Progress in Non-Traditional Processing for Fabricating Superhydrophobic Surfaces
Abstract
:1. Introduction
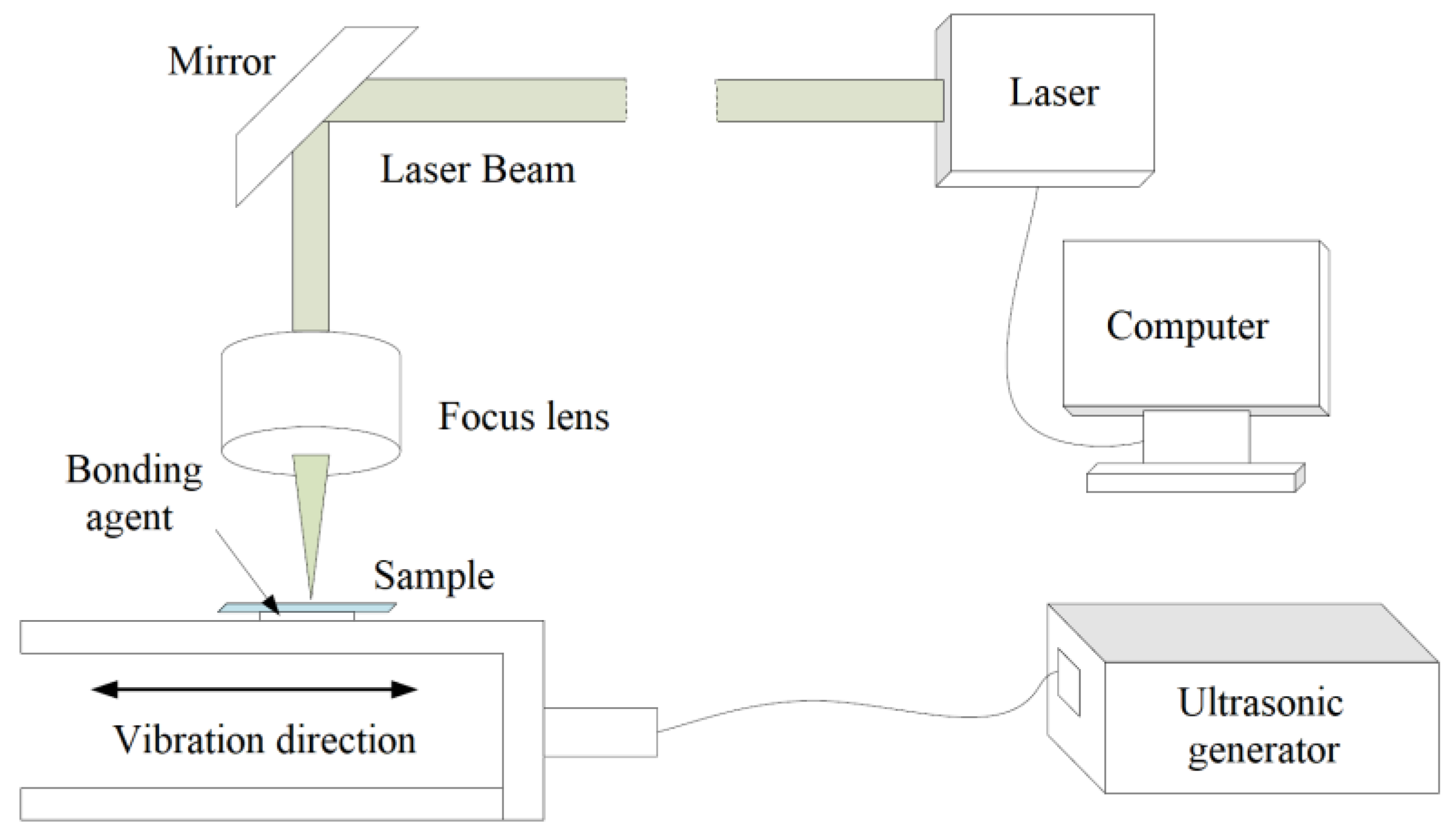
2. Laser Beam Machining
2.1. Principle of the LBM
2.2. Machining Performances of the LBM
2.3. Summary
3. Electrical Discharge Machining
3.1. Principle of the EDM
3.2. Machining Performances of the EDM
3.3. Summary
4. Electrochemical Machining
4.1. Principle of the ECM
4.2. Machining Performances of the ECM
4.2.1. Electrochemical Oxidation Process
4.2.2. Electrochemical Reduction Process
4.3. Summary
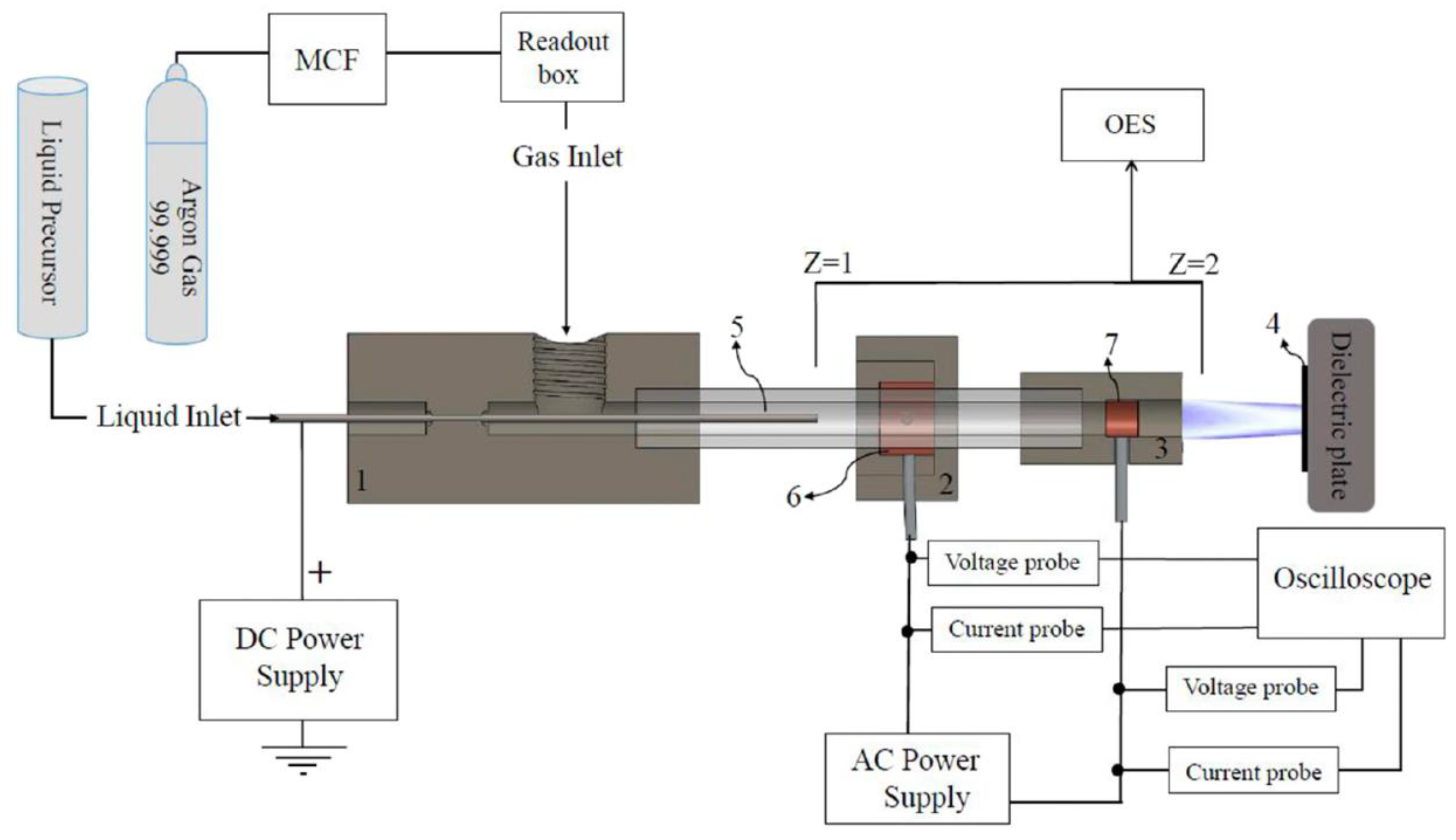
5. Other Non-Traditional Machining
5.1. USM
5.2. WJM
5.3. PSM
5.4. Summary
6. Discussion
7. Outlook
- For laser processing, femtosecond laser and picosecond laser have relatively little thermal effect on material removal processing. However, compared with nanosecond laser, their equipment purchase costs are high, and the machining costs are also high. For the manufacture of superhydrophobic micro-structures, femtosecond laser and picosecond laser methods must consider their enthusiasm and reduce manufacturing costs. In the future, manufacturing costs need to be considered as a constraint or an optimization goal.
