3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Microgels
2.3. Morphological Characterization
2.4. Chemical Characterization
2.5. In Vitro Biocompatibility
3. Results and Discussion
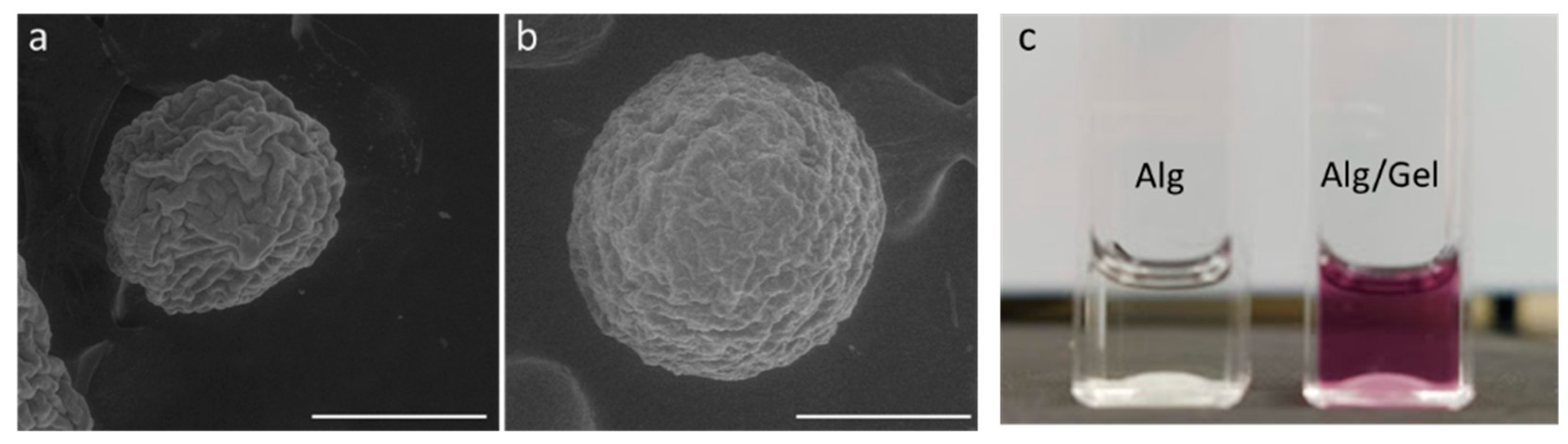
3.1. Microgels Characterization
3.2. In Vitro Characterization of 3D Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Koch, S.; Schwinger, C.; Kressler, J.; Heinzen, C.; Rainov, N.G. Alginate encapsulation of genetically engineered mammalian cells: Comparison of production devices, methods and microcapsule characteristics. J. Microencapsul. 2003, 20, 303–316. [Google Scholar] [CrossRef]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef]
- Serrano-Bello, J.; Cruz-Maya, I.; Suaste-Olmos, F.; González-Alva, P.; Altobelli, R.; Ambrosio, L.; Medina, L.A.; Guarino, V.; Alvarez-Perez, M.A. In vivo Regeneration of Mineralized Bone Tissue in Anisotropic Biomimetic Sponges. Front. Bioeng. Biotechnol. 2020, 8, 587. [Google Scholar] [CrossRef]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized Alginate-Gelatin Hydrogel: A Favorable Matrix for Growth and Osteogenic Differentiation of Adipose-Derived Stem Cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef]
- Abdul Khodir, W.; Abdul Razak, A.; Ng, M.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Saracino, E.; Cirillo, V.; Marrese, M.; Guarino, V.; Benfenati, V.; Zamboni, R.; Ambrosio, L. Structural and functional properties of astrocytes on PCL based electrospun fibres. Mater. Sci. Eng. C 2021, 118, 111363. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Alvarez, M.A. Protein based devices for oral tissue repair and regeneration. AIMS Mater. Sci. 2018, 5, 156–170. [Google Scholar] [CrossRef]
- Altobelli, R.; Guarino, V.; Ambrosio, L. Micro- and nanocarriers by electrofludodynamic technologies for cell and molecular therapies. Process Biochem. 2016, 51, 2143–2154. [Google Scholar] [CrossRef]
- Kang, S.-M.; Lee, J.-H.; Huh, Y.S.; Takayama, S. Alginate Microencapsulation for Three-Dimensional In Vitro Cell Culture. ACS Biomater. Sci. Eng. 2020, 7, 2864–2879. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2016, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Vedicherla, S.; Gansau, J.; McIntyre, T.; Doherty, M.; Buckley, C.T. Living Cell Factories-Electrosprayed Microcapsules and Microcarriers for Minimally Invasive Delivery. Adv. Mater. 2016, 28, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Caputo, T.; Calcagnile, P.; Altobelli, R.; Demitri, C.; Ambrosio, L. Core/shell cellulose-based microspheres for oral administration of Ketoprofen Lysinate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2636–2644. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Ambrosio, L. Chitosan Microgels and Nanoparticles via Electrofluidodynamic Techniques for Biomedical Applications. Gels 2016, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Guarino, V.; Cirillo, V.; Oliviero, O.; Ambrosio, L. Optimization of Bicomponent Electrospun Fibers for Therapeutic Use: Post-Treatments to Improve Chemical and Bio-logical Stability. J. Funct. Biomater. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Altobelli, R.; Marrese, M.; Guarino, V. Design of alginate based micro-gels via electro fluid dynamics to construct microphysiological cell culture systems. Polym. Adv. Technol. 2021, 32, 2981–2989. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-J.; Chiu, K.-H.; Chen, C.-Y.; Huang, C.-H.; Yao, C.-H. Alginate-gelatin based core-shell capsule enhances the osteogenic potential of human osteoblast-like MG-63 cells. Mater. Des. 2021, 210, 110109. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L.; Bellini, D. Process for the Preparation of Microspheres Comprising Semisynthetic Polymers. WIPO Patent WO2009143947A8, 3 December 2009. [Google Scholar]
- Feng, Q.; Li, Q.; Wen, H.; Chen, J.; Liang, M.; Huang, H.; Lan, D.; Dong, H.; Cao, X. Injection and self-assembly of bioinspired stem cell-Laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019, 29, 1906690. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, H.; Wen, H.; Huang, H.; Li, Q.; Liang, M.; Liu, Y.; Dong, H.; Cao, X. Engineering the cellular mechanical microenvironment to regulate stem cell chondrogenesis: Insights from a microgel model. Acta Biomater. 2020, 113, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Girón-Hernández, J.; Gentile, P.; Benlloch-Tinoco, M. Impact of heterogeneously crosslinked calcium alginate networks on the encapsulation of β-carotene-loaded beads. Carbohydr. Polym. 