1. Introduction
Hydrogen sulfide (H
2S), which is a toxic, harmful, corrosive, and colorless gas, is produced by oil deposits, as well as biogas and natural gas fields. Thus, developing an H
2S sensor with excellent performances, such as good response, selectivity, stability, and a fast response and recovery time, is crucial for the health and safety of industrial workers and the general population. With the advent of the internet of things era, high-performance H
2S sensors driven at a low voltage and low power have been examined with semiconducting metal oxide (SMO) as the sensing material [
1,
2,
3]. In particular, SMOs such as tin dioxide (SnO
2), zinc oxide (ZnO), tungsten trioxide (WO
3), nickel oxide (NiO), and copper oxide (CuO) have been identified as the most promising H
2S sensing materials. Among these, SnO
2 is most widely utilized as an H
2S-sensing material in SMO-based gas sensors because of its excellent gas-detection properties (a good compromise between price, stability, and reliability of material, together with a relatively low operating temperature, and a fast response and recovery time) and numerous fabrication advantages; that is, low-cost, simple fabrication, and good compatibility with the micro-electromechanical (MEMS) process [
4,
5,
6,
7]. SnO
2 has been applied to H
2S gas sensors in various forms, such as thin films, thick films, pellets, and hot-wire type. SnO
2 is an n-type semiconductor and an H
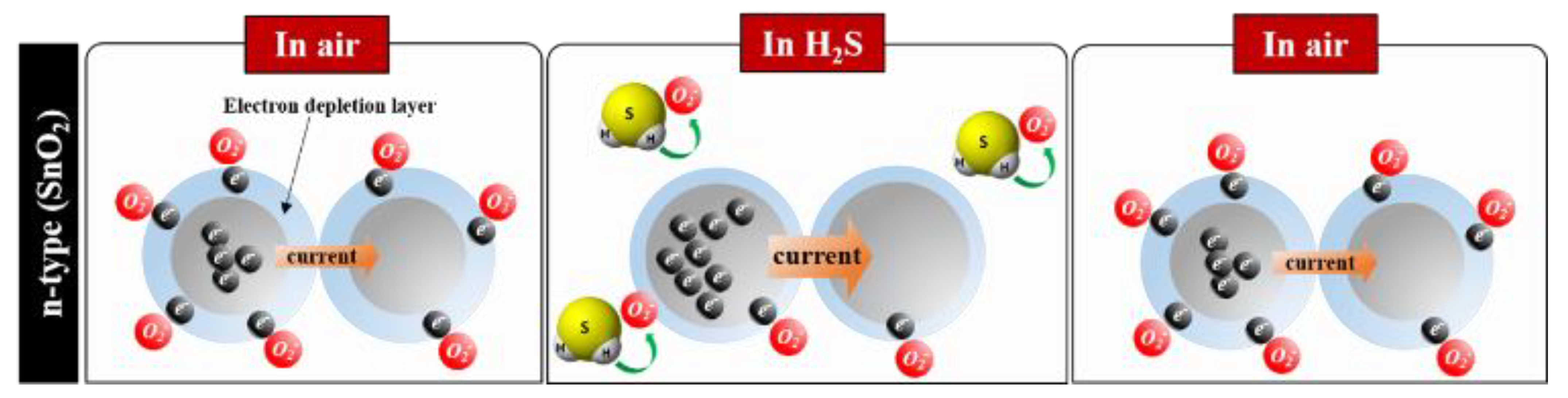
2S-sensor, based on its utilization of resistance–change mechanisms wherein there is an induced variation of the depletion region, owing to the adsorption of ionized oxygen species (O
2−, O
−, and O
2−) on the SnO
2 surface, as shown in
Figure 1. The oxygen-related gas-sensing mechanism involves the absorption of oxygen molecules on the SnO
2 surface to generate chemisorbed oxygen species (O
2−, O
− and O
2−) by capturing electrons from the conductance band, which makes the SnO
2 surface highly resistive.
When the SnO
2 surface is exposed to a reductive gas (H
2S), the reductive gas (H
2S) upon reacting with the oxygen species (O
2−, O
−, and O
2−) reduces the concentration of the oxygen species on this surface, thereby increasing the electron concentration [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. Oxygen species with different forms (O
2−, O
− and O
2−), which are adsorbed on the SnO
2 surface, are reliant on sensing temperature; therefore, controlling the temperature of H
2S sensor is vital. In general, the adsorption of O
2− is dominant in the range of 150–200 °C (1), and the adsorption of O
− dominates above 200 °C (2). A further increase in temperature above 400 °C tends to result in the domination of the adsorption of O
2− (3) [
19]. The process flow is detailed as follows:
As mentioned above, the SnO
2 interacts well with H
2S in a wide range of temperatures; however, it is not suitable for the selective detection of H
2S. This is because various reducing gases, such as hydrogen, carbon monoxide, ammonia, and others, interact with SnO
2 in similar ways. Despite the many advantages of SnO
2, the pristine SnO
2 gas sensor usually suffers from poor selectivity. Consequently, diverse effective approaches have been conducted to improve the selectivity of SnO
2-based gas sensors [
19,
20], such as noble metal doping, composite hetero-structure design, and controlling the reaction temperature. Among these methods, controlling the optimal reaction temperature is the simplest and most effective method. In general, pristine SnO
2 has an excellent response and a good selectivity at 150–200 °C for H
2S. Thus, developing an SnO
2-based H
2S microsensor which operates well at 150–200 °C is important [
21,
22,
23]. Microsensors for detecting H
2S comprise a micro-heater, inter-digitated electrode (IDE), and sensing material. The micro-heater (which elevates temperature) embedded in the gas sensor has an important role to play, as aforementioned, in improving the performance of an H
2S microsensor. It supplies sufficient thermal energy for the reaction between the target gas (H
2S), oxygen species (O
2−, O
− and O
2−), and sensing material, thereby boosting the H
2S microsensor performance. To apply the fabricated H
2S microsensor with a built-in micro-heater to portable applications, a well-designed sensor interface circuit that can supply an appropriate voltage to the sensor is essential. Common sensor interface circuits utilized in commercial portable application supply voltage in the range of 1.8–5 V [
24,
25,
26]. However, additional circuitry, such as a charge pump circuit, is required to supply a high voltage when an H
2S microsensor with a high operating voltage is used. This results in additional power consumption and a larger footprint. Therefore, it is important to develop a low-voltage-driven H
2S microsensor. In this study, low-voltage-driven SnO
2-based H
2S microsensors with an optimized micro-heater were designed, fabricated, and characterized based on experimental requirements. To investigate the relationship between the H
2S-detection performance and the heating performance influenced by the geometric design of the micro-heater, micro-heaters with different patterns were fabricated in the proposed H
2S microsensor platform and characterized. Finally, a low-voltage-driven (3–4 V) SnO
2-based H
2S microsensor with an optimized micro-heater was developed, and it was used to detect H
2S at a low concentration.
