Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix
2.3. Fabrication of HApCh and MgHApCh Coatings
2.4. Physico-Chemical Studies
2.5. Biological Evaluation
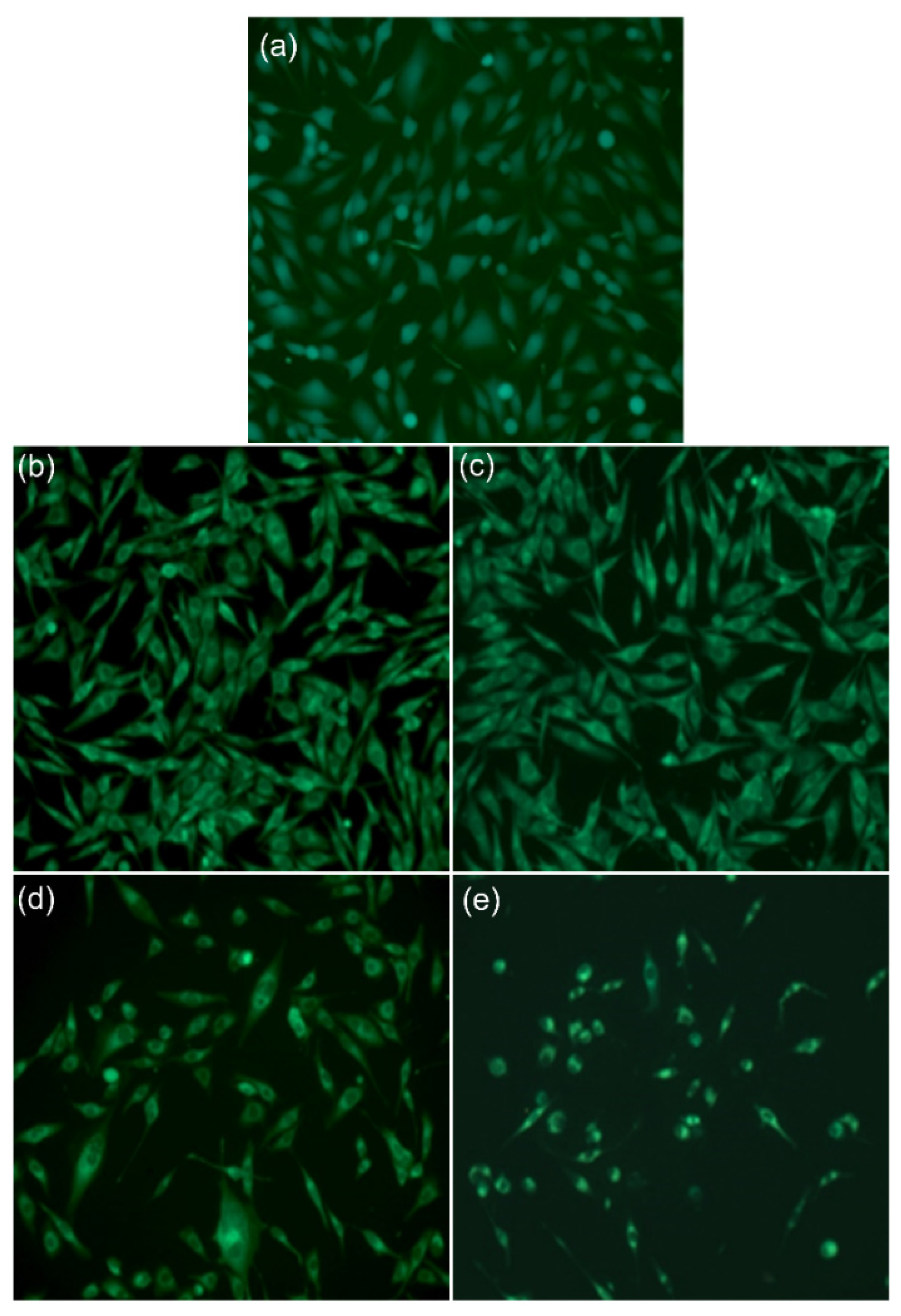
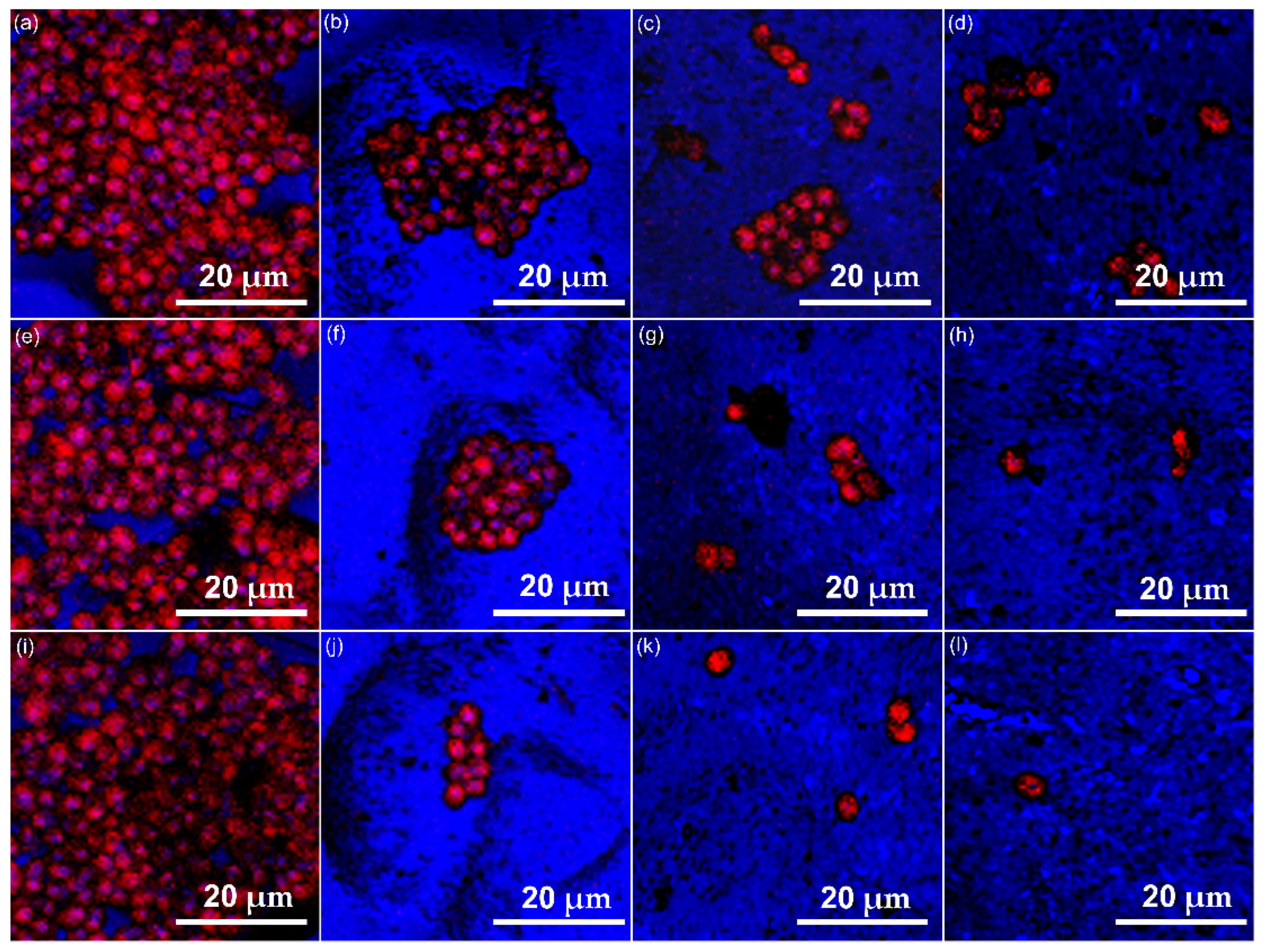
2.5.1. Fluorescein Diacetate (FDA)—Propidium Iodide (PI) Cell Staining
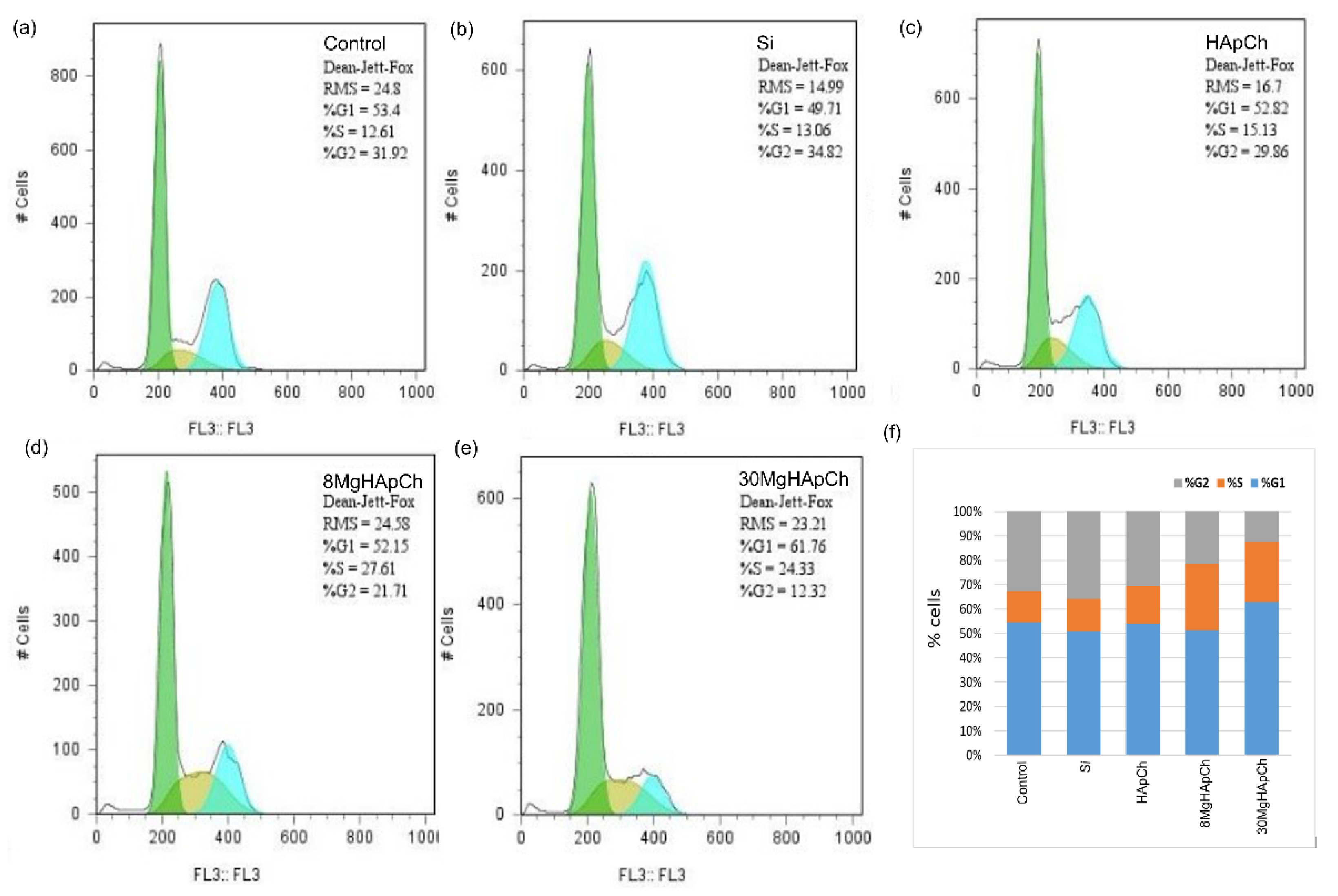
2.5.2. Analysis of Cell Cycle
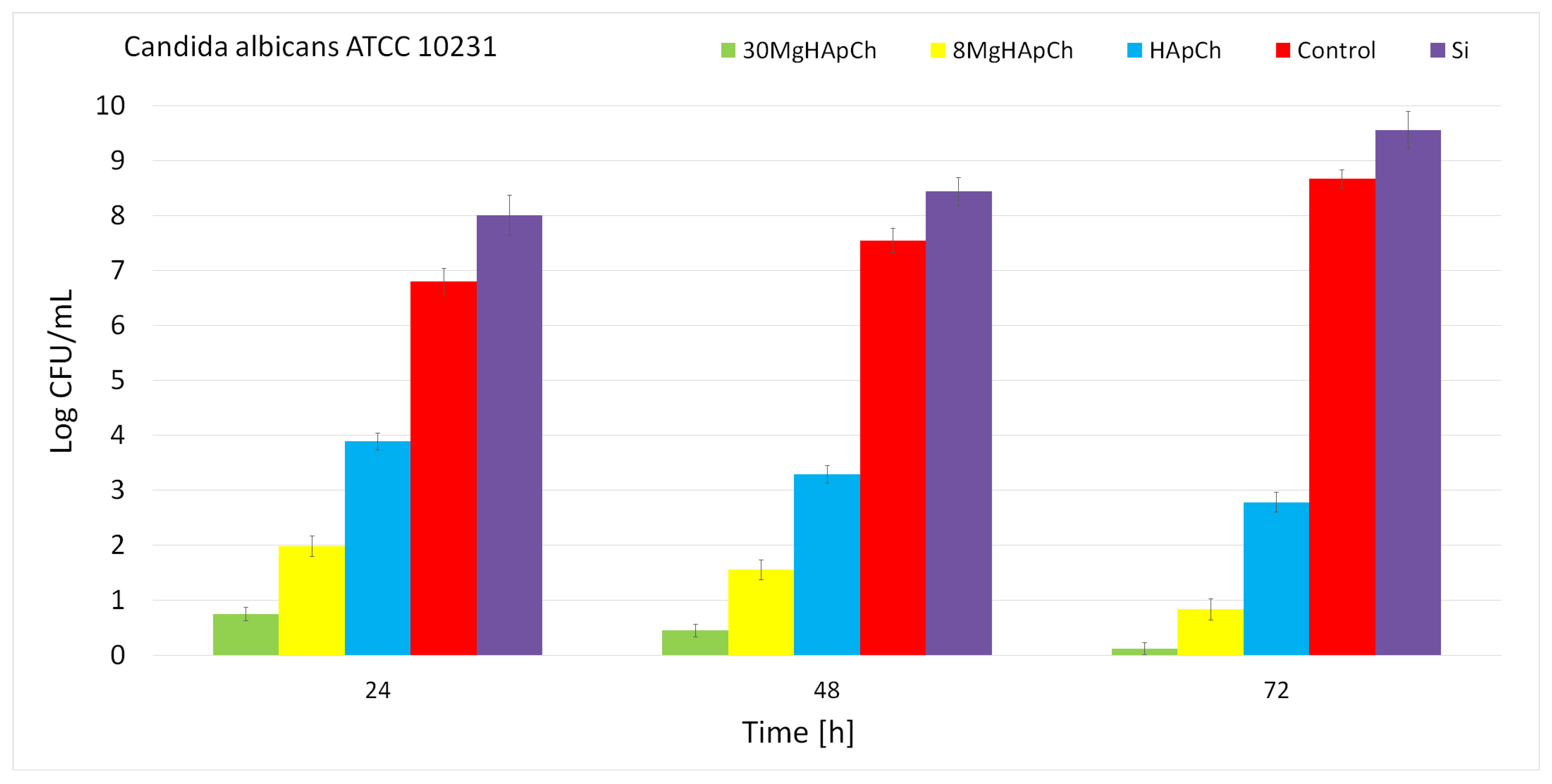
2.5.3. In Vitro Antifungal Assays
3. Results
3.1. Structural and Morphological Characterizations
3.2. Biological Evaluations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grandviewresearch. Available online: https://www.grandviewresearch.com/industry-analysis/biomaterials-industry (accessed on 11 September 2022).
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Morris, H.F.; Ochi, S. Hydroxyapatite-coated implants: A case for their use. J. Oral Maxillofac. Surg. 1998, 56, 1303–1311. [Google Scholar] [CrossRef]
