Application of Microfluidic Chips in the Detection of Airborne Microorganisms
Abstract
1. Introduction
2. Microfluidic Chips
2.1. Materials for the Fabrication of Microfluidic Chips
2.2. Methods for the Fabrication of Microfluidic Chips
3. Collection and Detection of Airborne Microorganisms
3.1. Collection of Airborne Microorganisms
3.2. Detection of Airborne Microorganisms
4. Detection of Airborne Microorganisms by Microfluidic Chips
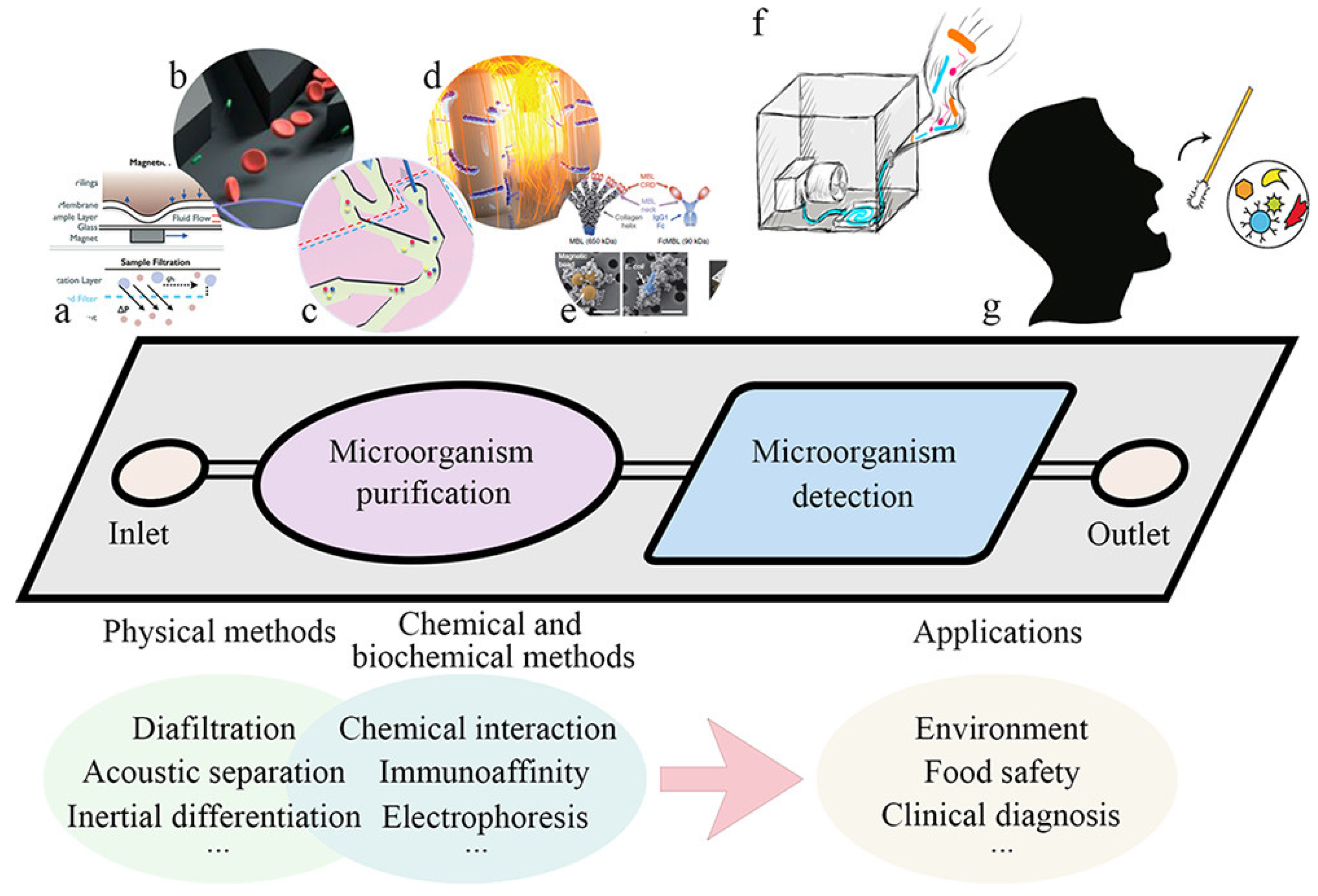
4.1. The Role of Microfluidic Chips in the Separation of Microorganisms
4.2. Application of Microfluidic Chips in Bacterial Detection
4.3. Application of Microfluidic Chips in Virus Detection
4.4. Application of Microfluidic Chips in Fungi Detection
5. Challenges and Future Trends
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stetzenbach, L.D. Airborne Infectious Microorganisms. In Encyclopedia of Microbiology, 3rd ed.; Academic Press: Oxford, UK, 2009; pp. 175–182. [Google Scholar]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6 (Suppl. S6), S737–S746. [Google Scholar] [CrossRef]
- Kilburn, K.H. Summary of the 5th international conference on bioaerosols, fungi, bacteria, mycotoxins, and human health. Arch. Environ. Health 2003, 58, 538–542. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Gutarowska, B.; Brochocka, A. Aspects of tests and assessment of filtering materials used for respiratory protection against bioaerosols. Part I: Type of active substance, contact time, microorganism species. Int. J. Occup. Saf. Ergon. 2010, 16, 263–273. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Zhang, Y.Z. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- Zhu, C.; Kawamura, K.; Kunwar, B. Organic tracers of primary biological aerosol particles at subtropical okinawa island in the Western North Pacific Rim. J. Geophys Res. Atmos. 2015, 120, 5504–5523. [Google Scholar] [CrossRef]
- Kanaani, H.; Hargreaves, M. Deposition rates of fungal spores in indoor environments, factors effecting them and comparison with non-biological aerosols. Atmos Environ. 2008, 42, 7141–7154. [Google Scholar] [CrossRef]
- Diaz, M.E.; Law, S.E.; Frank, J.F. Control of pathogenic microorganisms and turbidity in poultry-processing chiller water using UV-enhanced ozonation. Ozone Sci. Eng. 2001, 23, 53–64. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Shenqiti, A.; Bahashwan, S.A.; Ghanem, S.; Manzoor, N.; El, S. Nosocomial infections in intensive care and medical rehabilitation units, and evaluation of antibiotics prescription. Afr. J. Microbiol. Res. 2017, 11, 776–783. [Google Scholar] [CrossRef][Green Version]
- Roselló, G.M.; Bouza, J.E. Nosocomial respiratory viral infection. An. Sist. Sanit. Navar. 2014, 37, 265–279. [Google Scholar] [CrossRef]
- Garcíapatiño, M.G.; Garcíacontreras, R.; Liconalimón, P. The immune response against acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front. Immunol. 2017, 8, 441. [Google Scholar] [CrossRef]
- Guan, W.J.; Zhong, N.S. Clinical characteristics of COVID-19 in China. Reply. N. Engl. J. Med. 2020, 382, 1861–1862. [Google Scholar]
- Birgand, G.; Peiffer-Smadja, N.; Fournier, S.; Kerneis, S.; Lucet, J.C. Assessment of Air Contamination by SARS-CoV-2 in Hospital Settings. JAMA Netw. Open 2020, 3, e2033232. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Feng, S.; Jellicoe, M.; Raston, C.L. Application of microfluidic technology in food processing. Food Funct. 2020, 11, 5726–5737. [Google Scholar] [CrossRef]