2. Design and Fabrication of Micro-Heater Embedded in SnO2-Based H2S Microsensor
The performance of a micro-heater utilizing Joule heating is affected by various factors, such as electrical, mechanical, and material properties, as well as its geometric design. Materials used for a micro-heater are primarily metallic because of their high electrical conductivity, satisfactory specific heat capacity, and good compatibility with the MEMS process. Recently, with the majority of gas sensors being minimized for real-time monitoring and portable applications, the area wherein the micro-heater is fabricated has been limited and minimized as well. Therefore, an optimal geometric design of a micro-heater is certain to improve the heating performance; developing such a design, with an excellent heating performance in a small area, is critical. In this study, SnO
2-based H
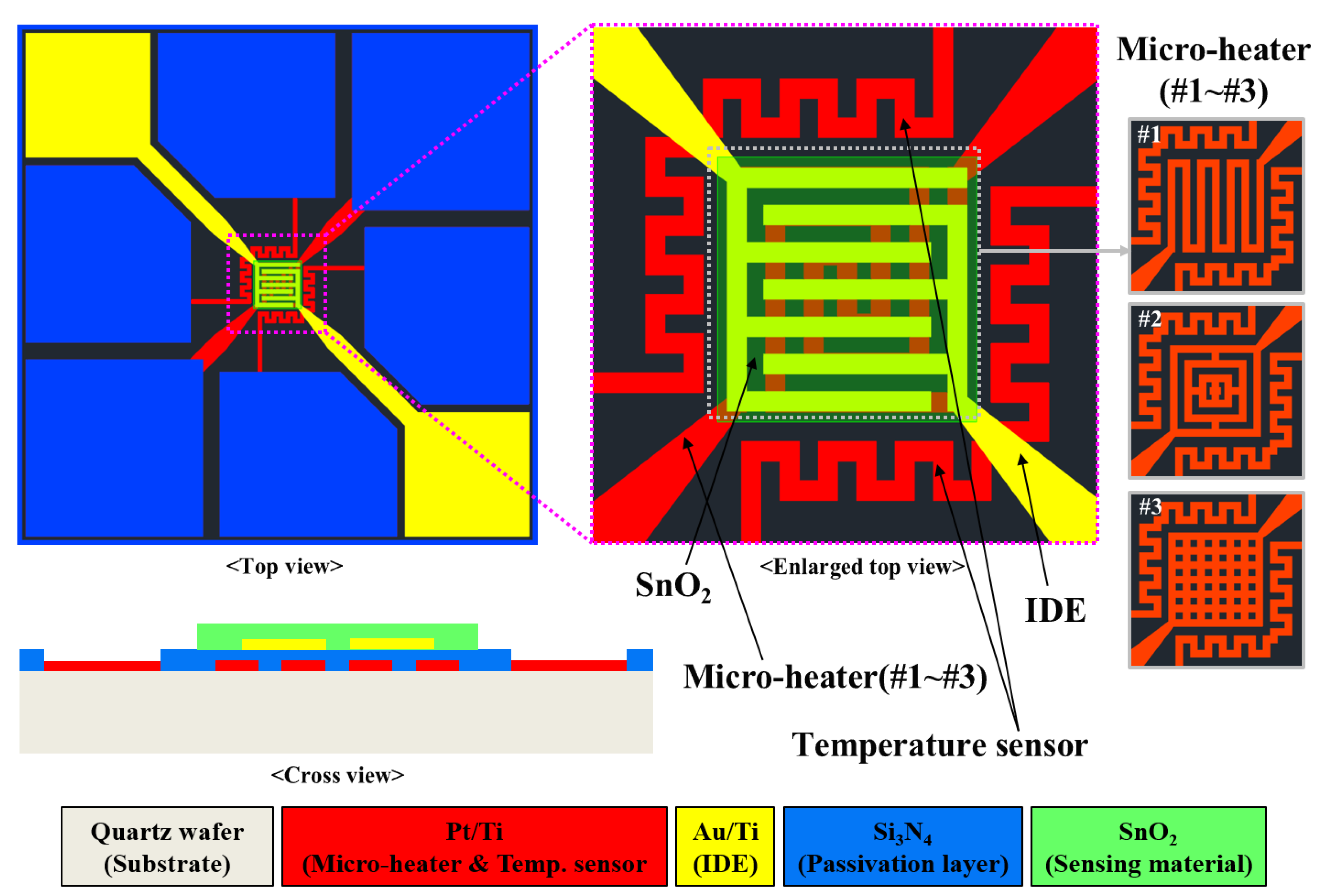
2S microsensors with three types of micro-heaters were proposed and designed as shown in
Figure 2.
The micro-heater types #1–#3 had patterns of meander, rectangular, and rectangular mesh, respectively. The proposed SnO2-based H2S microsensor comprised micro-heaters (types #1–#3), a temperature sensor, an IDE, and a sensing material (SnO2). The sensor and sensing area measured 3 mm × 3 mm and 100 μm × 100 μm, respectively. The width and thickness of a micro-heater, temperature sensor, and IDE were 20 μm and 200 nm, respectively. To minimize the loss of thermal energy produced by micro-heaters, a quartz wafer was used as the sensor substrate. Platinum (Pt), which exhibits a linear relationship between temperature and resistance, was used to fabricate the micro-heater and temperature sensor. Gold (Au) and SnO2 with thickness of 50.3 nm each were utilized as the IDE and sensing material, respectively.
