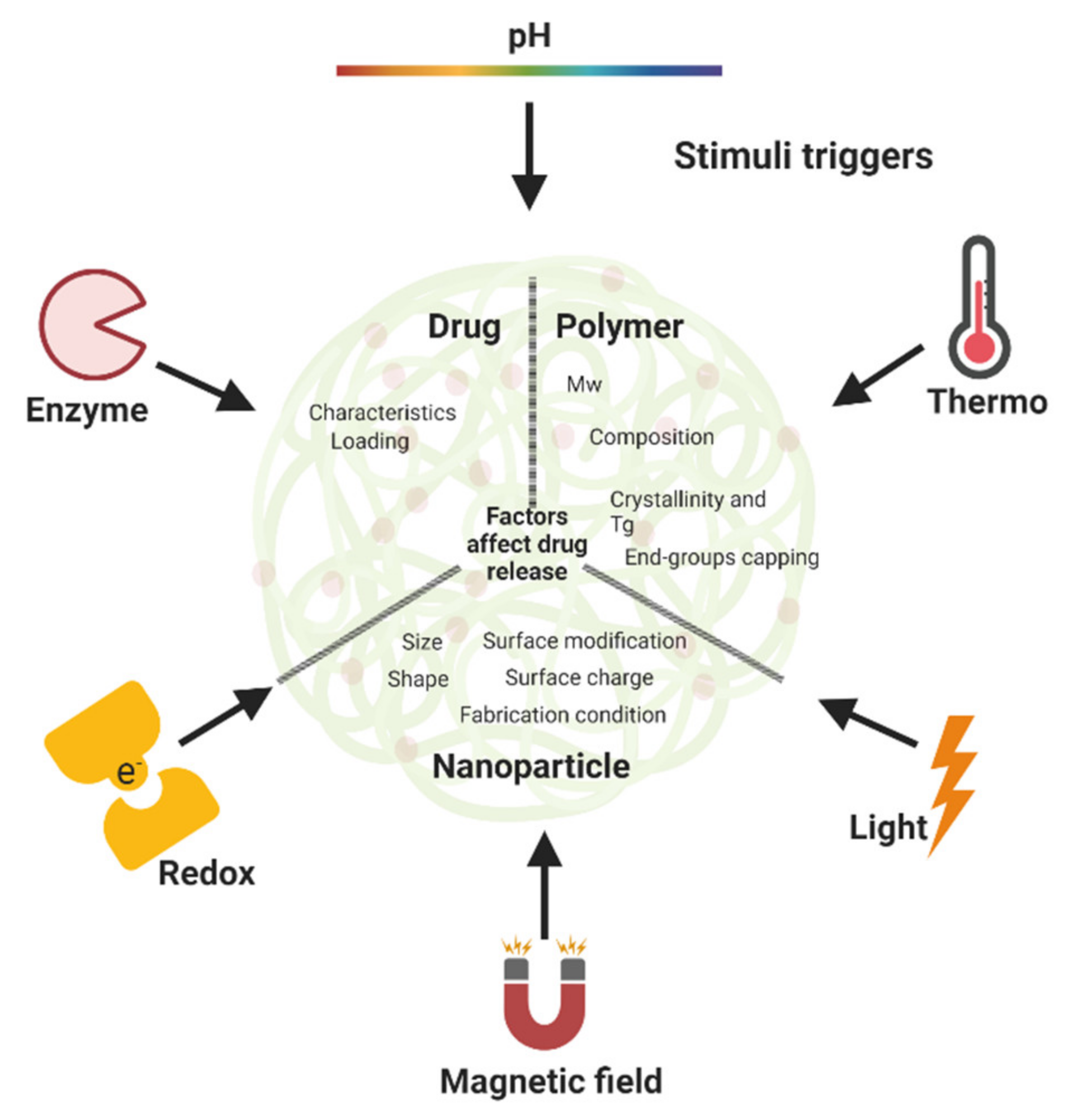
| Polymer Type | Definition | Features | Commonly Used Polymers |
|---|---|---|---|
| Natural polymers | Raw materials that naturally occur in the biological environment | Biocompatible, Biodegradable, Low immunogenic levels, Low-cost, Allowing chemical modifications | Gelatin, dextran, chitosan, collagen, albumin, heparin |
| Synthetic polymers | Macromolecules synthesized using different primary materials (e.g., natural products, oil). | Highly predictable physical properties such as solubility, permeability and rates of biodegradation. Increased the pharmacokinetics and circulation times of incorporated therapeutic substances. | PCL, HPMA, PLA, PLGA |
| Techniques | Preparation Methods | Type of Drug | Particular Features/Advantages | Disadvantages |
|---|---|---|---|---|
| w/o phase separation | Emulsify aqueous drug phase with polymer-dissolved organic phase | Hydrophilic | High encapsulation efficiencies for hydrophilic drugs, due to their insolubility in organic solvents. | Need to handle and dispose of oil; difficult to control condensation. |
| w1/o/w2 emulsion | Emulsify aqueous drug phase (w1) with polymer-dissolved water-immiscible phase (o). Mix w1/o with aqueous phase (w2). | Hydrophilic | Manufactured using high- or low-energy technologies; easily control the size distribution. | Poor EE% for small molecular weight (escape to the w2 phase) during the encapsulation process. |
| o/w solvent evaporation | Emulsify polymer-dissolved organic phase droplets into aqueous phase. Remove solvent by evaporation, emulsion droplets solidified into nanoparticles. | Hydrophobic | Easily adapted for hydrophobic drugs; good reproducibility; ease of scaling up the manufacturing process. | Use of volatile halogenated organic solvents, toxic solvents used; solvent residual. |
| o/w solvent extraction | Add excess of quench solvent such as water to the o/w emulsion to promote quenching organic solvent into the aqueous phase | Hydrophobic | Use of non-halogenated solvents. | High volumes of waste stream produced; difficult to remove solvent completely; nanoparticle aggregation. |
| o/o emulsion | Emulsify drug and polymer-dissolved organic phase (o1) with a continuous oil phase (o2) | Hydrophobic | Preparation method for water-insoluble drug, using non-halogenated solvent. | Issues to dispose and/or recycle of waste oil during manufacturing. |