- Park, J.; Han, D.H.; Park, J.K. Towards practical sample preparation in point-of-care testing: User-friendly microfluidic devices. Lab Chip 2020, 20, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef]
- Ryan, D.S.; Eric, S.; Casey, C.G.; Sung-Yueh, W.; Chen, Y.; Michael, R.; Liwei, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2017, 189, 52–68. [Google Scholar]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H., III; Volckens, J.; Henry, C.S. Paper-based analytical devices for environmental analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Yang, Y.; Noviava, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-based microfluidic devices: Emerging themes and applications. Anal. Chem. 2016, 89, 71–91. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in microfluidics for environmental analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef]
- Fan, Z.H.; Harrison, D.J. Micromachining of capillary electrophoresis injectors and separators on glass chips and evaluation of flow at capillary intersections. Anal. Chem. 1994, 66, 177–184. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Moore, A.W.; Ramsey, J.M. Fused quartz substrates for microchip electrophoresis. Anal. Chem. 1995, 67, 2059–2063. [Google Scholar] [CrossRef]
- Joh, D.Y.; Hucknall, A.M.; Wei, Q.; Mason, K.A.; Lund, M.L.; Fontes, C.M.; Hill, R.T.; Blair, R.; Zimmers, Z.; Achar, R.K.; et al. Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7054–E7062. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Morales-Narváez, E.; Merkoi, A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014, 54, 279–284. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. A miniaturized immunosensor platform for automatic detection of carcinoembryonic antigen in EBC. Sens. Actuat B-Chem. 2014, 205, 94–101. [Google Scholar] [CrossRef]
- Chen, G.; Chai, H.H.; Yu, L.; Fang, C. Smartphone supported backlight illumination and image acquisition for microfluidic-based point-of-care testing. Biomed. Opt. Express 2018, 9, 4604–4612. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Spehar, A.M.; Koster, S.; Linder, V.; Kulmala, S.; de Rooij, N.F.; Verpoorte, E.; Sigrist, H.; Thormann, W. Electrokinetic characterization of poly(dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3674–3678. [Google Scholar] [CrossRef]
- Abdullah, B.; Nesrin, H.; Ulku, M.A.; Volkan, K.; Ertugrul, S.M. Colorimetric bisphenol-a Detection with a portable smartphone-based spectrometer. IEEE Sens. J. 2018, 18, 5948–5955. [Google Scholar]
- Potluri, V.; Kathiresan, P.S.; Kandula, H.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Kota Sai Pavan, S.; Yarravarapu, D.; Soundararajan, A.; Baskar, K.; Gupta, R.; et al. An inexpensive smartphone-based device for point-of-care ovulation testing. Lab Chip 2019, 19, 59–67. [Google Scholar] [CrossRef]
- Soper, S.A.; Ford, S.M.; Qi, S.; Mccarley, R.L.; Kelly, K.; Murphy, M.C. Peer reviewed: Polymeric microelectromechanical systems. Anal. Chem. 2000, 72, 642–651. [Google Scholar] [CrossRef]
- Jönsson, C.; Aronsson, M.; Rundström, G.; Pettersson, C.; Mendel-Hartvig, I.; Bakker, J.; Martinsson, E.; Liedberg, B.; MacCraith, B.; Ohman, O.; et al. Silane—dextran chemistry on lateral flow polymer chips for immunoassays. Lab Chip 2008, 8, 1191–1197. [Google Scholar] [CrossRef]