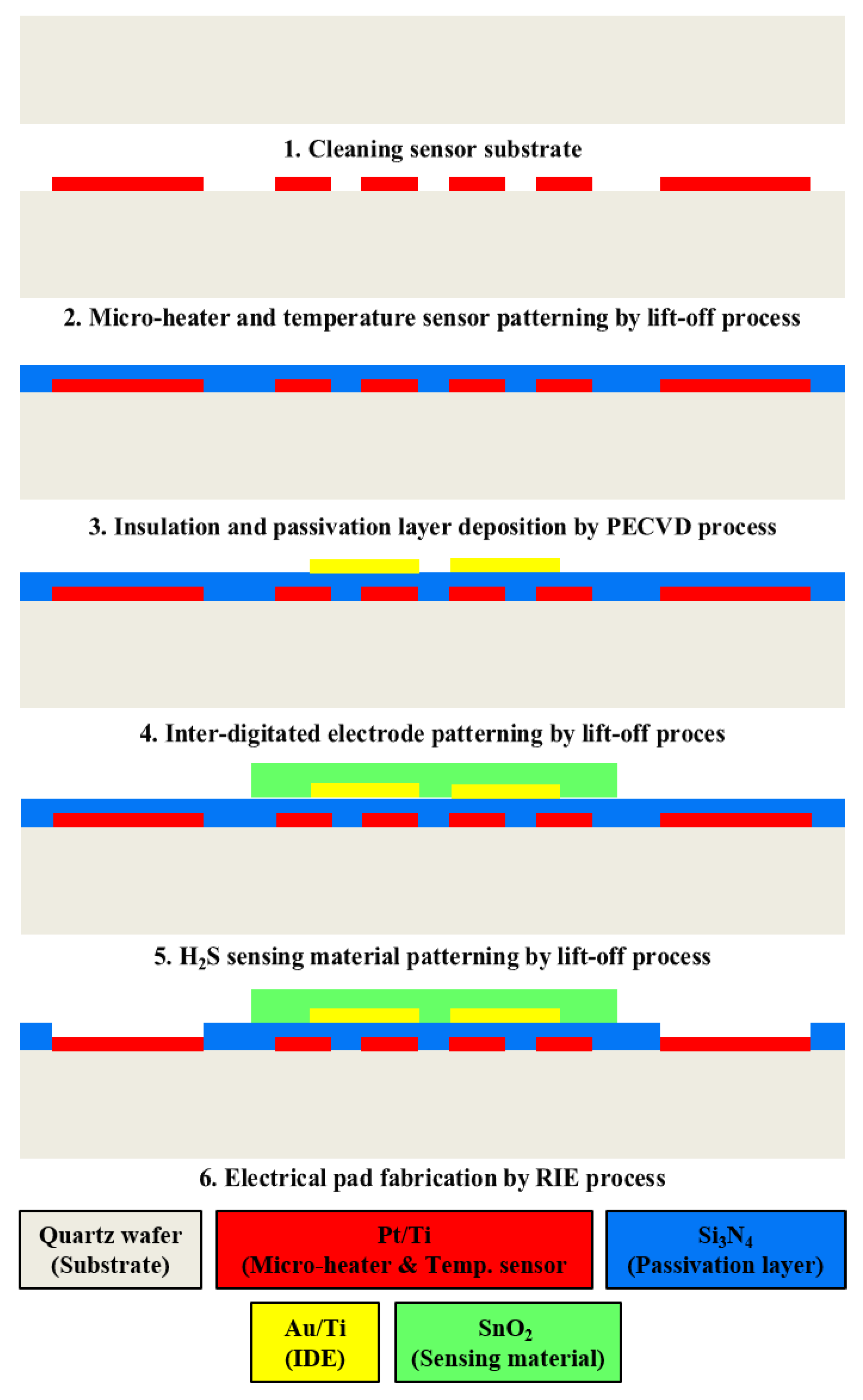
Figure 3 shows the fabrication process of the proposed SnO
2-based H
2S microsensor. First, the quartz wafer (sensor substrate) was cleaned with an acetone and methanol solution for 10 min. Then, the proposed micro-heaters of three types and the temperature sensor were fabricated through photolithography (for the patterning of the desired geometric designs) and e-beam evaporation (Pt deposition) processes. Silicon nitride (Si
3N
4) was deposited via a plasma-enhanced chemical-vapor-deposition process. Subsequently, the deposited Si
3N
4 was used for electrical insulation and passivation. The IDE was fabricated through photolithography and e-beam evaporation processes for Au deposition. Finally, SnO
2, used as the H
2S-sensing material, was deposited via a sputtering process, and Si
3N
4 was etched to fabricate the electrical pads of the micro-heaters and the temperature sensor.