- As the EDM process is restricted by its technology [97], it is difficult to fabricate surfaces with superhydrophobic micro-structures from non-conductive materials (glass and non-conductive ceramic materials). In the future, a breakthrough in the EDM technology should be addressed. In addition, special superhydrophobic micro-structures with good preparation properties should combine the EDM process with other machining method, such as laser processing and ultrasonic machining methods.
- When the ECM process is used to manufacture superhydrophobic surfaces, many researches mainly use template-free processing technique, which is simple and economical. However, the template-free electrochemical machining method is difficult for micro-channels, array micro-holes, and other surfaces with high-precision requirements. It is necessary to prepare high-precision electrodes and to overcome most of the problems related to processing technology. Therefore, the cost of large-scale manufacturing of superhydrophobic materials can be further reduced.
- It is not very common for the USM or WJM processes to independently fabricate superhydrophobic surfaces. It is necessary to further expand the study of related mechanisms/processes and establish related simulation models to improve the quality of machining process. The machining mechanism of USM or WJM processes combined with other non-traditional machining methods also needs to be elaborated through the establishment of related models. In addition, the machining economy of the PSM process also needs attention in future research.
- Due to the particularity of non-traditional processing, some processing techniques have a negative impact on the environment. For example, the harmless treatment of waste electrolyte in electrochemical machining, and the toxic gas of oily dielectric in EDM machining. For the future manufacturing of large-scale superhydrophobic materials, not only processing time and processing costs, but also environmental impacts need to be considered, such as the use of environmentally friendly electrolytes, optimization of laser processing energy, and so on.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
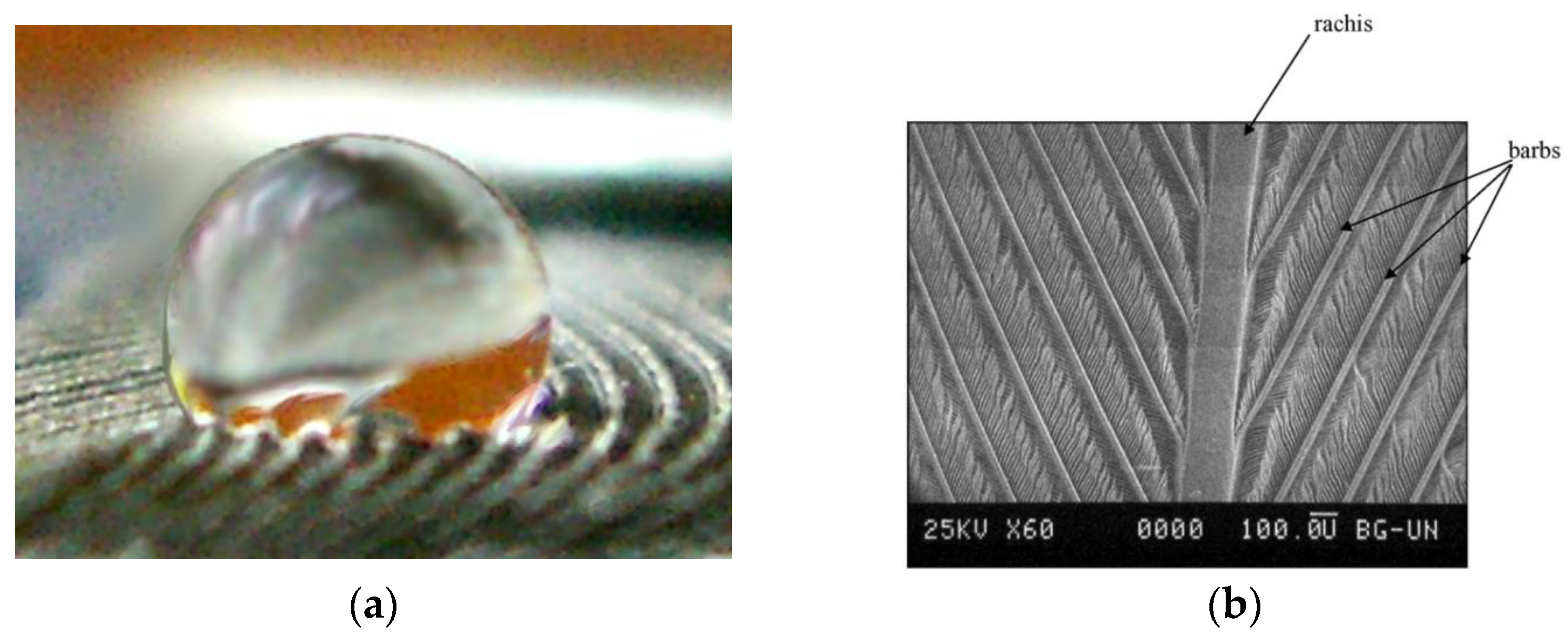
- Bormashenko, E.; Bormashenko, Y.; Stein, T.; Whyman, G. Why do pigeon feathers repel water? Hydrophobicity of pennae, Cassie-Baxter wetting hypothesis and Cassie-Wenzel capillarity-induced wetting transition. J. Colloid Interface Sci. 2007, 311, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, X.; Liu, H.; Quéré, D.; Jiang, L. Self-removal of condensed water on the legs of water striders. Proc. Natl. Acad. Sci. USA 2015, 112, 9247–9252. [Google Scholar] [CrossRef] [Green Version]
- Ball, P. Engineering shark skin and other solutions. Nature 1999, 400, 507–509. [Google Scholar] [CrossRef]
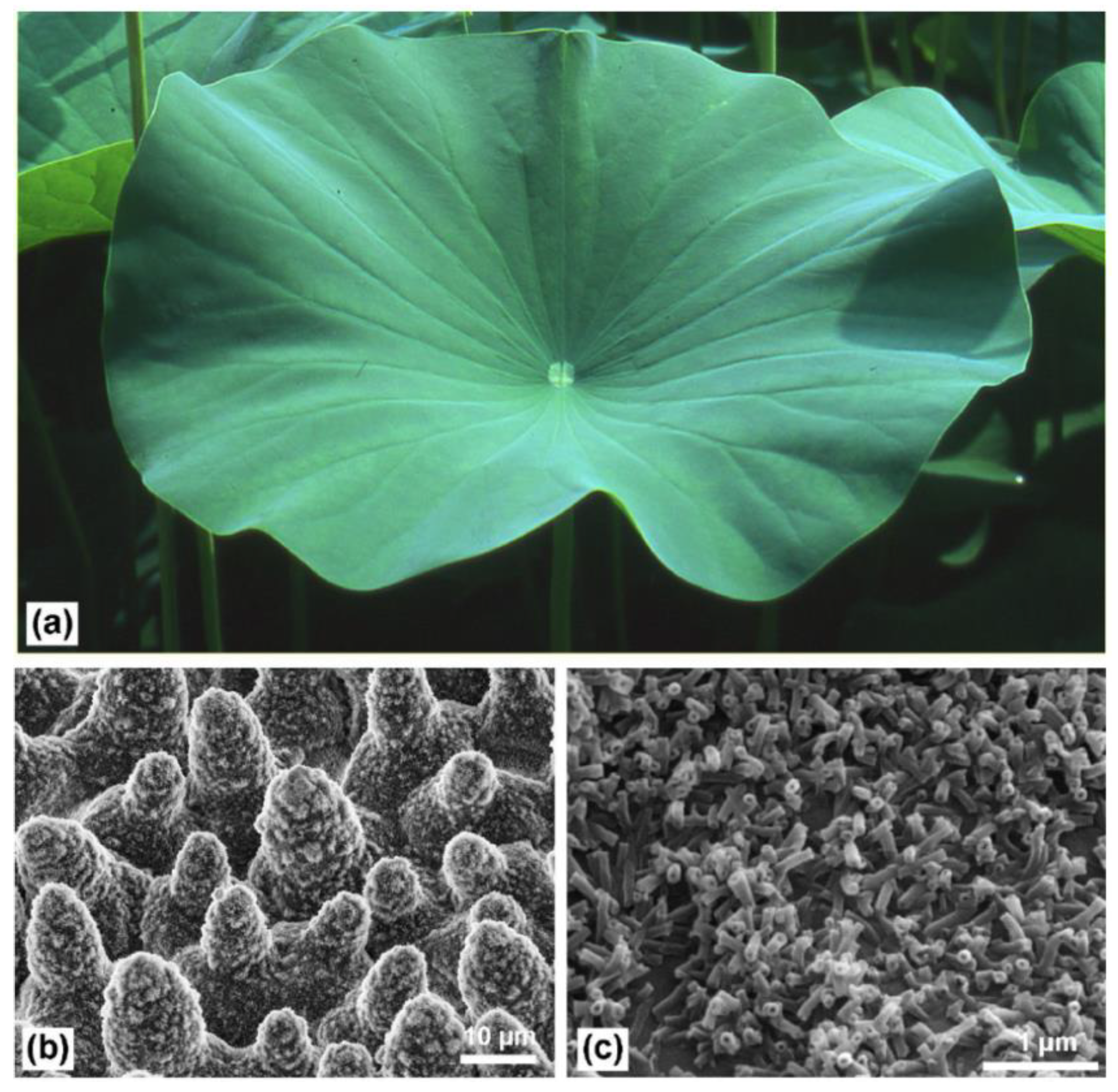
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Neinhuis, C. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Development and Military Application of Superhydrophobic Material Technology. 2021. Available online: https://www.sohu.com/a/233229801_465915 (accessed on 2 July 2021).