- Fredrickson, C.K.; Xia, Z.; Das, C.; Ferguson, R.; Tavares, F.T.; Fan, Z.H. Effects of fabrication process parameters on the properties of cyclic olefin copolymer microfluidic devices. J. Microelectromech. Syst. 2006, 15, 1060–1068. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Sun, P.; Tang, Q.; Li, H.; Wang, X.; Guo, G.; Pu, Q. Ionic polymer enhanced electrophoresis in plastic microchips for rapid and robust determination of rhodamine dyes. Sens. Actuat B-Chem. 2017, 250, 250–258. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Wang, Z.; Wu, J.; Wang, W.; Zhou, L.; Xiao, Q.; Pu, Q. Mobile phone mediated point-of-care testing of HIV p24 antigen through plastic micro-pit array chips. Sens. Actuat B-Chem. 2018, 271, 189–194. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef]
- Wang, P.; Ge, L.; Yan, M.; Song, X.; Ge, S.; Yu, J. Paper-based three-dimensional electrochemical immunodevice based on multi-walled carbon nanotubes functionalized paper for sensitive point-of-care testing. Biosens. Bioelectron. 2012, 32, 238–243. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Ge, S.; Yan, M.; Huang, J.; Yu, J. Battery-triggered ultrasensitive electrochemiluminescence detection on microfluidic paper-based immunodevice based on dual-signal amplification strategy. Anal. Chim. Acta. 2013, 767, 66–74. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.J.; Wei, J.F.; Xu, J.R.; Wang, Y.H.; Zheng, G.X. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 2014, 406, 2799–2807. [Google Scholar] [CrossRef]
- Charbaji, A.; Smith, W.; Anagnostopoulos, C.; Faghri, M. Zinculose: A new fibrous material with embedded zinc particles. Eng. Sci. Technol. Int. J. 2021, 24, 571–578. [Google Scholar] [CrossRef]
- Ireta-Muñoz, L.A.; Cueva-Perez, I.; Saucedo-Dorantes, J.J.; Pérez-Cruz, A. A Paper-Based Cantilever Beam Mini Actuator Using Hygro-Thermal Response. Actuators 2022, 11, 94. [Google Scholar] [CrossRef]
- Carrio, A.; Sampedro, C.; Sanchez-Lopez, J.L.; Pimienta, M.; Campoy, P. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors 2015, 24, 29569–29593. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based microfluidic device for improved detection of nitrate in water. Sensors 2020, 26, 102. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.; Zhang, Z.; Li, J.; Yuan, L.; Zhang, Z.; Chen, L. Microfluidic paper-based chips in rapid detection: Current status, challenges, and perspectives. TrAC Trend. Anal. Chem. 2021, 143, 116371. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19, detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly (lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Keraite, I.; Liga, A.; Conoscenti, G.; Coles, S.; Schulze, H.; Bachmann, T.T.; Parvez, K.; Casiraghi, C.; Howarth, N.M. Laser ablation of poly (lactic acid) sheets for the rapid prototyping of sustainable, single-use, disposable medical microcomponents. ACS Sustain. Chem. Eng. 2018, 6, 4899–4908. [Google Scholar] [CrossRef]
- Morgan, A.J.; Hidalgo San Jose, L.; Jamieson, W.D.; Wymant, J.M.; Song, B.; Stephens, P.; Barrow, D.A.; Castell, O.K. Simple and versatile 3D printed microfluidics using fused filament fabrication. PLoS ONE 2016, 11, e0152023. [Google Scholar]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Borenstein, J.T. Biomaterials-based microfluidics for engineered tissue constructs. Soft Matter 2010, 6, 4999–5015. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, X.; Xiao, Z.; Zhang, Q.; Zhou, J.; Xu, F.; Lu, T. Cell-encapsulating microfluidic hydrogels with enhanced mechanical stability. Soft Matter 2012, 8, 10687–10694. [Google Scholar] [CrossRef]