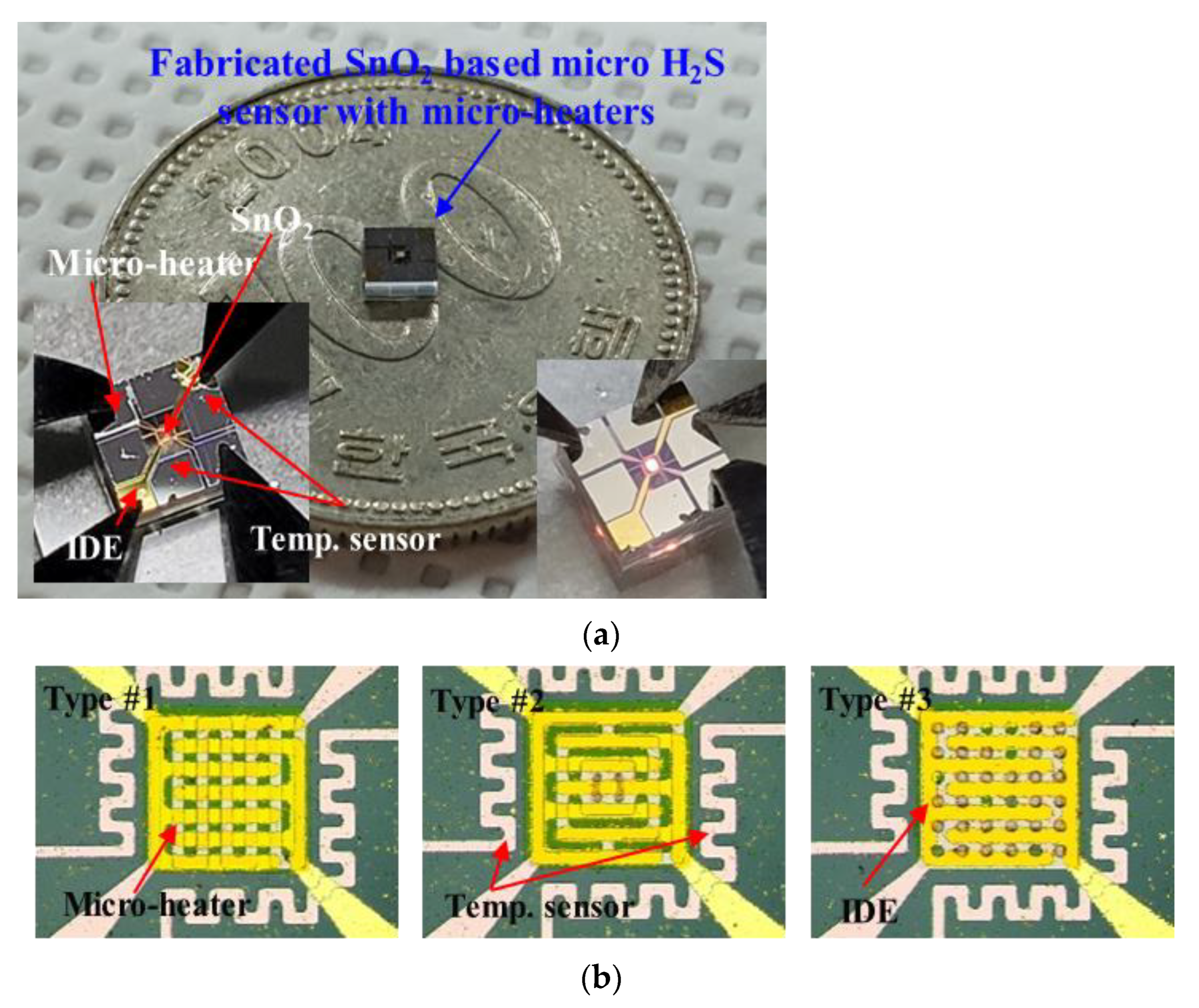
Figure 4a,b show the fabricated SnO
2-based H
2S microsensor.
3. Characterization of Micro-Heater Embedded in SnO2-Based H2S Microsensor
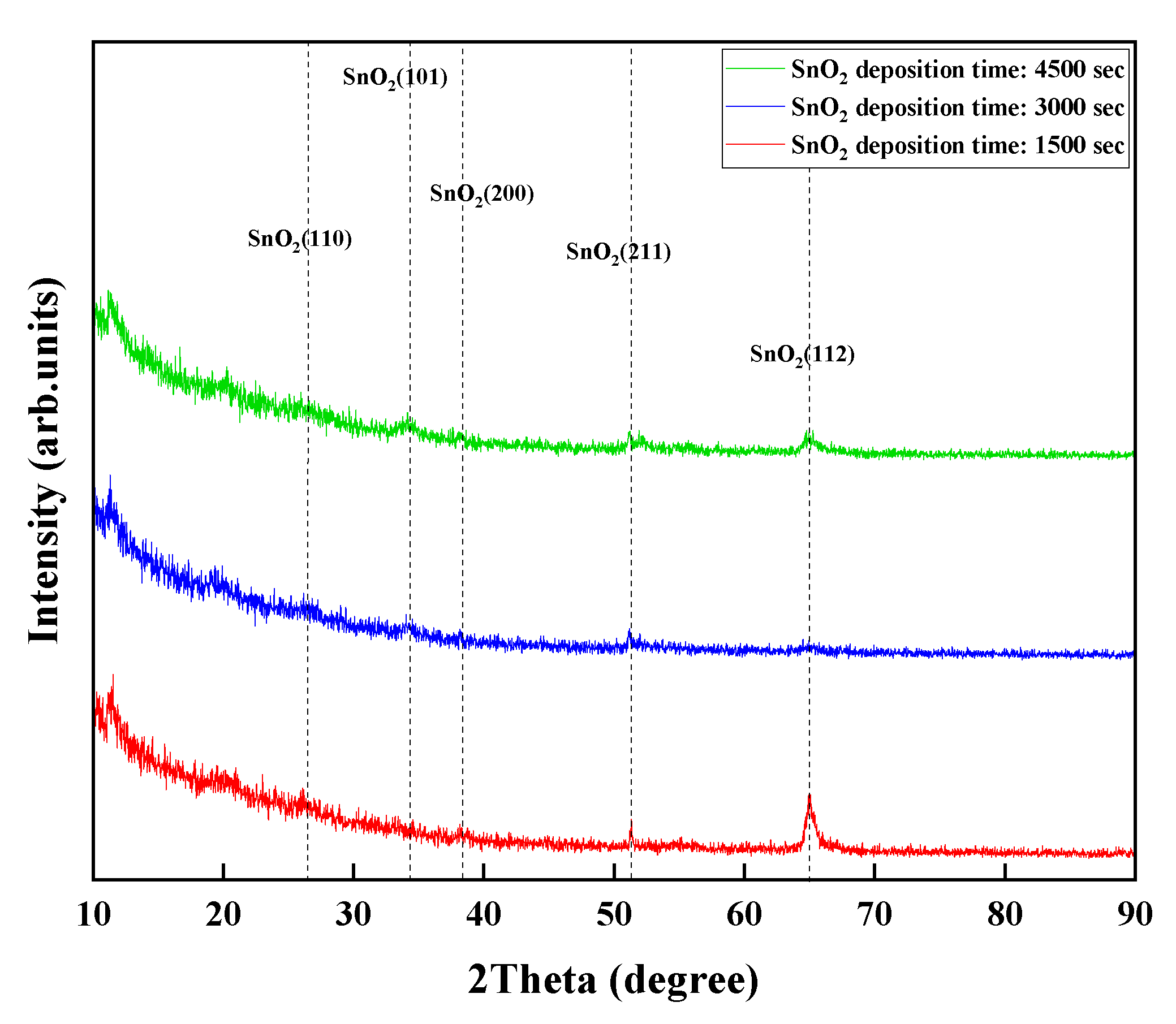
The fabricated sensing material (SnO
2) for detecting H
2S was examined via X-ray diffraction (XRD), and the XRD curves are shown in
Figure 5. The results of the diffraction peaks in the 2-theta range from 10° to 90° verified that the crystal structure of the sensing material (SnO
2) with a varying thickness is the standard tetragonal–rutile crystal phase of SnO
2. The observed peaks of the deposited SnO
2 matched well with the standard JCPDS data of SnO
2.
