Fabrication of 3D Micro-Blades for the Cutting of Biological Structures in a Microfluidic Guillotine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microchannel Design
2.2. Surface Treatment of Substrates for Adhesion Test
2.3. Scanning Electron Microscopy
2.4. Confocal Microscopy
2.5. Stentor Coeruleus Culture and Cell Preparation
2.6. Experiments on Cutting Stentor Coeruleus
2.7. Measuring Cell Survival
2.8. Sytox Green Assay to Measure the Degree of Wounding
3. Results and Discussion
3.1. Fabrication of 3D Micro-Blades Using High Resolution Two-Photon Laser Lithography in the Nanoscribe System
3.2. IP-S Resist Adhesion to Substrates with Different Surface Treatments
3.3. Double Replication Process to Generate Microfluidic Channels in PDMS
3.4. Cell Cutting Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, H.J. Cell dissection by hand. Anat. Rec. 1924, 28, 371–377. [Google Scholar] [CrossRef]
- Chambers, R. The microvivisection method. Biol. Bull. 1918, 34, 121–136. [Google Scholar] [CrossRef]
- Thalhammer, M.; Thalhammer, S.; Lahr, G.; Clement-Sengewald, A.; Heckl, W.M.; Burgemeister, R.; Schutze, K. Laser microtools in cell biology and molecular medicine. Laser Phys. 2003, 13, 681–691. [Google Scholar]
- König, K.; Riemann, I.; Fischer, P.; Halbhuber, K.J. Intracellular nanosurgery with near infrared femtosecond laser pulses. Cell Mol. Biol. 1999, 45, 195–201. [Google Scholar]