- Technology Development and Military Application Prospect of Superhydrophobic Materials Abroad. 2018. Available online: http://www.ecorr.org/dhTJDAOHANG/xinxiziyuan/kepuqikan/2018-09-18/3414.html (accessed on 2 July 2021).
- Wang, Z.; Zheng, H.; Lam, Y. Investigation on femtosecond laser irradiation energy in inducing hydrophobic polymer surfaces. Appl. Surf. Sci. 2011, 257, 10427–10433. [Google Scholar] [CrossRef]
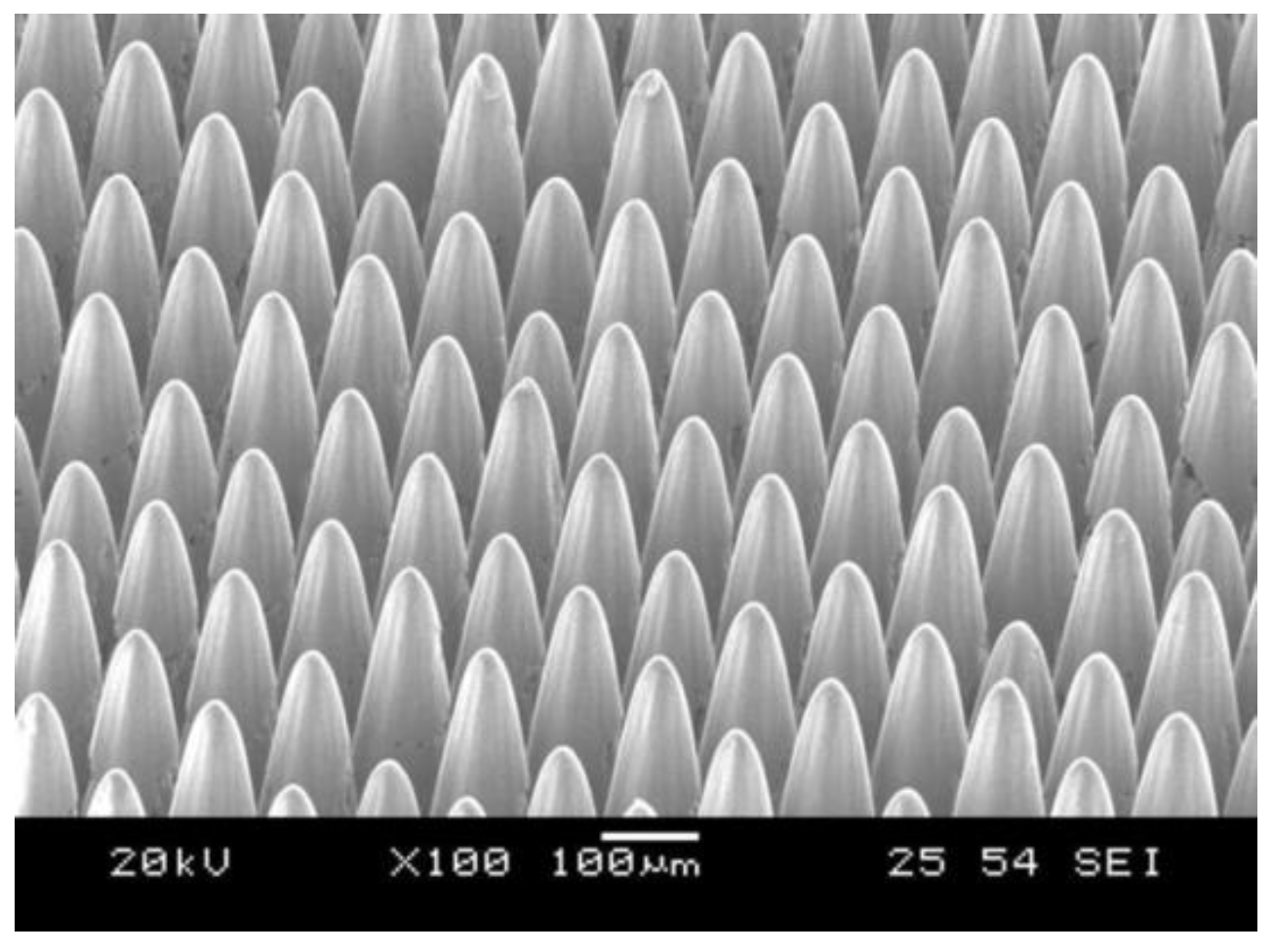
- Lee, S.W.; Shin, H.S.; Chu, C.N. Fabrication of micro-pin array with high aspect ratio on stainless steel using nanosecond laser beam machining. Appl. Surf. Sci. 2013, 264, 653–663. [Google Scholar] [CrossRef]
- Xiao, S.; Hao, X.; Yang, Y.; Li, L.; He, N.; Li, H. Feasible fabrication of a wear-resistant hydrophobic surface. Appl. Surf. Sci. 2018, 463, 923–930. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Y.; Yang, Z.; Yang, C.; Li, L.; Wang, S.; Wang, M. The simulation of droplet impact on the super-hydrophobic surface with micro-pillar arrays fabricated by laser irradiation and silanization processes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 612, 125966. [Google Scholar] [CrossRef]
- Sun, X.; Wang, K.; Fan, Z.; Wang, R.; Mei, X.; Lu, Y. Regulation of hydrophobicity on yttria stabilized zirconia surface by femtosecond laser. Ceram. Int. 2020, 47, 9264–9272. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, W.; Luo, X.; Sousa, A.M.; Lau, K.H.A.; Qin, Y. Superhydrophobic structures on 316L stainless steel surfaces machined by nanosecond pulsed laser. Precis. Eng. 2018, 52, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, Z.; Deng, X.; Peng, M.; Wan, Y.; Zhou, J.; Ouyang, C.; Yao, F.; Luo, H. Fabrication of a gradient hydrophobic surface with parallel ridges on pyrolytic carbon for artificial heart valves. Colloids Surf. B Biointerfaces 2021, 205, 111894. [Google Scholar] [CrossRef]
- Wang, Z.; Nandyala, D.; Colosqui, C.E.; Cubaud, T.; Hwang, D.J. Glass surface micromachining with simultaneous nanomaterial deposition by picosecond laser for wettability control. Appl. Surf. Sci. 2021, 546, 149050. [Google Scholar] [CrossRef]
- Bae, W.-G.; Kim, D.; Song, K.Y.; Jeong, H.E.; Chu, C.N. Engineering stainless steel surface via wire electrical discharge machining for controlling the wettability. Surf. Coat. Technol. 2015, 275, 316–323. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Gao, H.; Jie, X.; Lian, W. Experimental study on the anti-fouling effects of EDM machined hierarchical micro/nano structure for heat transfer surface. Appl. Therm. Eng. 2019, 162. [Google Scholar] [CrossRef]
- Deng, D.; Zheng, J.; Chen, X.; Sun, W. Fabrication and characterization of CuO nanowires on V-shaped microgroove surfaces. Curr. Appl. Phys. 2021, 28, 26–34. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Z.; Zhou, H.; Han, F.; Zhao, L.; Yan, H. One-step fabrication of the wear-resistant superhydrophobic structure on SiCp/Al composite surface by WEDM. Surf. Coat. Technol. 2021, 409, 126876. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Wu, F.; Zhang, H.; Gao, X.; Jiang, L. Copper-based high-efficiency condensation heat transfer interface consisting of superhydrophobic hierarchical microgroove and nanocone structure. Mater. Today Phys. 2021, 19, 100407. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Liang, Z.; Yue, T.; Guo, Z.; Liu, J.; Chen, X. Fabrication of a superhydrophobic mesh via magnetically aided electrode electric discharge machining. Colloids Surf. A Physicochem. Eng. Asp. 2020, 612, 125963. [Google Scholar] [CrossRef]