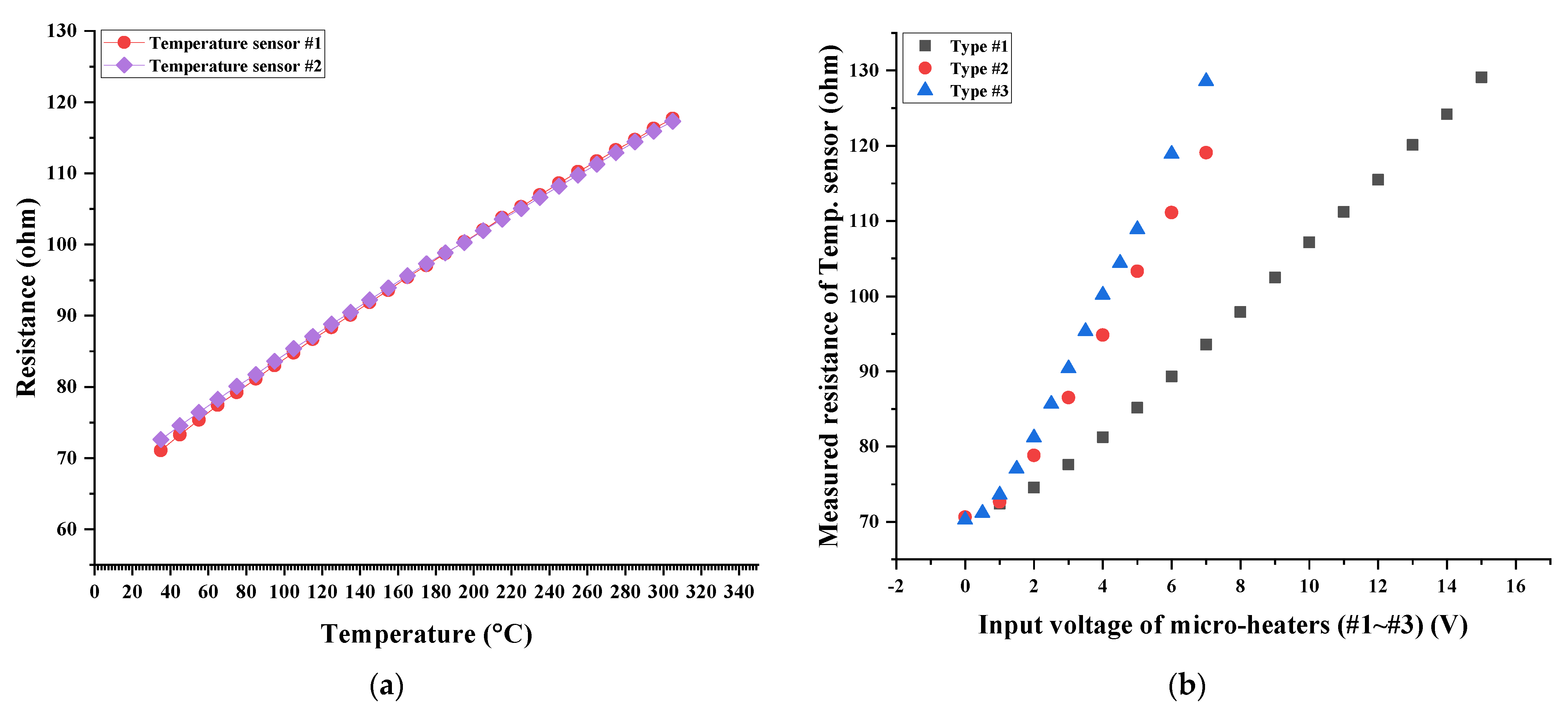
The performance of the fabricated temperature sensor and micro-heaters with different geometric designs was verified before the SnO
2-based H
2S microsensor was characterized, along with the H
2S concentration. The fabricated temperature sensor’s resistance was measured by modulating the gas chamber temperature. The measured resistance values of the temperature sensor at 30, 100, 200, and 300 °C were approximately 70, 83, 101, and 115 ohm, respectively. These measured resistance values of the temperature sensor were linearly increased by increasing the gas chamber temperature, as shown in
Figure 6a. This implied that the heating performance of the micro-heaters embedded in the H
2S microsensor could be estimated in real time. Next, various input voltage values were applied to the micro-heaters and their heating performance was characterized by measuring the resistance of the temperature sensor (closely fabricated to the micro-heaters). The fabricated micro-heaters of types #1–#3 exhibited different heating performances, implying that the generated thermal energy differed based on the geometric design of a micro-heater for the same input voltage applied to the micro-heaters, as shown in
Figure 6b. As the heating performance of a micro-heater was affected by Joule heating, which is closely related to the current traveling through the micro-heater, increasing the current traveling through the micro-heater was very important. The measured initial resistance value of micro-heaters of types #1–#3 were 107.44, 30.27, and 22.35 ohm, respectively. The micro-heater of type #3 exhibited the lowest initial resistance value, thereby enabling a greater flow of current at an equal input voltage of the micro-heater. Therefore, the micro-heater with the rectangular mesh pattern (Type #3) produced more heat energy than the others employing different patterns (Type #1–#2) (for the equal input voltage value being applied). Thus, the micro-heater with the rectangular mesh pattern can produce thermal energy effectively. Simultaneously, the heating performance, along with the micro-heater’s pattern, was also confirmed by estimating the H
2S-detection performance of the SnO
2-based H
2S microsensor.