- Berns, M.W.; Aist, J.; Edwards, J.; Strahs, K.; Girton, J.; McNeill, P.; Rattner, J.B.; Kitzes, M.; Hammer-Wilson, M.; Liaw, L.H.; et al. Laser microsurgery in cell and developmental biology. Science 1981, 213, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Magidson, V.; Loncarek, J.; Hergert, P.; Rieder, C.L.; Khodjakov, A. Laser microsurgery in the GFP era: A cell biologist’s perspective. Laser Manip. Cells Tissues 2007, 82, 239–266. [Google Scholar]
- Blauch, L.R.; Gai, Y.; Khor, J.W.; Sood, P.; Marshall, W.F.; Tang, S.K.Y. Microfluidic guillotine for single-cell wound repair studies. Proc. Natl. Acad. Sci. USA 2017, 114, 7283–7288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.S.; Blauch, L.R.; Huang, W.; Marshall, W.F.; Tang, S.K.Y. Microfluidic guillotine reveals multiple timescales and mechanical modes of wound response in Stentor coeruleus. BMC Biol. 2021, 19, 63. [Google Scholar] [CrossRef]
- Toda, S.; Blauch, L.R.; Tang, S.K.Y.; Morsut, L.; Lim, W.A. Programming self-organizing multicellular structures with synthetic cell-cell signaling. Science 2018, 361, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyssat, E.; Tallinen, T.; Le Merrer, M.; Mahadevan, L. Slicing softly with shear. Phys. Rev. Lett. 2012, 109, 244301. [Google Scholar] [CrossRef]
- Atkins, A.G.; Xu, X.; Jeronimidis, G. Cutting, by ‘pressing and slicing,’ of thin floppy slices of materials illustrated by experiments on cheddar cheese and salami. J. Mater. Sci. 2004, 39, 2761–2766. [Google Scholar] [CrossRef]