| o/w salting-out | Emulsify drug and polymer-dissolved organic phase with an electrolyte-saturated aqueous phase | Hydrophobic | Using non-halogenated solvent; low energy mixing device | Using large quantities of salting-out agents which need to be recycled such as salts/electrolytes. |
| Nanoprecipitation (solvent dialysis method) | Mix polymer-dissolved organic phase with an aqueous phase through a low energy mixing device. | Hydrophobic | Use of non-halogenated solvent; one-step method for loading water-insoluble drugs; low-energy mixing device. | Low concentration of polymer in the dispersed phase; nanoparticles may aggregate because of unremoved solvent. |
| SESD | Dissolve polymers in mixture of water miscible and water immiscible solvent; nanoparticles are formed by emulsification and solvent evaporation | Hydrophobic | Use of pharmaceutically acceptable organic solvents with no need of high-pressure homogenizers. | The binary solution contains a halogenated solvent. |
| Spray drying | Spray solid-in-oil dispersion or water-in-oil emulsion in a stream of heated air | Hydrophilic | Fast and easy method with a small number of parameters; suitable for industrial fabrication | Nanoparticles may adhere and/or agglomerate to the inner walls of the spray dryer. |
| Microfluidics | Mix two phases of liquids in a microfluidic device with the microchannel at least one dimension smaller than 1000 μm | Hydrophilic/Hydrophobic | Control formation processes precisely; desired EE%; multiple drugs loading capacity; low energy mixing device | Limitation for scaling-up the process. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines 2022, 13, 1623. https://doi.org/10.3390/mi13101623
Bai X, Smith ZL, Wang Y, Butterworth S, Tirella A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines. 2022; 13(10):1623. https://doi.org/10.3390/mi13101623
Chicago/Turabian StyleBai, Xue, Zara L. Smith, Yuheng Wang, Sam Butterworth, and Annalisa Tirella. 2022. "Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review" Micromachines 13, no. 10: 1623. https://doi.org/10.3390/mi13101623
APA StyleBai, X., Smith, Z. L., Wang, Y., Butterworth, S., & Tirella, A. (2022). Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines, 13(10), 1623. https://doi.org/10.3390/mi13101623