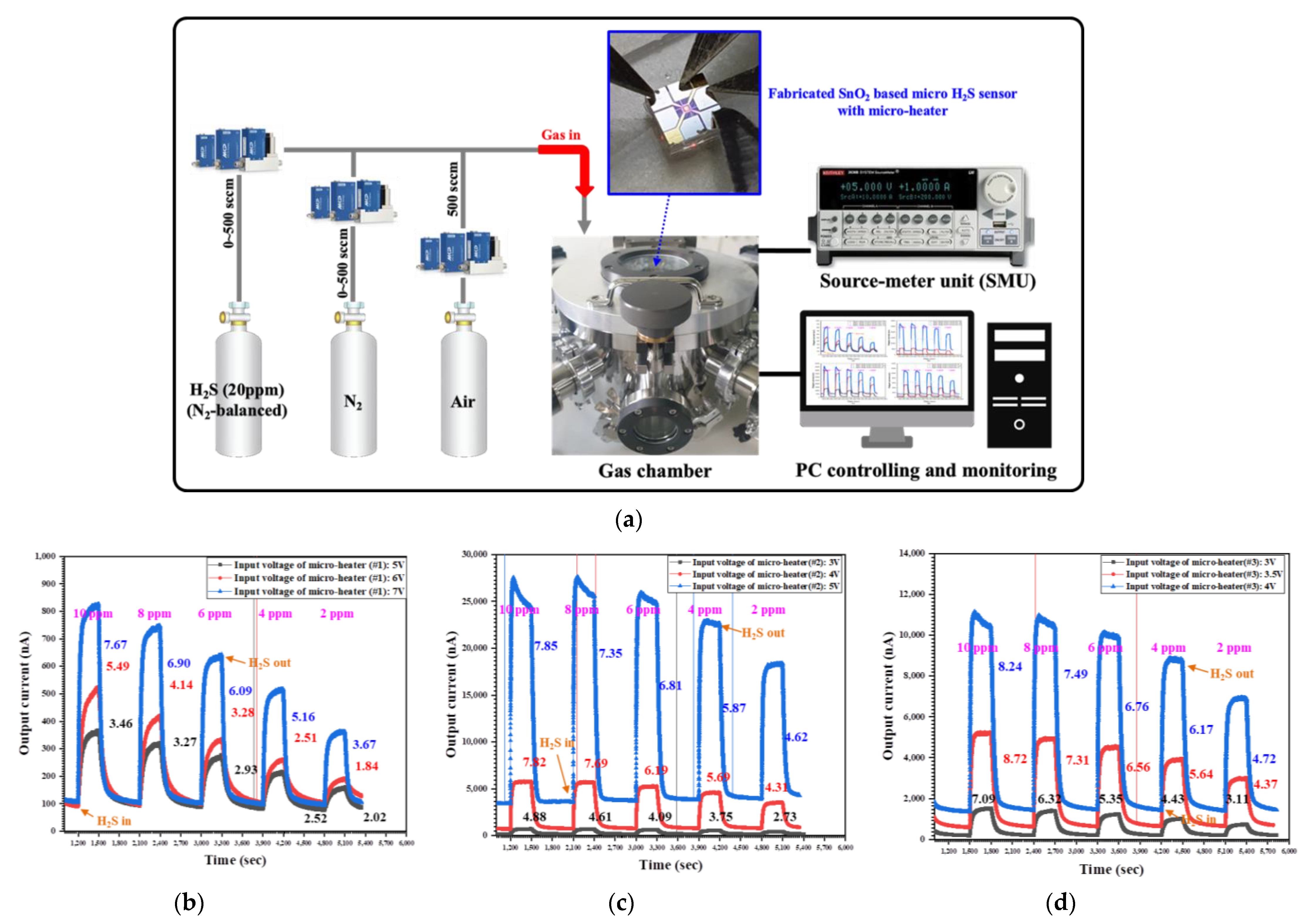
To characterize the fabricated SnO
2-based H
2S microsensor with micro-heaters (types #1–#3), it was placed in the prepared gas chamber and H
2S gas was injected at a concentration of 0 to 10 ppm. The operating temperature of the H
2S microsensor can be estimated via the measured resistance of the temperature sensor, along with the input voltage applied to micro-heaters.
Table 1 presents the expected operating temperature and power consumption along with the input voltage applied to micro-heaters.
The expected operating temperature was derived by using the relationship between the temperature and measured resistance of temperature sensor, whereas expected power consumption was derived using the power consumption formula (P = V
2/R). The temperature at which an excellent performance (high response and good selectivity) of SnO
2 for H
2S is ensured is 150–200 °C, as mentioned above. Therefore, the input voltage was applied to the fabricated micro-heaters (#1–#3) to elevate the optimal temperature (150–200 °C) and initiate a reaction between H
2S and SnO
2. The resistance of SnO
2 used as an H
2S-sensing material changed when it was exposed to H
2S, as mentioned above. The output current of the fabricated H
2S microsensor was measured by injecting H
2S gas in the range of 0 to 10 ppm, as shown in
Figure 7.
The variation in the output current was closely related to the chemical properties of the fabricated SnO
2 surface oxygen. Oxygen was absorbed on the SnO
2 surface in different forms depending on the operating temperature, and it was converted into molecular (physisorption) or dissociative (chemisorption) forms by the increasing operating temperature. The oxygen species with different forms (O
2−, O
− and O
2−) generated on the SnO
2 surface induced an electron-depletion layer, resulting in the decrease in carrier concentration and increase in resistance on the SnO
2 surface. Output currents of SnO
2-based H
2S microsensors increased when H
2S was injected into the chamber because the oxygen species adsorbed on the SnO
2-sensing material surface were consumed by the chemical reaction and the electrons donated back to the SnO
2 surface, resulting in a decreased electrical resistance. The response of an SnO
2-based H
2S microsensor is typically defined as
where
Rair and
Rgas are the resistances and
Iair and
Igas are the conductance values of the sensor regarding air and reducing gas (H
2S), respectively. The response dramatically improved when the operating temperature was increased by increasing the input voltage of the micro-heater. This is because increasing the operating temperature causes the oxidation of several H
2S molecules by producing a multitude of electrons. Therefore, the output current varies greatly, thus indicating a significant improvement in the response for H
2S detection. However, the response is not constantly improved by the increasing operating temperature. In
Figure 7b–d, the measured response of the fabricated H
2S microsensor slightly increased or saturated because the oxygen species were desorbed from the SnO
2 surface [
27]. In addition, at a higher operating temperature, the carrier concentration increased, owing to the intrinsic thermal excitation while the Debye length decreases. These are primarily responsible for the decrease in gas response at higher temperatures [
28]. The micro-heater of type #3 exhibited a superior H
2S-detecting performance at a lower operating voltage compared to micro-heaters of type #1 and #2 because of its lower resistance value, which aided in increasing the current traveling through micro-heater type #3. Thus, the micro-heater of type #3 operated at a relatively lower input voltage, had a superior heating performance, and actively provided sufficient energy for the reaction between H
2S and the oxygen species (O
2−, O
−, and O
2−) on the SnO
2 surface.