- Chaudhury, M. A cut above the rest. Physics 2012, 5, 139. [Google Scholar] [CrossRef] [Green Version]
- LaFratta, C.; Baldacchini, T. Two-Photon Polymerization Metrology: Characterization Methods of Mechanisms and Microstructures. Micromachines 2017, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Selimis, A.; Mironov, V.; Farsari, M. Direct laser writing: Principles and materials for scaffold 3D printing. Microelectron. Eng. 2015, 132, 83–89. [Google Scholar] [CrossRef]
- Yin, K.; Wu, Z.; Wu, J.; Zhu, Z.; Zhang, F.; Duan, J.-A. Solar-driven thermal-wind synergistic effect on laser-textured superhydrophilic copper foam architectures for ultrahigh efficient vapor generation. Appl. Phys. Lett. 2021, 118, 211905. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Yin, K.; Zhu, Z.; Xiao, S.; Wu, Z.; Duan, J.-A. Robust Hierarchical Porous PTFE Film Fabricated via Femtosecond Laser for Self-Cleaning Passive Cooling. Nano Lett. 2021, 21, 4209–4216. [Google Scholar] [CrossRef]
- Tang, S.K.Y.; Marshall, W.F. Self-repairing cells: How single cells heal membrane ruptures and restore lost structures. Science 2017, 356, 1022–1025. [Google Scholar] [CrossRef] [Green Version]
- Lillie, F.R. On the smallest parts of stentor capable of regeneration; a contribution on the limits of divisibility of living matter. J. Morphol. 1896, 12, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Tartar, V. The Biology of Stentor. In International Series of Monographs on Pure and Applied Biology; Pergammon Press: Oxford, UK, 1961; Volume 5, ISBN 9780080093437. [Google Scholar]