- Cabodi, M.; Choi, N.W.; Gleghorn, J.P.; Lee, C.; Bonassar, L.J. A Microfluidic Biomaterial. J. Am. Chem. Soc. 2005, 127, 13788–13789. [Google Scholar] [CrossRef]
- Wlodarczyk, K.L.; Hand, D.P.; Maroto-Valer, M.M. Maskless, rapid manufacturing of glass microfluidic devices using a picosecond pulsed laser. Sci. Rep. 2019, 9, 20215. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 2000, 21, 12–26. [Google Scholar] [CrossRef]
- Yang, Y.; Kameoka, J.; Wachs, T.; Henion, J.D.; Craighead, H.G. Quantitative mass spectrometric determination of methylphenidate concentration in urine using an electrospray ionization source integrated with a polymer microchip. Anal. Chem. 2004, 76, 2568–2574. [Google Scholar] [CrossRef]
- McCormick, R.M.; Nelson, R.J.; Alonso-Amigo, M.G.; Benvegnu, D.J.; Hooper, H.H. Microchannel electrophoretic separations of DNA in injection-molded plastic substrates. Anal. Chem. 1997, 69, 2626–2630. [Google Scholar] [CrossRef]
- Matellan, C.; Del Río Hernández, A.E. Cost-effective rapid prototyping and assembly of poly (methyl methacrylate) microfluidic devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef]
- Roberts, M.A.; Rossier, J.S.; Bercier, P.; Girault, H. UV laser machined polymer substrates for the development of micro-diagnostic systems. Anal. Chem. 1997, 69, 2035–2042. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Geschke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef]
- Younan, X.; Whitesides, G. Soft lithography. Angew. Chem. Int. Engl. 1998, 37, 550–575. [Google Scholar]
- Wei, X.; Pu, Q. Microchip electrophoresis for fast and interference-free determination of trace amounts of glyphosate and glufosinate residues in agricultural products. Methods Mol. Biol. 2015, 1274, 21–29. [Google Scholar]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef]
- Balakrishnan, H.K.; Doeven, E.H.; Merenda, A.; Dumée, L.F.; Guijt, R.M. 3D printing for the integration of porous materials into miniaturised fluidic devices: A review. Anal. Chim. Acta 2021, 1185, 338796. [Google Scholar] [CrossRef]
- Lal, H.; Punia, T.; Ghosh, B.; Srivastava, A.; Jain, V.K. Comparative study of bioaerosol during monsoon and post-monsoon seasons at four sensitive sites in Delhi region. Int. J. Adv. Earth Environ. 2013, 1, 1–7. [Google Scholar]
- Xu, Z.; Yao, M. Monitoring of bioaerosol inhalation risks in different environments using a six-stage andersen sampler and the PCR-DGGE method. Environ. Monit. Assess. 2013, 185, 3993–4003. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Stentiford, E.I. Generation and dispersion of airborne microorganisms from composting facilities. Process. Saf. Environ. Protect. 2003, 81, 166–170. [Google Scholar]
- Wang, Z.; Reponen, T.; Grinshpun, S.A.; Górny, R.L.; Willeke, K. Effect of sampling time and air humidity on the bioefficiency of filter samplers for bioaerosol collection. J. Aerosol. Sci. 2001, 32, 661–674. [Google Scholar]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar]
- Jing, W.; Jiang, X.; Zhao, W.; Liu, S.; Cheng, X.; Sui, G. Microfluidic platform for direct capture and analysis of airborne Mycobacterium tuberculosis. Anal. Chem. 2014, 86, 5815–5821. [Google Scholar]
- Jiang, X.; Jing, W.; Zheng, L.; Liu, S.; Wu, W.; Sui, G. A continuous-flow high-throughput microfluidic device for airborne bacteria PCR detection. Lab Chip 2014, 14, 671–676. [Google Scholar]
- Zare, R.N. My life with LIF: A personal account of developing laser-induced fluorescence. Annu. Rev. Anal. Chem. 2012, 5, 1–14. [Google Scholar]