Figure 8a–c show the measured response and recovery time of the H
2S microsensor for micro-heaters of types #1–#3. The response time is defined as the time required for decreasing the H
2S sensor resistance or increasing the H
2S sensor conductance by 90% of the total decrease
(Rair-Rgas) or total increase (
Igas-Iair). In contrast, the recovery time is defined as the time required to recover the H
2S sensor resistance or increase the H
2S sensor conductance by 90% of the total decrease
(Rair-Rgas) or total increase (
Igas-Iair) when the H
2S injection is stopped and air is injected into the chamber.
Response and recovery times considerably decreased with the increase in operating temperature, which is controlled by the input voltage of the micro-heater. At a lower input voltage, the micro-heater of type #3 had a shorter response and recovery time compared to that of type #1 and #2, as it produced a more adequate thermal energy for an active and rapid reaction between H
2S and oxygen species (O
2−, O
− and O
2−) on the SnO
2 surface, owing to its superior heating ability. To initiate a reaction between molecules, they must be close to each other, and each molecule must have an energy greater than the energy required for the reaction (i.e., activation energy,
Ea). The activation energy for the reaction between H
2S and the surface-adsorbed oxygen species (O
2−, O
− and O
2−) decreased with the increasing operating temperature. This decreased activation energy rapidly induced the reaction between H
2S and oxygen species (O
2−, O
− and O
2−) with an increasing operating temperature. It was confirmed that the fabricated SnO
2-based H
2S microsensors with micro-heaters of type #1–#3 actively react with H
2S at the operating temperature of approximately 170–180 °C; however, the SnO
2-based H
2S microsensor’s response for H
2S was saturated or degraded when the operating temperature was further increased above 180 °C, as shown in
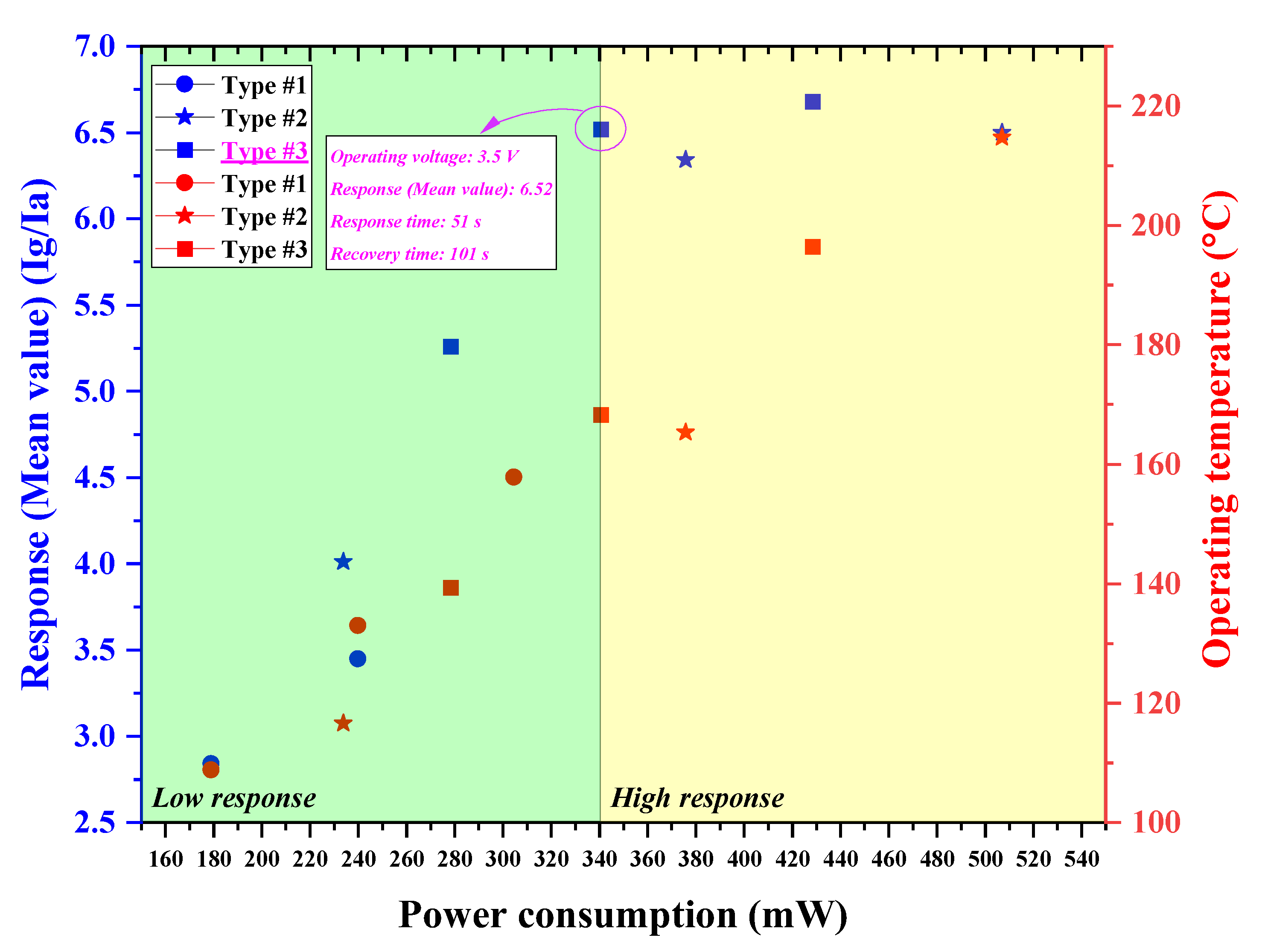
Figure 9. In the high response region, the SnO
2-based H
2S microsensor with a micro-heater of type #3 exhibited a low driven voltage (3.5 V) and a low power consumption (340.65 mW), while offering a high performance (response: 6.52, response time: 51 s, recovery time: 101 s) for portable gas sensor applications, as shown in
Figure 9. However, other H
2S microsensors with micro-heaters of type #1–#2 must be supplied with a higher operating voltage and require a greater power consumption to exhibit a similar H
2S-detection ability. This limits their applicability in portable gas sensor applications.