- Huang, C.; Wippold, J.A.; Stratis-Cullum, D.; Han, A. Eliminating air bubble in microfluidic systems utilizing integrated in-line sloped microstructures. Biomed. Microdevices 2020, 22, 76. [Google Scholar] [CrossRef]
- Structure Adhesion—NanoGuide. Available online: https://support.nanoscribe.com/hc/en-gb/articles/360001346514-Structure-Adhesion (accessed on 19 August 2021).
- Slabodnick, M.M.; Ruby, J.G.; Dunn, J.G.; Feldman, J.L.; DeRisi, J.L.; Marshall, W.F. The kinase regulator mob1 acts as a patterning protein for stentor morphogenesis. PLoS Biol. 2014, 12, e1001861. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Curing Printed Structures—NanoGuide. Available online: https://support.nanoscribe.com/hc/en-gb/articles/360002022373 (accessed on 14 August 2021).
- Izard, A.G.; Garcia, E.P.; Dixon, M.; Potma, E.O.; Baldacchini, T.; Valdevit, L. Enhanced adhesion in two-photon polymerization direct laser writing. AIP Adv. 2020, 10, 045217. [Google Scholar] [CrossRef]
- Desai, S.P.; Freeman, D.M.; Voldman, J. Plastic masters-rigid templates for soft lithography. Lab Chip 2009, 9, 1631–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
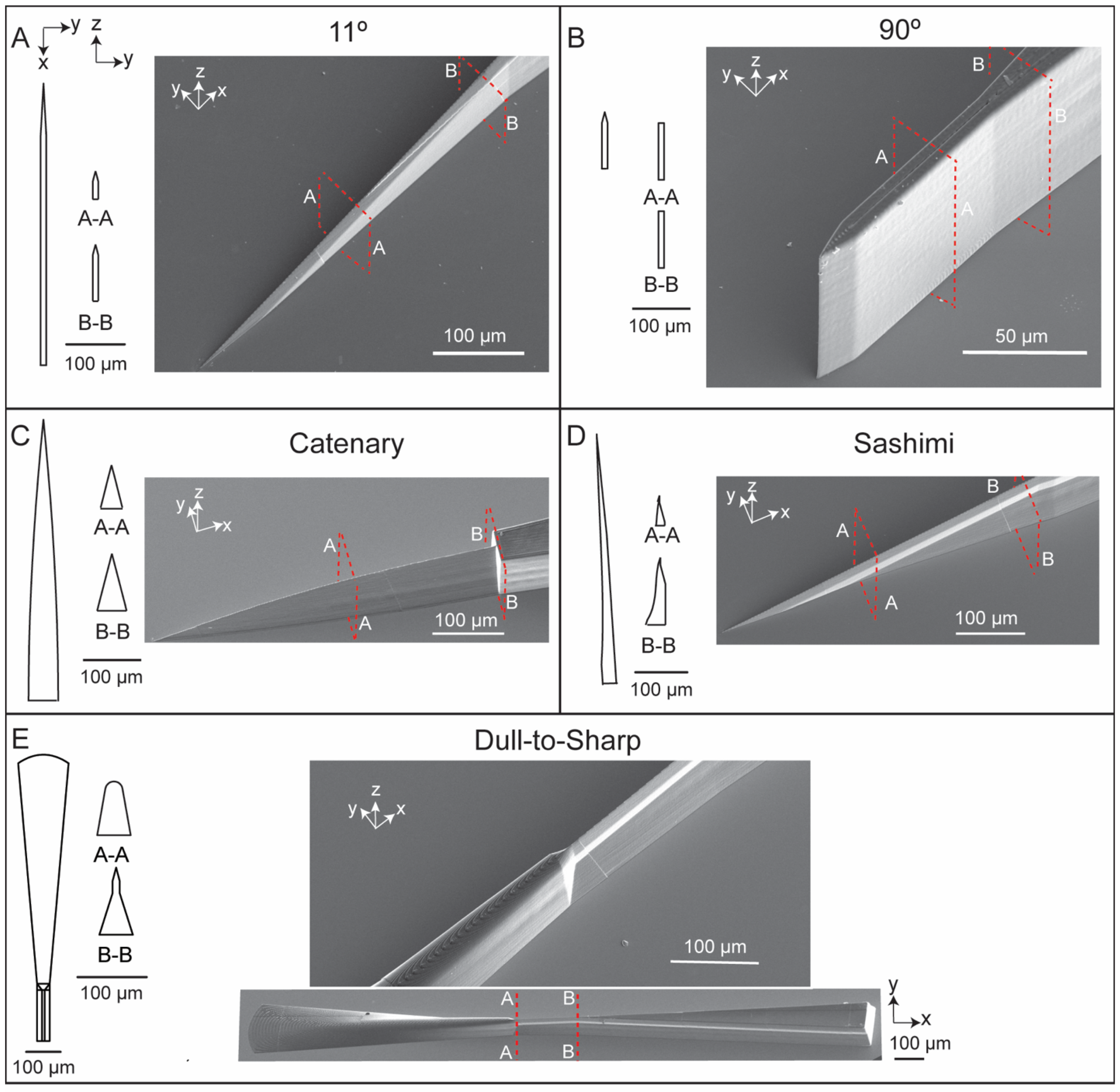
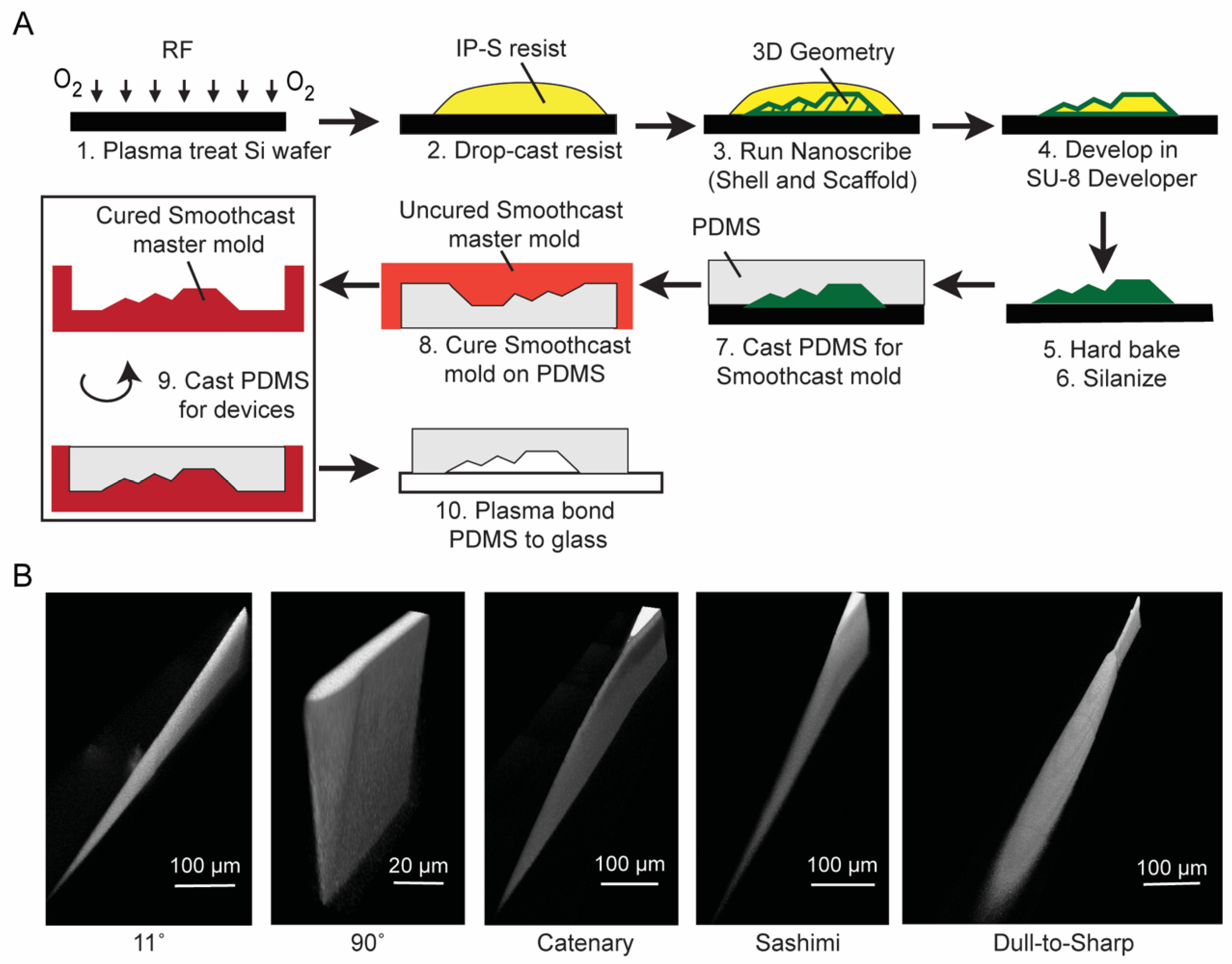
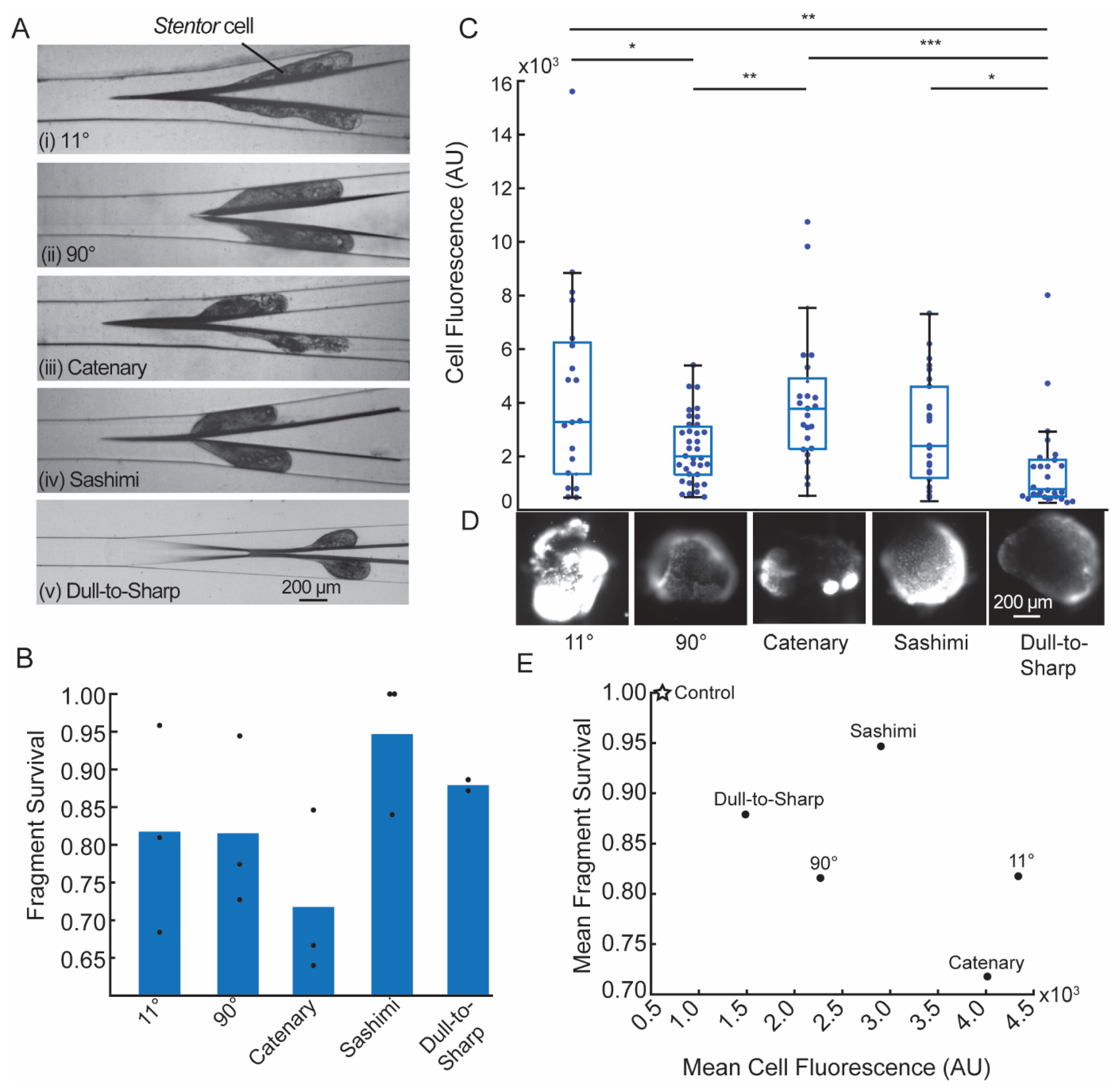