2021, 271, 118429. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Pal, R.; Moon, G.Y. Characteristics of sodium alginate membranes for the pervaporation dehydration of ethanol–water and isopropanol–water mixtures. J. Memb. Sci. 1999, 160, 101–113. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Al-Harbi, S.A. Preparation of a Calcium Alginate-Coated Polypyrrole/Silver Nanocomposite for Site-Specific Immobilization of Polygalacturonase with High Reusability and Enhanced Stability. Catal. Lett. 2022, 152, 28–42. [Google Scholar] [CrossRef]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated Trichoderma harzianum UPM40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Comparative Study on Protein-Rich Electrospun Fibers for In Vitro Applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Q.; Du, Y. Alginate/gelatin blend films and their properties for drug controlled release. J. Memb. Sci. 2006, 280, 37–44. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Jia-hui, Y.; Yu-min, D.; Hua, Z. Blend films of chitosan-gelatin. Wuhan Univ. J. Nat. Sci. 1999, 4, 476. [Google Scholar] [CrossRef]
- KarbalaeiMahdi, A.; Shahrousvand, M.; Javadi, H.R.; Ghollasi, M.; Kamali, M.; Salimi, A. Neural differentiation of human induced pluripotent stem cells on polycaprolactone/gelatin bi-electrospun nanofibers. Mater. Sci. Eng. C 2017, 78, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef]
- Guarino, V.; Khodir, W.K.W.A.; Ambrosio, L. Biodegradable microparticles and nanoparticles by electrospraying techniques. J. Appl. Biomater. Funct. Mate. 2012, 10, 191–196. [Google Scholar] [CrossRef]
- Vineis, C.; Maya, I.C.; Mowafi, S.; Varesano, A.; Ramírez, D.O.S.; Abou Taleb, M.; Tonetti, C.; Guarino, V.; El Sayed, H. Synergistic effect of sericin and keratin in gelatin based nanofibers for in vitro applications. Inter. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Fasolino, I.; Cirillo, V.; Ambrosio, L. Degradation and early in vitro activity of healthy hepatocytes onto bicomponent electrospun fibers. Inter. J. Polym. Mater. Polym. Biomater. 2018, 67, 961–966. [Google Scholar] [CrossRef]
- Nishter, S.R.; Fathima, N. A gelatin based antioxidant enriched biomaterial by grafting and saturation: Towards sustained drug delivery from antioxidant matrix. Colloids Surf. B Biointerfaces 2015, 128, 537–543. [Google Scholar] [CrossRef]
- Chui, C.Y.; Odeleye, A.; Nguyen, L.; Kasoju, N.; Soliman, E.; Ye, H. Electrosprayed genipin cross-linked alginate–chitosan microcarriers for ex vivo expansion of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2019, 107, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Schop, D.; Janssen, F.W.; Borgart, E.; de Bruijn, J.D.; van Dijkhuizen-Radersma, R. Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: Growth and metabolism. J. Tissue Eng. Regen. Med. 2008, 2, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Di Lisa, D.; de Rutte, J.; Sakib, M.N.; Alves e Silva, A.R.; Tamayol, A.; Di Carlo, D.; Khademhosseini, A.; Sheikhi, A. In situ forming microporous gelatin methacryloyl hydrogel scaffolds from thermostable microgels for tissue engineering. Bioeng. Transl. Med. 2020, 5, e10180. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Truong, V.; Fisch, P.; Levinson, C.; Glattauer, V.; Zenobi-Wong, M.; Thissen, H.; Forsythe, J.; Frith, J. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018, 77, 48–62. [Google Scholar] [CrossRef]
- Basiri, H.; Mohseni, S.S.; Abouei Mehrizi, A.; Rajabnejadkeleshteri, A.; Ghaee, A.; Farokhi, M.; Kumacheva, E. Composite Microgels for Imaging-Monitored Tracking of the Delivery of Vascular Endothelial Growth Factor to Ischemic Muscles. Biomacromolecules 2021, 22, 5162–5172. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Maya, I.; Guarino, V. 3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization. Micromachines 2022, 13, 1726. https://doi.org/10.3390/mi13101726
Cruz-Maya I, Guarino V. 3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization. Micromachines. 2022; 13(10):1726. https://doi.org/10.3390/mi13101726
Chicago/Turabian StyleCruz-Maya, Iriczalli, and Vincenzo Guarino. 2022. "3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization" Micromachines 13, no. 10: 1726. https://doi.org/10.3390/mi13101726
APA StyleCruz-Maya, I., & Guarino, V. (2022). 3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization. Micromachines, 13(10), 1726. https://doi.org/10.3390/mi13101726