- Nuchtavorn, N.; Bek, F.; Macka, M.; Suntornsuk, W.; Suntornsuk, L. Rapid separations of nile blue stained microorganisms as cationic charged species by chip-CE with LIF. Electrophoresis 2012, 33, 1421–1426. [Google Scholar]
- Ferguson, R.M.W.; Garcia-Alcega, S.; Coulon, F.; Dumbrell, A.J.; Whitby, C.; Colbeck, I. Bioaerosol biomonitoring: Sampling optimization for molecular microbial ecology. Mol. Ecol. Resour. 2019, 19, 672–690. [Google Scholar] [CrossRef]
- Prost, K.; Kloeze, H.; Mukhi, S.; Bozek, K.; Poljak, Z.; Mubareka, S. Bioaerosol and surface sampling for the surveillance of influenza A virus in swine. Transbound. Emerg. Dis. 2019, 66, 1210–1217. [Google Scholar] [CrossRef]
- Wang, L.; Qi, W.; Liu, Y.; Essien, D.; Zhang, Q.; Lin, J. Recent advances on bioaerosol collection and detection in microfluidic chips. Anal. Chem. 2021, 93, 9013–9022. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Review: Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta. 2017, 966, 11–33. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Y.; Cao, X.; Liu, C.; Zhang, N.; Shi, D. Droplet digital PCR as an emerging tool in detecting pathogens nucleic acids in infectious diseases. Clin. Chim. Acta. 2021, 517, 156–161. [Google Scholar] [CrossRef]
- Li, X.; Weng, Z.; Cao, A.; Liu, Q.; Sui, G. An overview about varieties and detection methods of pathogenic microorganisms in indoor air. Chin. Ence Bull. 2018, 63, 2116–2127. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef]
- Sui, G.; Cheng, X. Microfluidics for detection of airborne pathogens: What challenges remain? Bioanalysis 2014, 6, 5–7. [Google Scholar] [CrossRef]
- Moon, H.S.; Nam, Y.W.; Park, J.C.; Jung, H.I. Dielectrophoretic separation of airborne microbes and dust particles using a microfluidic channel for real-time bioaerosol monitoring. Environ. Sci. Technol. 2009, 43, 5857–5863. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, K.S.; Kim, S.S.; Bae, G.N.; Jung, J.H. Real-time detection of an airborne microorganism using inertial impaction and mini-fluorescent microscopy. Lab Chip 2014, 14, 244–251. [Google Scholar] [CrossRef]
- Meltzer, R.H.; Krogmeier, J.R.; Kwok, L.W.; Allen, R.; Crane, B.; Griffis, J.W.; Knaian, L.; Kojanian, N.; Malkin, G.; Nahas, M.K.; et al. A lab-on-chip for biothreat detection using single-molecule DNA mapping. Lab Chip 2011, 11, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Kang, J.S.; Lee, J.E.; Kim, S.S.; Jung, J.H. Continuous aerosol size separator using inertial microfluidics and its application to airborne bacteria and viruses. Lab Chip 2015, 15, 1889–1897. [Google Scholar] [CrossRef]
- Choi, J.; Hong, S.C.; Kim, W.; Jung, J.H. Highly enriched, controllable, continuous aerosol sampling using inertial microfluidics and its application to real-time detection of airborne bacteria. ACS Sens. 2017, 2, 513–521. [Google Scholar] [CrossRef]
- Jing, W.; Zhao, W.; Liu, S.; Li, L.; Tsai, C.T.; Fan, X.; Wu, W.; Li, J.; Yang, X.; Sui, G. Microfluidic device for efficient airborne bacteria capture and enrichment. Anal. Chem. 2013, 85, 5255–5262. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Lan, Y.; Wang, B.; Zhang, Y.S.; Liu, B.; Yang, P.; Zhang, W.; Qiao, L. Microfluidic air sampler for highly efficient bacterial aerosol collection and identification. Anal. Chem. 2016, 88, 11504–11512. [Google Scholar] [CrossRef] [PubMed]