| Substrate | Development Time (Minutes) | Hard Bake Time at 190 °C (Minutes) | Number of Times PDMS Can Be Cured and Peeled off without Detaching Any IP-S Features from the Substrate |
|---|---|---|---|
| Si | 16 | 5 | 2 |
| Si + Plasma treatment | 16 | 5 | 2 |
| Si + HMDS | 15 | 2 | 1 |
| Si + 3-(trimethoxysilyl)propyl methacrylate (30 min) | 10 | 0.5 | 1 |
| Si + Plasma treatment + 3-(trimethoxysilyl)propyl methacrylate (2 h) | 10 | 0.5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppaka, S.; Zhang, K.S.; Kurosu Jalil, M.; Blauch, L.R.; Tang, S.K.Y. Fabrication of 3D Micro-Blades for the Cutting of Biological Structures in a Microfluidic Guillotine. Micromachines 2021, 12, 1005. https://doi.org/10.3390/mi12091005
Koppaka S, Zhang KS, Kurosu Jalil M, Blauch LR, Tang SKY. Fabrication of 3D Micro-Blades for the Cutting of Biological Structures in a Microfluidic Guillotine. Micromachines. 2021; 12(9):1005. https://doi.org/10.3390/mi12091005
Chicago/Turabian StyleKoppaka, Saisneha, Kevin S. Zhang, Myra Kurosu Jalil, Lucas R. Blauch, and Sindy K. Y. Tang. 2021. "Fabrication of 3D Micro-Blades for the Cutting of Biological Structures in a Microfluidic Guillotine" Micromachines 12, no. 9: 1005. https://doi.org/10.3390/mi12091005
APA StyleKoppaka, S., Zhang, K. S., Kurosu Jalil, M., Blauch, L. R., & Tang, S. K. Y. (2021). Fabrication of 3D Micro-Blades for the Cutting of Biological Structures in a Microfluidic Guillotine. Micromachines, 12(9), 1005. https://doi.org/10.3390/mi12091005