- Peta, K.; Bartkowiak, T.; Galek, P.; Mendak, M. Contact angle analysis of surface topographies created by electric discharge machining. Tribol. Int. 2021, 107139. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Zhao, H.; Xu, B.; Zhu, L.; Liu, Y.; Gao, C. Effect of sub-millimetre morphologies on the hydrophobicity of a copper surface prepared by WEDM. Surf. Coat. Technol. 2020, 385, 125455. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Liu, Z.; Ma, J.; Zhang, Z.; Ruan, W.; Ren, S.; Peng, T.; Wu, X.; Shi, H. Ultrasonic injection molding of glass fiber reinforced polypropylene parts using tungsten carbide-cobalt mold core. Mater. Des. 2021, 205, 109771. [Google Scholar] [CrossRef]
- Chu, X.; Zeng, X.; Zhuang, W.; Zhou, W.; Quan, X.; Fu, T. Vibration assisted high-speed wire electric discharge machining for machining surface microgrooves. J. Manuf. Process. 2019, 44, 418–426. [Google Scholar] [CrossRef]
- Shirsath, G.; Muralidhar, K.; Pala, R.G.S.; Ramkumar, J. Condensation of water vapor underneath an inclined hydrophobic textured surface machined by laser and electric discharge. Appl. Surf. Sci. 2019, 484, 999–1009. [Google Scholar] [CrossRef]
- Ming, W.; Shen, F.; Zhang, Z.; Huang, H.; Du, J.; Wu, J. A comparative investigation on magnetic field assisted EDM of magnetic and non-magnetic materials. Int. J. Adv. Manuf. Technol. 2020, 109, 1103–1116. [Google Scholar] [CrossRef]
- Ming, W.; Jia, H.; Zhang, H.; Zhang, Z.; Liu, K.; Du, J.; Shen, F.; Zhang, G. A comprehensive review of electric discharge machining of advanced ceramics. Ceram. Int. 2020, 46, 21813–21838. [Google Scholar] [CrossRef]
- Ming, W.; Ma, J.; Zhang, Z.; Huang, H.; Shen, D.; Zhang, G.; Huang, Y. Soft computing models and intelligent optimization system in electro-discharge machining of SiC/Al composites. Int. J. Adv. Manuf. Technol. 2016, 1–17. [Google Scholar] [CrossRef]
- Gotoh, H.; Tani, T.; Mohri, N.; Fukuzawa, Y. Machining of insulating ceramics by EDM with Torque control axis: Machining characteristics of insulating Si3N4 ceramics. Proc. Manuf. Mach. Tool Conf. 2004, 2004.5, 287–288. [Google Scholar] [CrossRef]
- Gotoh, H.; Tani, T.; Mohri, N. EDM of insulating ceramics by electrical conductive surface layer control. Procedia CIRP 2016, 42, 201–205. [Google Scholar] [CrossRef]
- McGeough, J.A.; Barker, M.B. Electrochemical Machining Development and Application; American Chemical Society: Washington, DC, USA, 1989; pp. 578–588. [Google Scholar] [CrossRef]
- Kozak, J.; Rajurkar, K.P.; Makkar, Y. Selected problems of micro-electrochemical machining. J. Mater. Process. Technol. 2004, 149, 426–431. [Google Scholar] [CrossRef]
- Sivaprasad, P.V.; Panneerselvam, K.; Haq, A.N. A comparative assessment in sequential μ-drilling of Hastelloy-X using laser in combination with μ-EDM and μ-ECM. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 1–19. [Google Scholar] [CrossRef]
- Wu, T.; Suzuki, Y. Design, microfabrication and evaluation of robust high-performance superlyophobic surfaces. Sens. Actuators B Chem. 2011, 156, 401–409. [Google Scholar] [CrossRef]
- Feng, L.; Song, Y.; Zhai, J.; Liu, B.; Xu, J.; Jiang, L.; Zhu, D. Creation of a superhydrophobic surface from an amphiphilic polymer. Angew. Chem. Int. Ed. 2003, 42, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Z.; Cheng, B.; DeSimone, J.M.; Samulski, E.T. Superhydrophobic behavior of a perfluoropolyether lotus-leaf-like topography. Langmuir 2006, 22, 8576–8580. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.; Joseph, K.R.; Brant, W.R. On the superhydrophobic properties of nickel nanocarpets. Phys. Chem. Chem. Phys. 2009, 11, 9537–9544. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.; Bhavitha, K.; Bracco, G.; Luciano, G.; Cavallo, D.; Paolini, G.; Passaglia, S.; Carraro, G.; Vattuone, L.; Masini, R.; et al. Correlating hydrophobicity to surface chemistry of microstructured aluminium surfaces. Appl. Surf. Sci. 2020, 542, 148574. [Google Scholar] [CrossRef]