- Fuchiwaki, Y.; Nagai, H.; Saito, M.; Tamiya, E. Ultra-rapid flow-through polymerase chain reaction microfluidics using vapor pressure. Biosens. Bioelectron. 2011, 27, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Jing, W.; Liu, S.; Zhang, D.; Sui, G. First airborne pathogen direct analysis system. Analyst 2016, 141, 1637–1640. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Jung, J.H. Fully integrated optofluidic SERS platform for real-time and continuous characterization of airborne microorganisms. Biosens. Bioelectron. 2020, 169, 112611. [Google Scholar] [CrossRef]
- Stachwiak, J.C.; Shugard, E.E.; Mosier, B.P.; Renzi, R.F.; Caton, P.F.; Ferko, S.M.; Van de Vreugde, J.L.; Yee, D.D.; Haroldsen, B.L.; VanderNoot, V.A. Autonomous microfluidic sample preparation system for protein profile-based detection of aerosolized bacterial cells and spores. Anal. Chem. 2007, 79, 5763–5770. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, W.; Zheng, J.; Ni, Z.; Sun, H. Spatial varying profiling of air PM constituents using paper-based microfluidics. Biomicrofluidics 2019, 13, 054103. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Tan, M.; Wang, Z.; Yao, M.; Xu, Z.; Wu, Y.; Wang, J.; Guo, X.; Zhu, T. Integrating silicon nanowire field effect transistor, microfluidics and air sampling techniques for real-time monitoring biological aerosols. Environ. Sci. Technol. 2011, 45, 7473–7480. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Fronczek, C.F.; Angus, S.V.; Nicolini, A.M.; Yoon, J.Y. Rapid and sensitive detection of H1N1/2009 virus from aerosol samples with a microfluidic immunosensor. J. Lab. Autom. 2014, 19, 322–331. [Google Scholar] [CrossRef]
- Xiong, H.; Ye, X.; Li, Y.; Qi, J.; Fang, X.; Kong, J. Efficient microfluidic-based air sampling/monitoring platform for detection of aerosol SARS-CoV-2 on-site. Anal. Chem. 2021, 93, 4270–4276. [Google Scholar] [CrossRef]
- Breshears, L.E.; Nguyen, B.T.; Mata Robles, S.; Wu, L.; Yoon, J.Y. Biosensor detection of airborne respiratory viruses such as SARS-CoV-2. SLAS Technol. 2022, 27, 4–17. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, R.; Yang, N.; Kwabena Oppong, P.; Sun, J.; Wang, P. High-precision extraction and concentration detection of airborne disease microorganisms based on microfluidic chip. Biomicrofluidics 2019, 13, 024110. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, Q.; Zhao, W.; Liu, S.; Sui, G. Microfluidic system for rapid detection of airborne pathogenic fungal spores. ACS Sens. 2018, 3, 2095–2103. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, J.; Zhang, T.; Zhang, X.; Chen, X.; Zhao, W.; Yao, Y.; Zhao, W.; Sui, G. A rapid multiplex nucleic acid detection system of airborne fungi by an integrated DNA release device and microfluidic chip. Talanta 2022, 246, 123467. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, L.; Wang, H.; Wang, L. Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines 2022, 13, 1576. https://doi.org/10.3390/mi13101576
Wang J, Yang L, Wang H, Wang L. Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines. 2022; 13(10):1576. https://doi.org/10.3390/mi13101576
Chicago/Turabian StyleWang, Jinpei, Lixia Yang, Hanghui Wang, and Lin Wang. 2022. "Application of Microfluidic Chips in the Detection of Airborne Microorganisms" Micromachines 13, no. 10: 1576. https://doi.org/10.3390/mi13101576
APA StyleWang, J., Yang, L., Wang, H., & Wang, L. (2022). Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines, 13(10), 1576. https://doi.org/10.3390/mi13101576