- Luo, J.; Mamat, B.; Yue, Z.; Zhang, N.; Xu, X.; Li, Y.; Su, Z.; Ma, C.; Zang, F.; Wang, Y. Multi-metal ions doped hydroxyapatite coatings via electrochemical methods for antibacterial and osteogenesis. Colloids Interface Sci. Commun. 2021, 43, 100435. [Google Scholar] [CrossRef]
- Rodríguez-Valencia, C.; López-Álvarez, M.; Cochón-Cores, B.; Pereiro, I.; Serra, J.; González, P. Novel selenium-doped hydroxyapatite coatings for biomedical applications. J. Biomed. Mater. Res. Part A 2013, 101, 853–861. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.-A.; Buton, N.; Megier, C.; Beuran, M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Fabrication of Silver- and Zinc-Doped Hydroxyapatite Coatings for Enhancing Antimicrobial Effect. Coatings 2020, 10, 905. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Perez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B 2020, 191, 110996. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Konrad, M. Chapter 21—Magnesium homeostasis. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 509–525. [Google Scholar]
- Elin, R.J. Magnesium metabolism in health and disease. Dis. Mon. 1988, 34, 161–218. [Google Scholar]
- Melkikh, A.V.; Sutormina, M.I. Model of active transport of ions in cardiac cell. J. Theor. Biol. 2008, 252, 247–254. [Google Scholar]
- Avioli, L.V.; Berman, M. Mg28 kinetics in man. J. Appl. Physiol. 1966, 21, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, J.K. Magnesium: Its Biological Significance; CRC Press: Boca Raton, FL, USA, 1981. [Google Scholar]
- Fox, C.; Ramsoomair, D.; Carter, C. Magnesium: Its proven and potential clinical significance. South Med. J. 2001, 94, 1195–1201. [Google Scholar] [CrossRef]
- Maguire, M.E. Magnesium: A regulated and regulatory cation. Met. Ions Biol. Syst. 1990, 26, 135–153. [Google Scholar]
- Rude, R. Magnesium disorders. In Fluids and Electrolytes; Kokko, J., Tannen, R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 421–445. [Google Scholar]
- Siddiqui, N.; Madala, S.; Parcha, S.R.; Mallick, S.P. Osteogenic differentiation ability of human mesenchymal stem cells on Chitosan/Poly(Caprolactone)/nano beta Tricalcium Phosphate composite scaffolds. Biomed. Phys. Eng. Express 2020, 6, 015018. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Kundu, B.; Chanda, A.; Nandi, S.K. MG63 osteoblast cell response on Zn doped hydroxyapatite (HAp) with various surface features. Ceram. Int. 2017, 43, 3752–3760. [Google Scholar] [CrossRef]
- Ramires, P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef]
- Geng, F.; Tan, L.L.; Jin, X.X.; Yang, J.Y.; Yang, K. The preparation, cytocompatibility, and in vitro biodegradation study of pure β-TCP on magnesium. J. Mater. Sci. Mater. Med. 2009, 20, 1149–1157. [Google Scholar] [CrossRef]
- Ghosh, R.; Das, S.; Mallick, S.P.; Beyene, Z. A Review on the Antimicrobial and Antibiofilm Activity of Doped Hydroxyapatite and its Composites for Biomedical Applications. Mater. Today Commun. 2022, 31, 103311. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Kong, F.; Wang, J.; Han, R.; Ji, S.; Yue, J.; Wang, Y.; Ma, L. Antifungal activity of magnesium oxide nanoparticles: Effect on the growth and key virulence factors of Candida albicans. Mycopathologia 2020, 185, 485–494. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chitosan (accessed on 10 September 2022).