- Wang, M.-F.; Raghunathan, N.; Ziaie, B. A Nonlithographic top-down electrochemical approach for creating hierarchical (micro-nano) superhydrophobic silicon surfaces. Langmuir 2007, 23, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Nenzi, P.; Giacomello, A.; Bolognesi, G.; Chinappi, M.; Balucani, M.; Casciola, C.M. Superhydrophobic porous silicon surfaces. Sens. Transducers 2011, 13, 62. [Google Scholar]
- Wang, D.; Wang, X.; Liu, X.; Zhou, F. Engineering a titanium surface with controllable oleophobicity and switchable oil adhesion. J. Phys. Chem. C 2010, 114, 9938–9944. [Google Scholar] [CrossRef]
- Wu, X.; Shi, G. Production and characterization of stable superhydrophobic surfaces based on copper hydroxide nanoneedles mimicking the legs of water striders. J. Phys. Chem. B 2006, 110, 11247–11252. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zheng, M.; Yao, L.; Yuan, X.; Li, M.; Ma, L.; Shen, W. Preparation and properties of ZnO nanostructures by electrochemical anodization method. Appl. Surf. Sci. 2010, 256, 2557–2562. [Google Scholar] [CrossRef]
- Yang, S.; Habazaki, H.; Fujii, T.; Aoki, Y.; Skeldon, P.; Thompson, G.E. Control of morphology and surface wettability of anodic niobium oxide microcones formed in hot phosphate–glycerol electrolytes. Electrochim. Acta 2011, 56, 7446–7453. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Yan, J.; Ye, Q.; Zhou, F. Template-free and direct electrochemical deposition of hierarchical dendritic gold microstructures: Growth and their multiple applications. J. Phys. Chem. C 2010, 114, 15617–15624. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Z.; Zhang, X. Combining a layer-by-layer assembling technique with electrochemical deposition of gold aggregates to mimic the legs of water striders. Adv. Mater. 2005, 17, 1005–1009. [Google Scholar] [CrossRef]
- Ren, H.-X.; Huang, X.-J.; Yarimaga, O.; Choi, Y.-K.; Gu, N. A cauliflower-like gold structure for superhydrophobicity. J. Colloid Interface Sci. 2009, 334, 103–107. [Google Scholar] [CrossRef]
- Shepherd, J.L.; Clement, J.; McGillivary, L. Friction titration measurements of electrochemically generated mixed alkylthiol monolayers on polycrystalline gold. Electrochim. Acta 2020, 340, 135937. [Google Scholar] [CrossRef]
- Zhao, N.; Shi, F.; Wang, Z.; Zhang, X. Combining layer-by-layer assembly with electrodeposition of silver aggregates for fabricating superhydrophobic surfaces. Langmuir 2005, 21, 4713–4716. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, T.-Y. Electrochemical synthesis of silver polyhedrons and dendritic films with superhydrophobic surfaces. Langmuir 2008, 24, 12010–12016. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sarkar, D.K.; Chen, X.-G. Fabrication of superhydrophobic surfaces on aluminum alloy via electrodeposition of copper followed by electrochemical modification. Nano-Micro Lett. 2011, 3, 160–165. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jiang, C.; Yin, H.; Chen, J.; Yuan, W. Superhydrophobic silicon surfaces with micro–nano hierarchical structures via deep reactive ion etching and galvanic etching. J. Colloid Interface Sci. 2011, 364, 219–229. [Google Scholar] [CrossRef]
- Huang, S.; Hu, Y.; Pan, W. Relationship between the structure and hydrophobic performance of Ni-TiO2 nanocomposite coatings by electrodeposition. Surf. Coat. Technol. 2011, 205, 3872–3876. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Zhao, G.; Taleb, A.; Jin, Y. Fabrication of NiCo coating by electrochemical deposition with high super-hydrophobic properties for corrosion protection. Surf. Coat. Technol. 2019, 363, 352–361. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Ban, C.; Xue, Y.; Taleb, A.; Jin, Y. Fabrication of robust tungsten carbide particles reinforced CoNi super-hydrophobic composite coating by electrochemical deposition. Surf. Coat. Technol. 2020, 385, 125390. [Google Scholar] [CrossRef]