To develop an H2S gas sensor that requires a lower-driven voltage and power consumption than the developed H2S microsensor, a membrane structure must be fabricated. This must be considered depending on applicable fields, as a gas sensor with a membrane structure can incur high costs, encounter difficulties in fabrication, and possess weak mechanical properties.
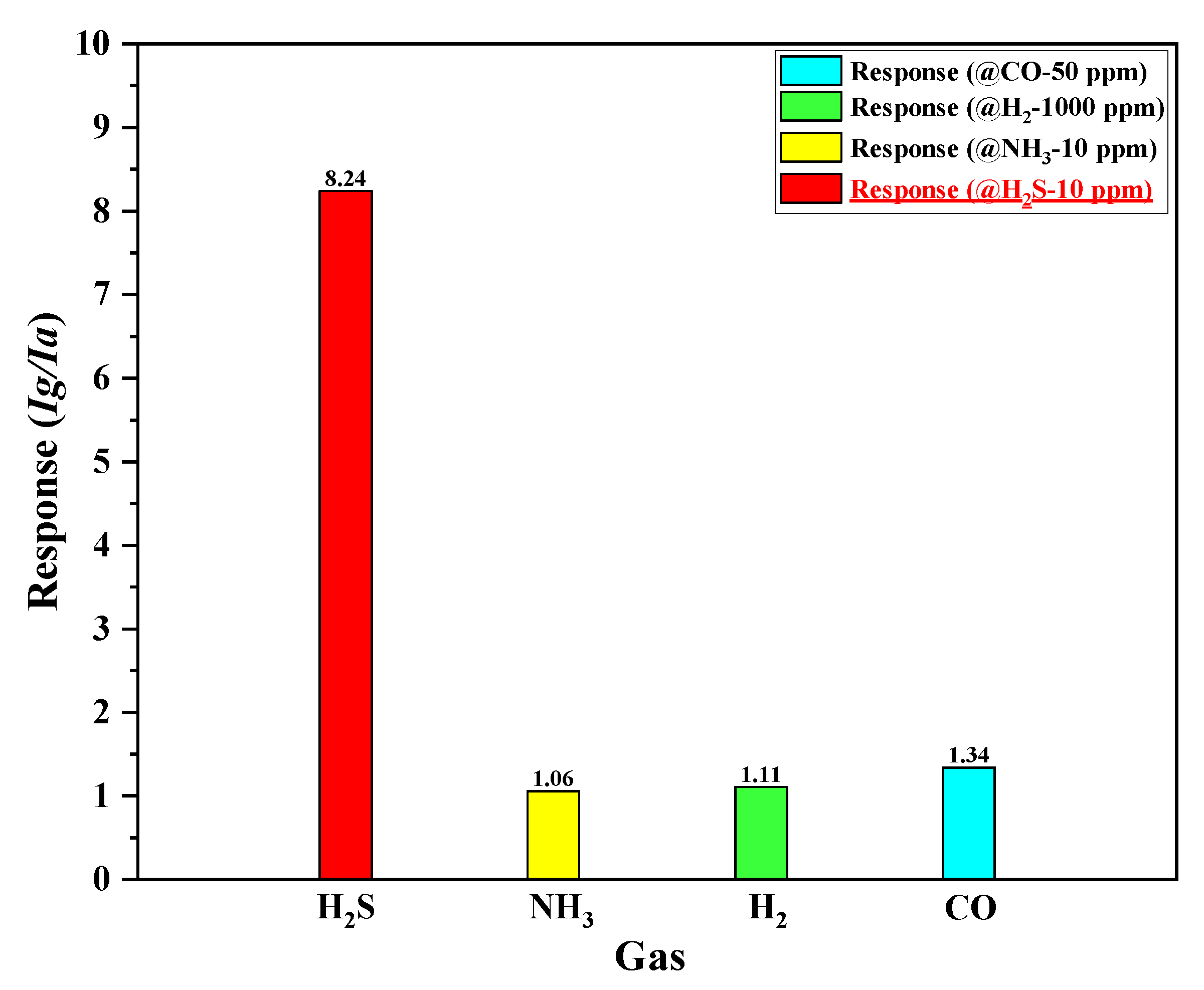
For practical applications, gas sensors should exhibit a strong response, as well as good selectivity, toward the targeted gas (H
2S in this study). To estimate the selectivity of the proposed SnO
2-based H
2S microsensor with micro-heater type #3 (input voltage is 3.5 V, power consumption is 340.65 mW), the fabricated H
2S microsensor was exposed to different types of gases, including ammonia (NH
3), hydrogen (H
2), and carbon monoxide (CO) gases. As shown in
Figure 10, the fabricated H
2S microsensor displayed great selectivity toward H
2S. In case of an increasing operating temperature at the higher input voltage (>3.5–4 V), the fabricated H
2S microsensor with the micro-heater of type #3 actively reacted with different types of gases, as mentioned in
Section 1. Thus, the selectivity of fabricated H
2S microsensors is poor, and unnecessary power consumption is required. Based on the experimental results, we confirmed that it is important to provide sufficient thermal energy to reach an optimum operating temperature (170–180 °C) for the reaction between H
2S and oxygen species (O
2−, O
− and O
2−) on the SnO
2 surface using the micro-heater that exhibits a superior heating performance.
In summary, the H2S-detection ability of the proposed SnO2-based H2S microsensor can be significantly improved by supplying thermal energy, utilizing fabricated micro-heaters embedded in the H2S sensor. Further, the applied input voltage and power consumption can be minimized by optimizing the micro-heater design.