- Peter, M.G. Chitin and chitosan from animal sources. In Polysaccharides and Polyamides in the Food Industry; Steinbüchel, A., Rhee, S.K., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005; pp. 115–208. [Google Scholar]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Pigman, W.W.; Horton, D.; Wander, J.D. The Carbohydrates; Academic Press: New York, NY, USA, 1980; Volume IB, pp. 727–728. ISBN 9780125563512. [Google Scholar]
- Vasconcelos, M.W. Chitosan and chitooligosaccharide utilization in phytoremediation and biofortification programs: Current knowledge and future perspectives. Front. Plant Sci. 2014, 5, 616. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Raaen, S.; Badea, M.L.; Rokosz, K. Physicochemical and Biological Evaluation of Chitosan-Coated Magnesium-Doped Hydroxyapatite Composite Layers Obtained by Vacuum Deposition. Coatings 2022, 12, 702. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Predoi, D.; Trușcă, R.-D.; Prodan, A.M.; Groza, A.; Chifiriuc, M.C.; Beuran, M. Fabrication of Novel Chitosan–Hydroxyapatite Nanostructured Thin Films for Biomedical Applications. Coatings 2021, 11, 1561. [Google Scholar] [CrossRef]
- CasaXPS: Processing Software for XPS, AES, SIMS and More. Copyright© 2022 Casa Software Ltd. Available online: www.casaxps.com (accessed on 10 March 2022).
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 20 July 2021).
- Ciobanu, C.S.; Groza, A.; Iconaru, S.L.; Popa, C.L.; Chapon, P.; Chifiriuc, M.C.; Hristu, R.; Stanciu, G.A.; Negrila, C.C.; Ghita, R.V.; et al. Antimicrobial Activity Evaluation on Silver Doped Hydroxyapatite/Polydimethylsiloxane Composite Layer. BioMed Res. Int. 2015, 2015, 926513. [Google Scholar] [CrossRef]
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 10 July 2022).