- Thoe, T.; Aspinwall, D.; Wise, M. Review on ultrasonic machining. Int. J. Mach. Tools Manuf. 1998, 38, 239–255. [Google Scholar] [CrossRef]
- Singh, R.; Khamba, J. Ultrasonic machining of titanium and its alloys: A review. J. Mater. Process. Technol. 2006, 173, 125–135. [Google Scholar] [CrossRef]
- Bhosale, S.; Pawade, R.S.; Brahmankar, P. Effect of process parameters on MRR, TWR and surface topography in ultrasonic machining of alumina-zirconia ceramic composite. Ceram. Int. 2014, 40, 12831–12836. [Google Scholar] [CrossRef]
- Acherjee, B.; Maity, D.; Kuar, A.S. Ultrasonic machining process optimization by cuckoo search and chicken swarm optimization algorithms. Int. J. Appl. Metaheuristic Comput. 2020, 11, 1–26. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Zheng, H.; Thwe, A.; Lam, Y.C. Investigation on polycarbonate surface wetting property with femtosecond laser irradiation and ultrasonic treatment. Opt. Laser Technol. 2019, 115, 316–324. [Google Scholar] [CrossRef]
- Guo, F.; Yang, X.; Jiang, H.; Zhu, Y.; Li, C. An ultrasonic atomization spray strategy for constructing hydrophobic and hydrophilic synergistic surfaces as gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2020, 451, 227784. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.-J.; Chen, S.-G.; Ma, J.; Wu, X.-Y.; Shi, H.-Y.; Fu, L.-Y.; Xu, B. Fabrication of microplastic parts with a hydrophobic surface by micro ultrasonic powder moulding. J. Manuf. Process. 2020, 56, 180–188. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Z.; Zhao, J.; Shrotriya, P.; Wang, Y.; Cui, Y.; Guo, Z. Ultrasonic vibration assisted laser (UVAL) treatment of copper for superhydrophobicity. Surf. Coat. Technol. 2021, 421, 127386. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Wu, Z.; Luo, J.; Li, B.; Huang, X.; Xue, H.; Gao, J. Super-hydrophobic, durable and cost-effective carbon black/rubber composites for high performance strain sensors. Compos. Part B Eng. 2019, 176. [Google Scholar] [CrossRef]
- Kovacevic, R.; Hashish, M.; Mohan, R.; Ramulu, M.; Kim, T.J.; Geskin, E.S. State of the art of research and development in abrasive waterjet machining. J. Manuf. Sci. Eng. 1997, 119, 776–785. [Google Scholar] [CrossRef]
- Ramulu, M.; Kunaporn, S.; Arola, D.; Hashish, M.; Hopkins, J. Waterjet machining and peening of metals. J. Press. Vessel Technol. 1999, 122, 90–95. [Google Scholar] [CrossRef]
- Parikh, P.J.; Lam, S.S. Parameter estimation for abrasive water jet machining process using neural networks. Int. J. Adv. Manuf. Technol. 2008, 40, 497–502. [Google Scholar] [CrossRef]
- Wenjun, G.; Jianming, W.; Na, G. Numerical simulation for abrasive water jet machining based on ALE algorithm. Int. J. Adv. Manuf. Technol. 2010, 53, 247–253. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, S.; Wu, L.Y.; Zheng, H.Y.; Królczyk, G. Functional grading of surfaces through hybrid ultrasonic, abrasive water jet, and electric discharge machining processing. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 1–8. [Google Scholar] [CrossRef]
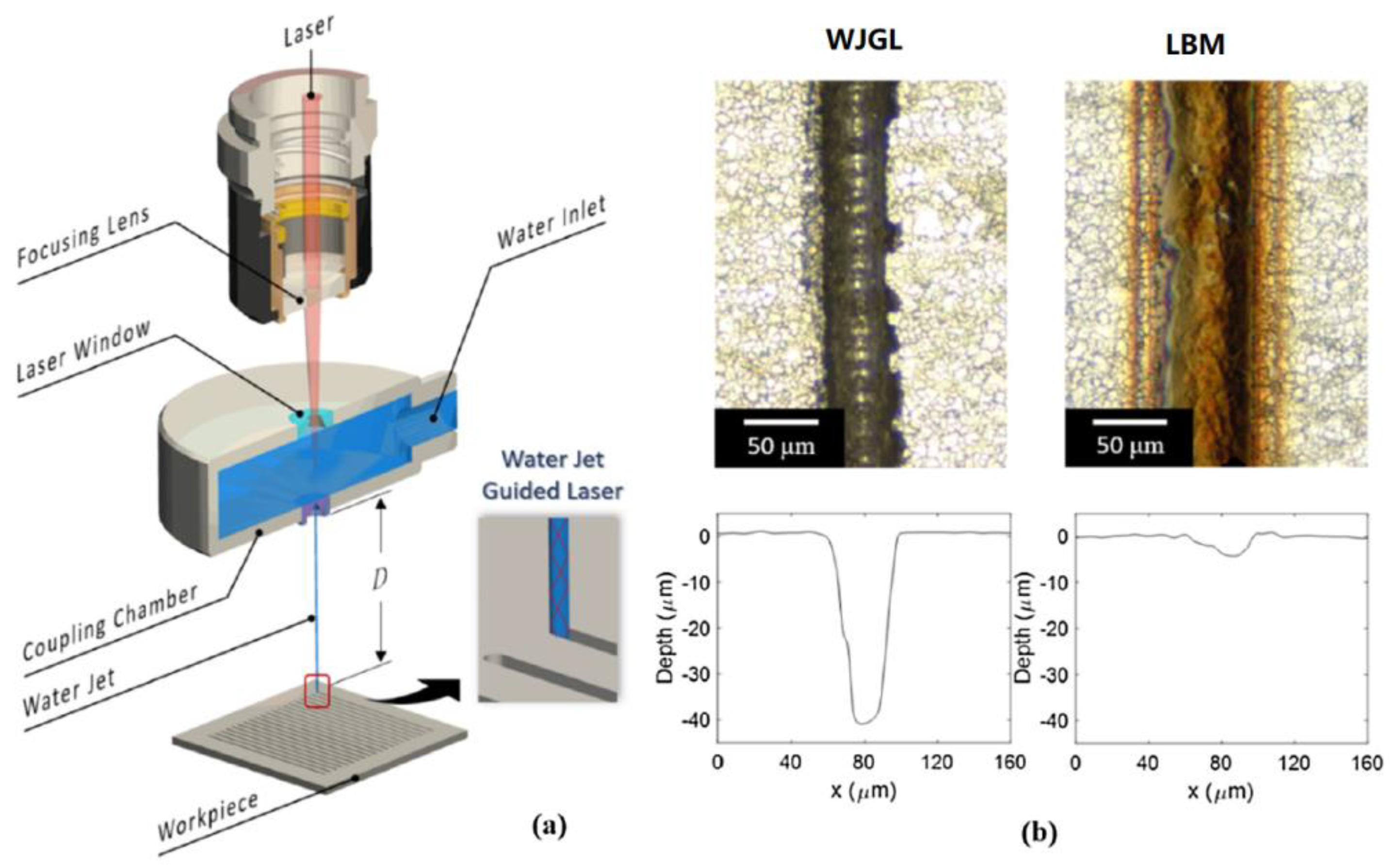
- Shi, Y.; Jiang, Z.; Cao, J.; Ehmann, K.F. Texturing of metallic surfaces for superhydrophobicity by water jet guided laser micro-machining. Appl. Surf. Sci. 2019, 500, 144286. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Nakashima, Y.; van der Heide, E. Microstructuring glass surfaces using a combined masking and microslurry-jet machining process. Precis. Eng. 2020, 67, 172–177. [Google Scholar] [CrossRef]
- Yang, L.-J.; Li, P.-H.; Fu, Q.-F. Liquid sheet formed by a Newtonian jet obliquely impinging on pro/hydrophobic surfaces. Int. J. Multiph. Flow 2019, 125, 103192. [Google Scholar] [CrossRef]
- Barletta, M.; Rubino, G.; Guarino, S.; Bolelli, G.; Lusvarghi, L.; Gisario, A. Fast regime-fluidized bed machining (FR-FBM) of atmospheric plasma spraying (APS) TiO2 coatings. Surf. Coat. Technol. 2008, 203, 855–861. [Google Scholar] [CrossRef]
- Lin, C.-M. Parameter optimisation of a vacuum plasma spraying process using boron carbide. J. Therm. Spray Technol. 2012, 21, 873–881. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Park, T.-S.; Adomako, N.; Ashong, A.-N.; Kim, Y.-K.; Yang, S.-M.; Kim, J.-H. Interfacial structure and physical properties of high-entropy oxide coatings prepared via atmospheric plasma spraying. Coatings 2021, 11, 755. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shakerinasab, E.; Eshghabadi, M.; Ghasemi, M. Characterization and performance of coupled atmospheric pressure argon plasma jet with n-hexane electrospray for hydrophobic layer coatings on cotton textile. Diam. Relat. Mater. 2018, 91, 34–45. [Google Scholar] [CrossRef]
- Ting, J.A.S.; Rosario, L.M.D.; Lee, H.V.; Ramos, H.J.; Tumlos, R.B. Hydrophobic coating on glass surfaces via application of silicone oil and activated using a microwave atmospheric plasma jet. Surf. Coat. Technol. 2014, 259, 7–11. [Google Scholar] [CrossRef]
- Asadollahi, S.; Profili, J.; Farzaneh, M.; Stafford, L. Multi-pass deposition of organosilicon-based superhydrophobic coatings in atmospheric pressure plasma jets. Thin Solid Films 2020, 714, 138369. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhang, Y.; Liu, D.; Wu, J.; Huang, Y.; Zhang, G. Study on machining characteristics of magnetically controlled laser induced plasma micro-machining single-crystal silicon. J. Adv. Res. 2020, 30, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kliuev, M.; Wiessner, M.; Büttner, H.; Maradia, U.; Wegener, K. Super-hydrophobic and Super-hydrophilic effect by means of EDM surface structuring of γ-TiAl. Procedia CIRP 2020, 95, 393–398. [Google Scholar] [CrossRef]
- Park, J.; Jeong, S.; Lee, H.; Jeong, H.; Lee, E.-S. A study on the chemical mechanical micro-machining (C3M) process and its application. J. Mater. Process. Technol. 2002, 130–131, 390–395. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, B.; Wang, J.; Jin, H.; Zhang, Y.; Dong, S. Chemical machining of Zerodur material with atmospheric pressure plasma jet. CIRP Ann. 2010, 59, 337–340. [Google Scholar] [CrossRef]
- Pandey, P.C.; Shan, H.S. Modern Machining Processes; McGraw Hill: New Delhi, India, 1980; ISBN 978-93-8676-183-5. [Google Scholar]
- Ho, K.; Newman, S. State of the art electrical discharge machining (EDM). Int. J. Mach. Tools Manuf. 2003, 43, 1287–1300. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, Y.; Kong, L.; Xu, M.; Ji, F. An Investigation into accumulative difference mechanism in time and space for material removal in micro-EDM milling. Micromachines 2021, 12, 711. [Google Scholar] [CrossRef]
- Quarto, M.; D’Urso, G.; Giardini, C.; Maccarini, G.; Carminati, M. A comparison between finite element model (FEM) simulation and an integrated artificial neural network (ANN)-particle swarm optimization (PSO) approach to forecast performances of micro electro discharge machining (micro-EDM) drilling. Micromachines 2021, 12, 667. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Hsu, C.-N.; Li, C.-R.; Lin, Y.-H.; Hsiao, W.-T.; Huang, K.-C.; Yeh, J. Surface wettability and electrical resistance analysis of droplets on indium-tin-oxide glass fabricated using an ultraviolet laser system. Micromachines 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S. Dynamics behaviors of droplet on hydrophobic surfaces driven by electric field. Micromachines 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, W.; Shen, F.; Zhang, G.; Liu, G.; Du, J.; Chen, Z. Green machining: A framework for optimization of cutting parameters to minimize energy consumption and exhaust emissions during electrical discharge machining of Al 6061 and SKD 11. J. Clean. Prod. 2020, 285, 124889. [Google Scholar] [CrossRef]