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M.J.C.P.B.E. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Pramanik, N.; Mishra, D.; Banerjee, I.; Maiti, T.K.; Bhargava, P.; Pramanik, P. Chemical synthesis, characterization, and biocompatibility study of hydroxyapatite/chitosan phosphate nanocomposite for bone tissue engineering applications. Int. J. Biomater. 2009, 2009, 512417. [Google Scholar] [CrossRef] [PubMed]
- Said, H.A.; Noukrati, H.; Youcef, H.B.; Bayoussef, A.; Oudadesse, H.; Barroug, A. Mechanical Behavior of Hydroxyapatite-Chitosan Composite: Effect of Processing Parameters. Minerals 2021, 11, 213. [Google Scholar] [CrossRef]
- Ismail, F.S.M.; Rohanizadeh, R.; Atwa, S.; Mason, R.S.; Ruys, A.J.; Martin, P.J.A. Bendavid. The influence of surface chemistry and topography on the contact guidance of MG63 osteoblast cells. J. Mater. Sci. Mater. Med. 2007, 18, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Linez, P.; Biehl, V.; Velten, D.; Breme, J.; Hildebrand, H.F. Cell orientation and cytoskeleton organisation on ground titanium surfaces. Biomed. Eng. 2002, 19, 233–237. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater Technol 2020, 35, 785–796. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Iqbal, S.; AL-Anazy, M.M.; Laref, A.; Tahir, M.A.; Channar, P.A.; Noreen, S.; Yasir, M.; Iqbal, A.; et al. Magnesium doped mesoporous bioactive glass nanoparticles: A promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells. J. Alloys Compd. 2021, 866, 159013. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Chen, X.-N.; Zhu, X.-D.; Cai, B.; Fan, H.-S.; Zhang, X.-D. Effect of Surface Topography of Hydroxyapatite on Human Osteosarcoma MG-63 Cell. J. Inorg. Mater. 2013, 28, 901–906. [Google Scholar] [CrossRef]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. 2006, 76, 169–178. [Google Scholar] [CrossRef]
- Murphy, M.F.; Lalor, M.J.; Manning, F.C.; Lilley, F.; Crosby, S.R.; Randall, C.; Burton, D.R. Comparative study of the conditions required to image live human epithelial and fibroblast cells using atomic force microscopy. Microsc. Res. Technol. 2006, 69, 757–765. [Google Scholar] [CrossRef]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, B.S.; Lee, J.; Cho, D.; Kwon, O.H.; Park, W.H. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater. Res. 2017, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Gudur, M.S.R.; Rao, R.R.; Peterson, A.W.; Caldwell, D.J.; Stegemann, J.P.; Deng, C.X. Noninvasive quantification of in vitro osteoblastic differentiation in 3D engineered tissue constructs using spectral ultrasound imaging. PLoS ONE 2014, 9, e85749. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Predoi, D.; Buton, N.; Megier, C.; Stan, G.E. Dextran-Thyme Magnesium-Doped Hydroxyapatite Composite Antimicrobial Coatings. Coatings 2020, 10, 57. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Fatima, Z.; Ahmad, A.; Hameed, S. Magnesium impairs Candida albicans immune evasion by reduced hyphal damage, enhanced β-glucan exposure and altered vacuole homeostasis. PLoS ONE 2022, 17, e0270676. [Google Scholar] [CrossRef]
- Guo, X.; Gough, J.E.; Xiao, P.; Liu, J.; Shen, Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J. Biomed. Mater. Res. 2007, 82, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Isloor, A.M.; Kumar, G.M.; Asiri, A.M. Nanohydroxyapatite reinforced chitosan composite hydrogel with tunable mechanical and biological properties for cartilage regeneration. Sci. Rep. 2019, 9, 15957. [Google Scholar] [CrossRef]
- Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers 2022, 14, 582. [Google Scholar] [CrossRef]

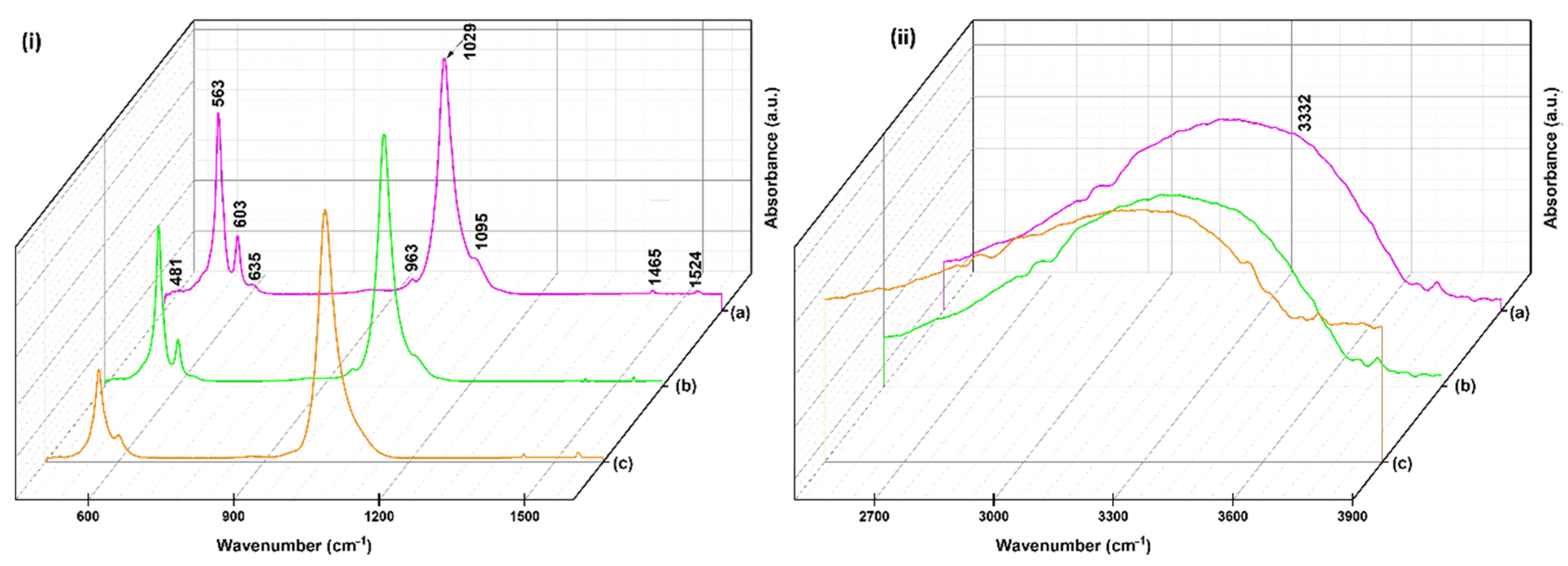



| Wavenumber (cm−1) | Assignments |
|---|---|
| 963 | v1 (PO43−) |
| 1095, 1029 | v3 (PO43−) |
| 481 | v2 (PO43−) |
| 563, 603, 635 | v4 (PO43−) |
| 3332 | v (OH−) |
| 1465 | v (C–H) |
| 1524 | v (NH2) |
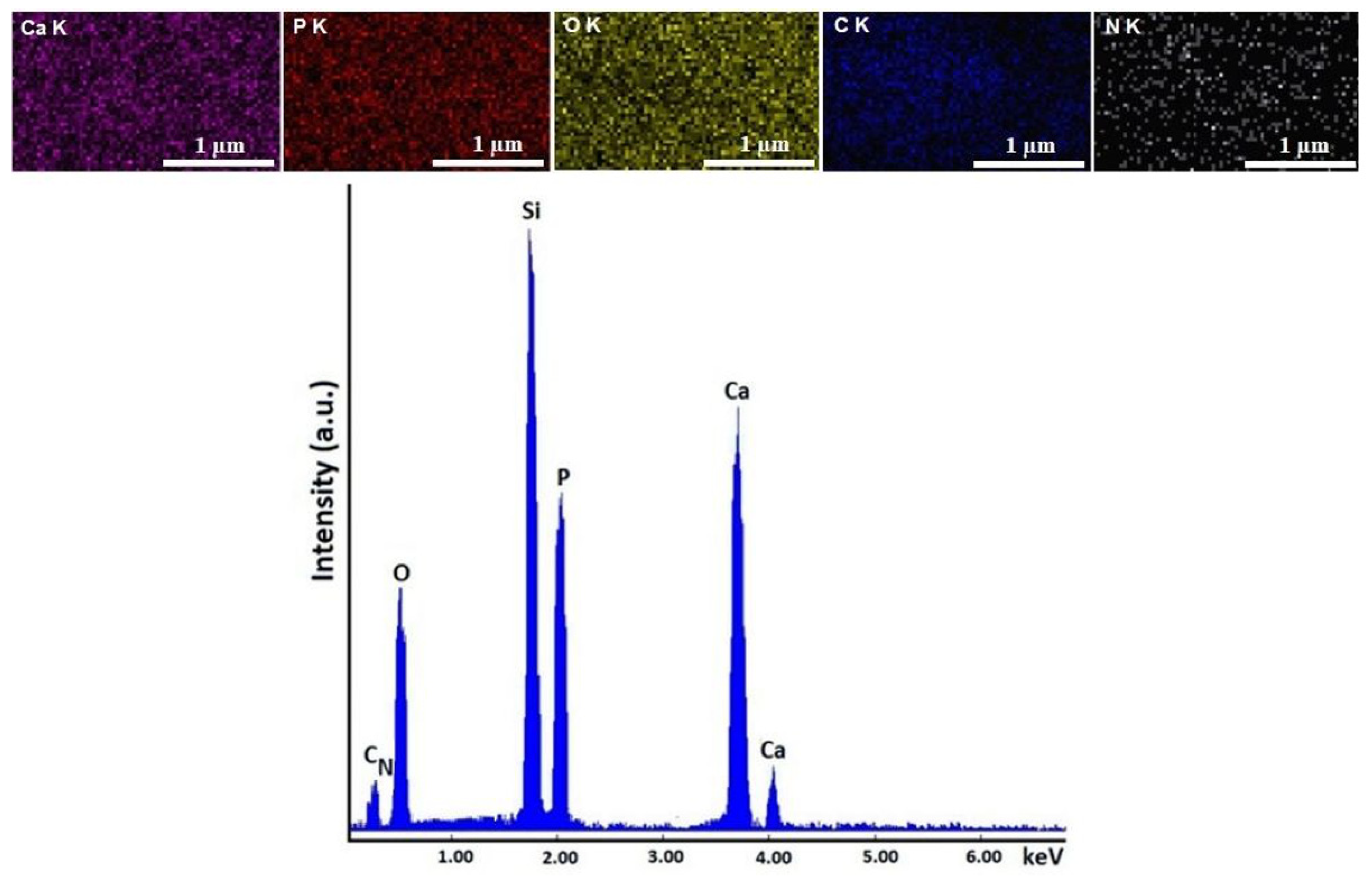
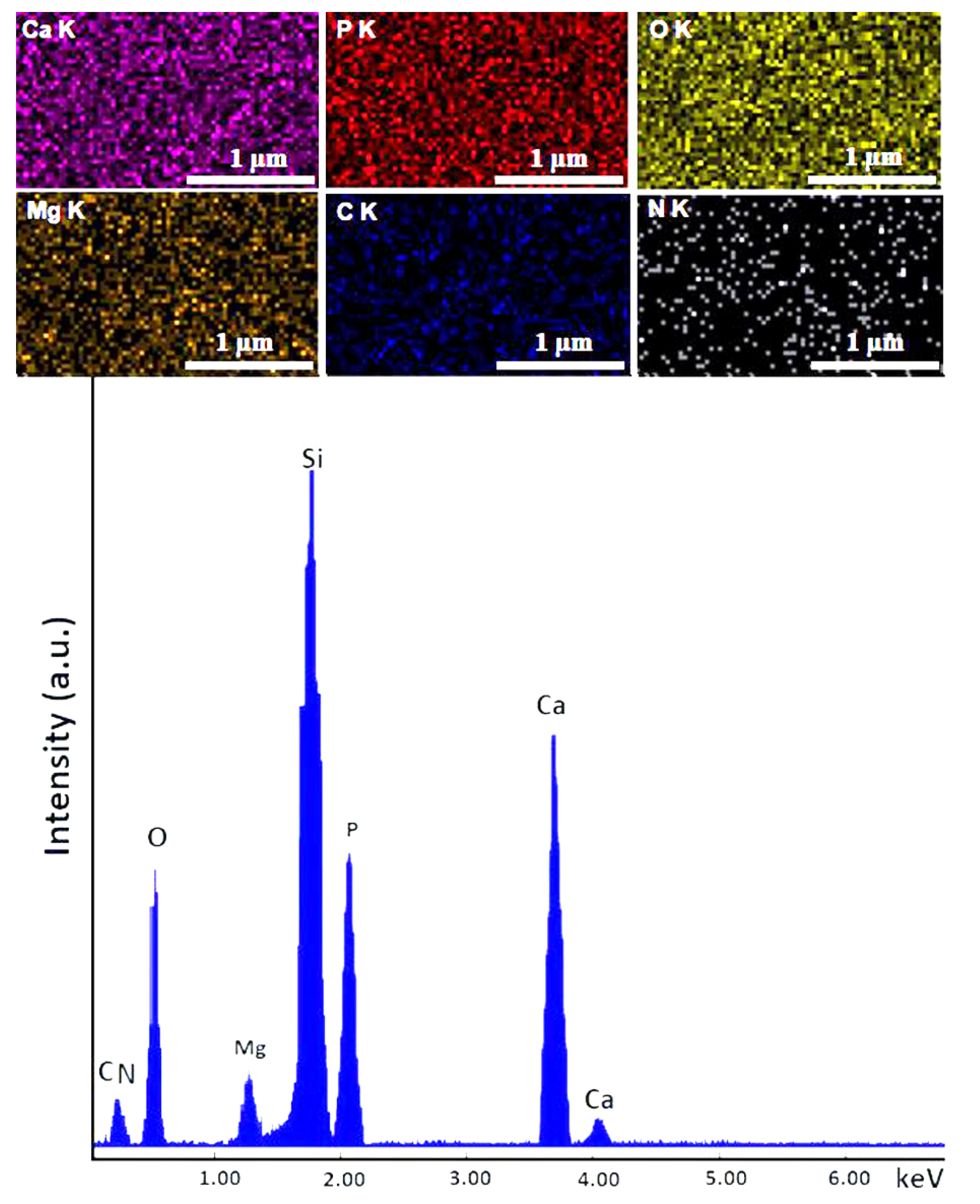
| HApCh | 30MgHApCh | ||
|---|---|---|---|
| Element | Atomic % | Element | Atomic % |
| Ca | 34.85 | Ca | 32.84 |
| P | 20.87 | P | 21.40 |
| O | 44.28 | O | 42.86 |
| Mg | 0 | Mg | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iconaru, S.L.; Ciobanu, C.S.; Predoi, G.; Rokosz, K.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Raaen, S.; Raita, S.M.; et al. Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines 2022, 13, 1574. https://doi.org/10.3390/mi13101574
Iconaru SL, Ciobanu CS, Predoi G, Rokosz K, Chifiriuc MC, Bleotu C, Stanciu G, Hristu R, Raaen S, Raita SM, et al. Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines. 2022; 13(10):1574. https://doi.org/10.3390/mi13101574
Chicago/Turabian StyleIconaru, Simona Liliana, Carmen Steluta Ciobanu, Gabriel Predoi, Krzysztof Rokosz, Mariana Carmen Chifiriuc, Coralia Bleotu, George Stanciu, Radu Hristu, Steinar Raaen, Stefania Mariana Raita, and et al. 2022. "Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix" Micromachines 13, no. 10: 1574. https://doi.org/10.3390/mi13101574
APA StyleIconaru, S. L., Ciobanu, C. S., Predoi, G., Rokosz, K., Chifiriuc, M. C., Bleotu, C., Stanciu, G., Hristu, R., Raaen, S., Raita, S. M., Ghegoiu, L., Badea, M. L., & Predoi, D. (2022). Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines, 13(10), 1574. https://doi.org/10.3390/mi13101574