| Laser Type | Object | Purpose | Findings | Remarks |
|---|---|---|---|---|
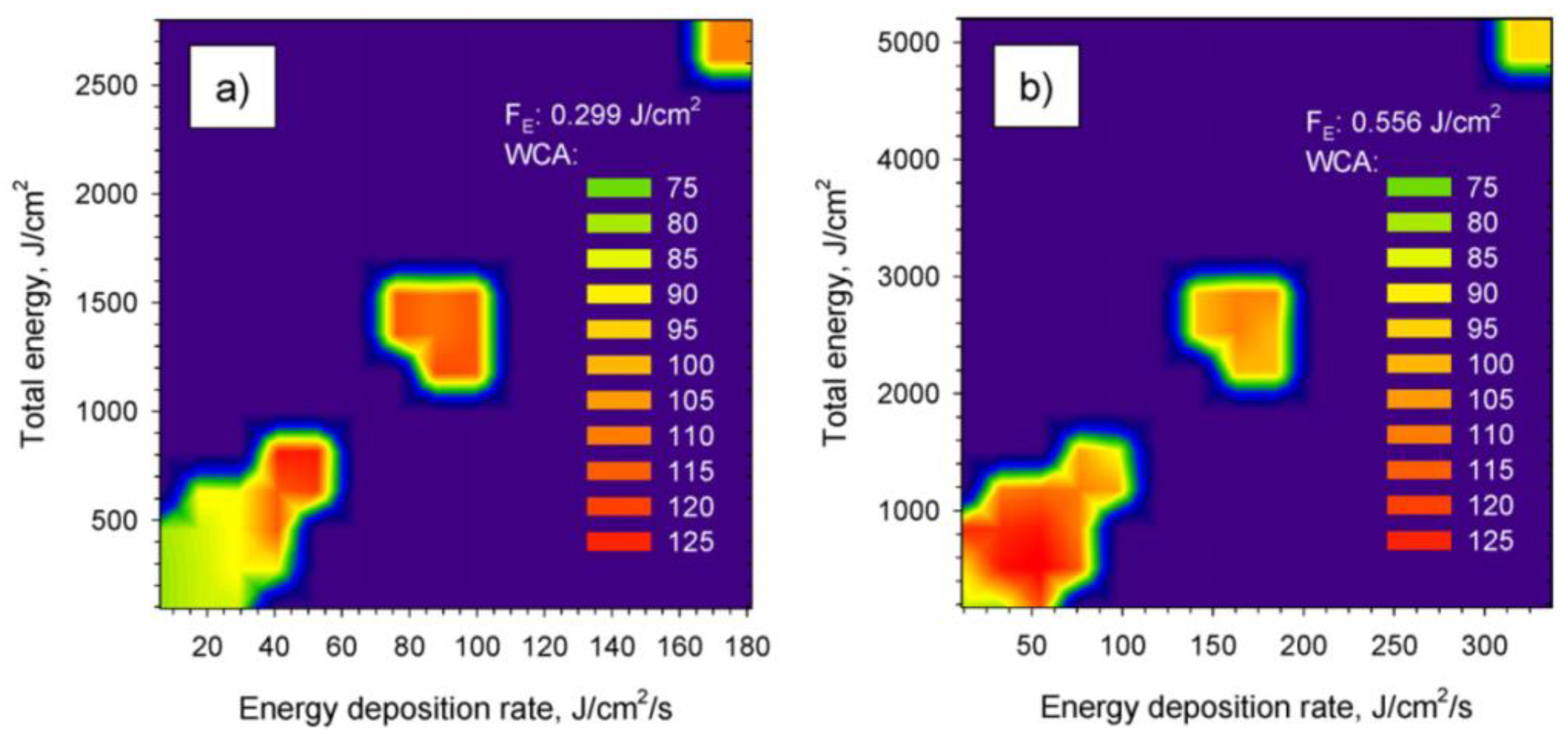
| Femtosecond laser | PMMA |  Inducing hydrophobic polymer surfaces | The WCA as a function of the energy deposition rate and the total energy deposited on the PMMA surface [14]. | Different energies have an effect on the ratio of induced polar groups and non-polar groups, resulting in different surface hydrophobicity. |
| YSZ surfaces |  Fabrication of micro/nano composite structures | Laser texturing greatly improved the hydrophobicity of the YSZ surface, and the WCA increased from 86.7° to 151.8° [18]. | Laser processing changes the ratio of polar groups to non-polar groups and ultimately improves the hydrophobicity of the surface. | |
| Picosecond laser | Glass | 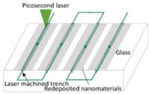 Fabricating multi-hierarchical micro/nano structures | A multi-level micro/nano structure could be fabricated in one step on any glass surface [21]. | Quickly and effectively fabricate a liquid injection surface on glass materials. |
| Nanosecond laser | AISI 304 | 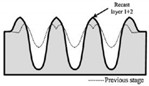 Fabrication of a micro-pin array with a high aspect ratio | The fabricated micro-pins array revealed adhesion on the vertical wall, and could produce 38.6 mN adhesion on the Ra 4.594 μm vertical wall [15]. | The developed micro-pin array can be used in vertical surface attachment scenarios. |
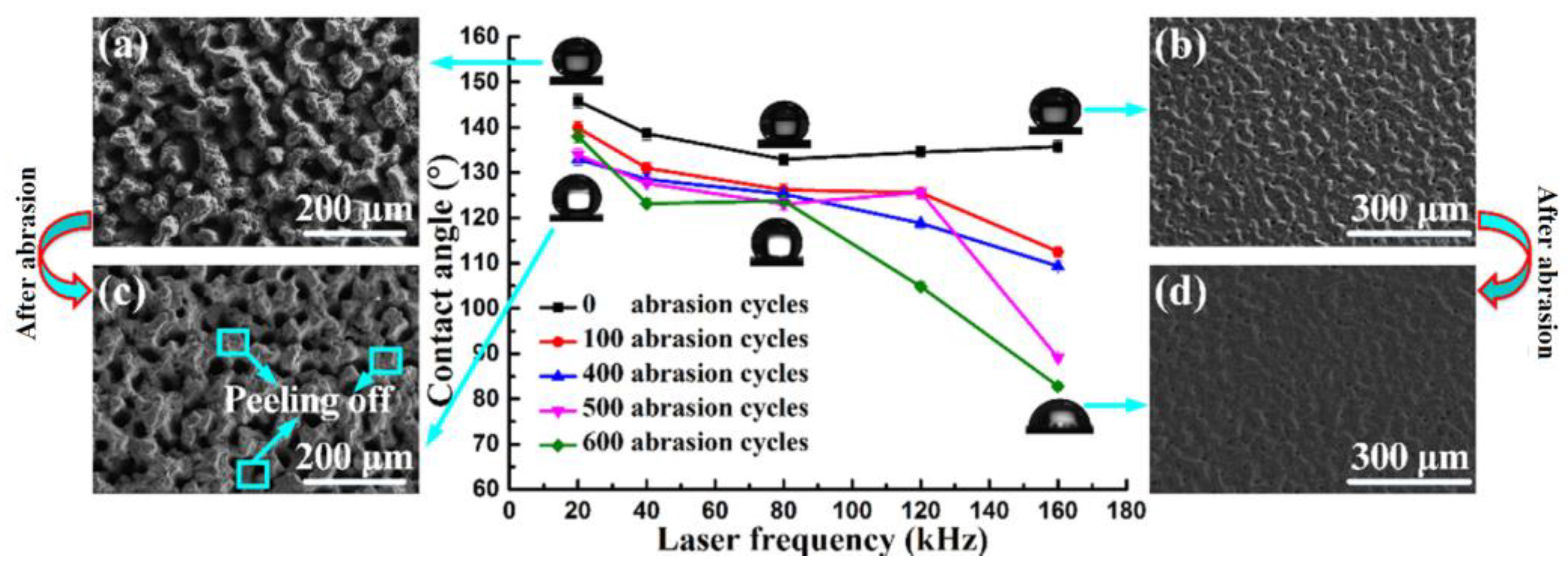
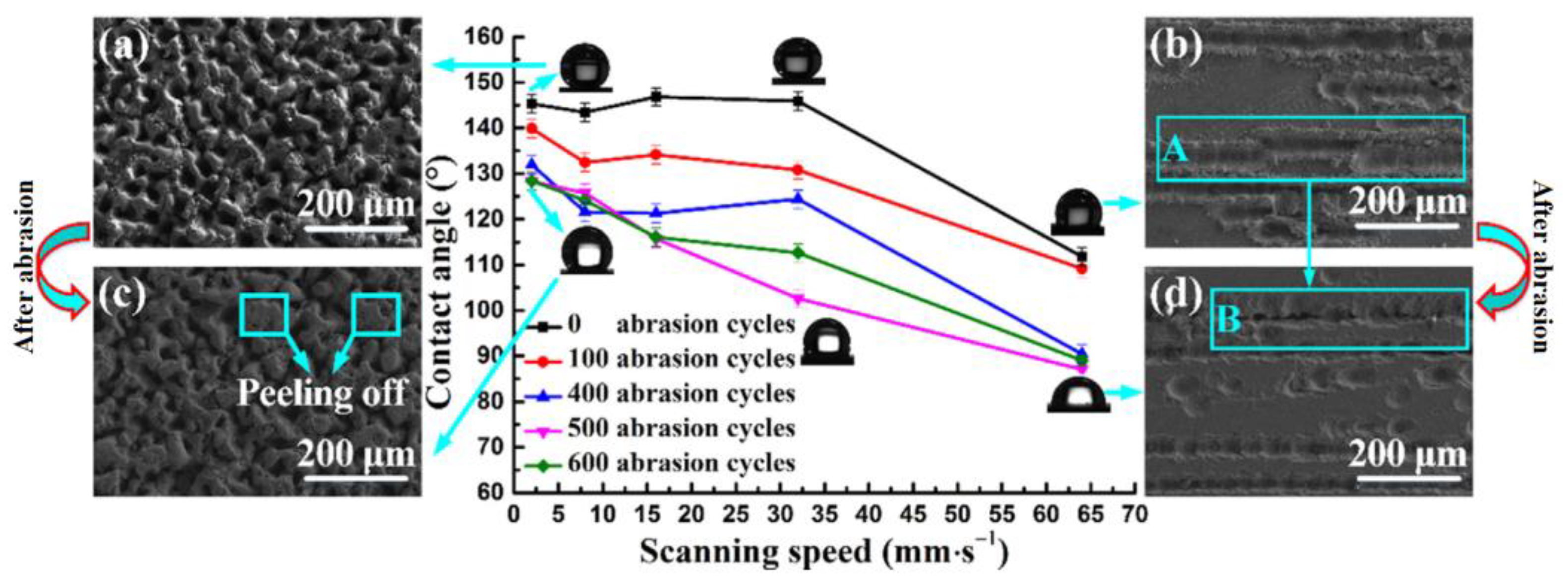
| Cemented carbide |  Obtaining a robust hydrophobic surface | The smaller the laser frequency, the smaller the decrease in the WCA; a relatively low scanning speed was more stable [16]. | The hydrophobic surface fabricated by the proposed new method, liquid-phase laser ablation, has excellent abrasion resistance. | |
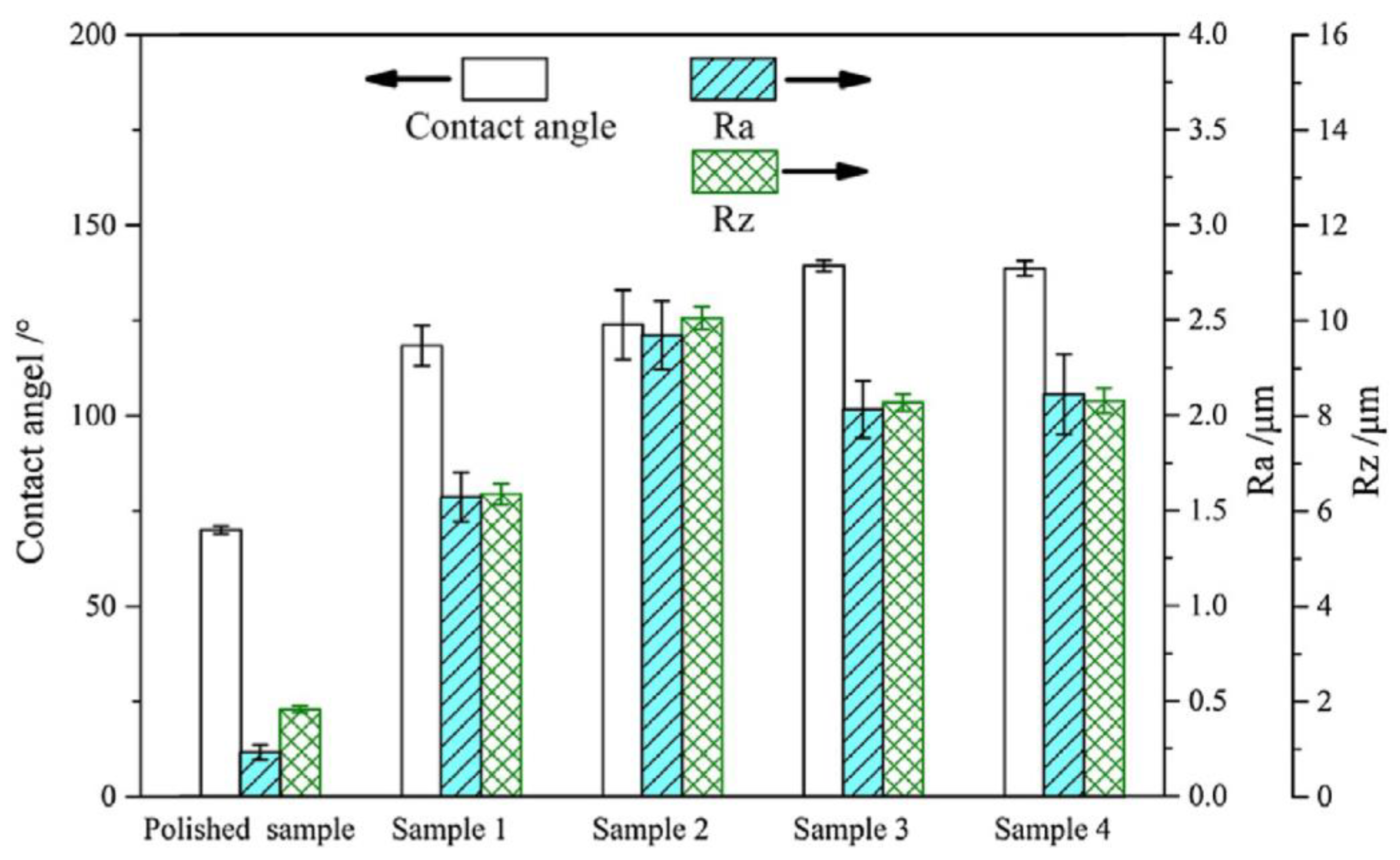
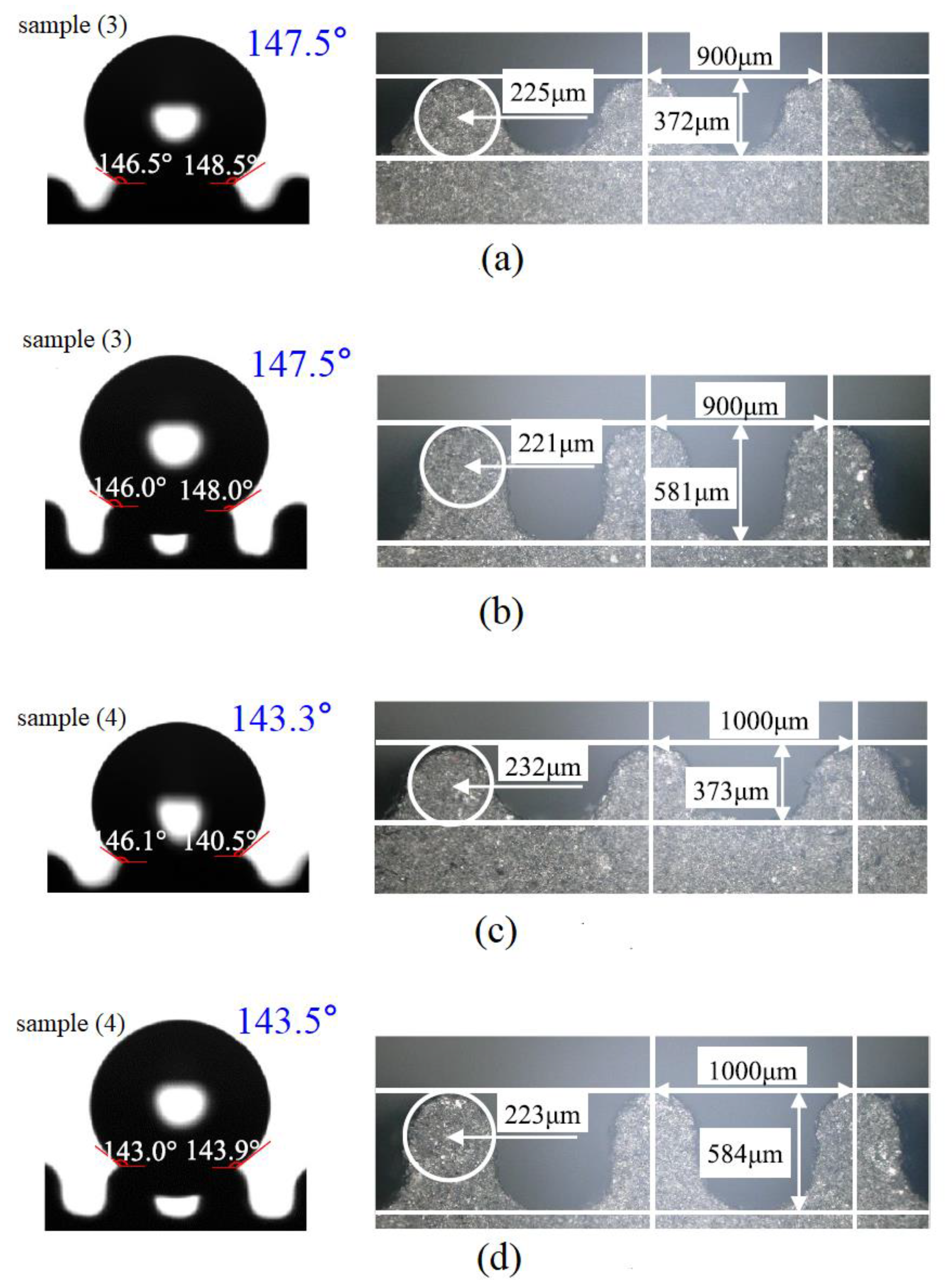
| Aluminum alloy substrates | 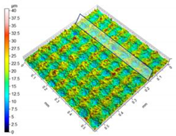 Fabrication of micro-pillar array | Experiments confirmed that the simulation using the proposed method was in good agreement with the results [17]. | An accurate, low-cost and meaningful reference for the selection of functional surface manufacturing methods can be provided. | |
| 316 L stainless |  Obtaining Gaussian micro-holes array | They established a geometric model for laser processing of Gaussian micro-holes array [19]. | The proposed model can optimize the geometric parameters of the micro-structure to achieve the maximum hydrophobicity. | |
| Pyrolytic carbon |  Fabricating a gradient hydrophobic surface with parallel ridges | A GHS was formed on the PYC [20]. | The proposed method helps to design the PYC for artificial heart valve with good blood compatibility. |
| Fabrication | Object | Purpose | Findings | Remarks |
|---|---|---|---|---|
| WEDM | AISI 304 |  To fabricate grooves for controlling the wettability | A commercial control system was successfully applied to manufacture a micro-grooved wettability-controlled surface [22]. | There is no need for any additional chemical treatment on the surface, which is conducive to low-cost industrial application. |
| SiCp/Al composite | 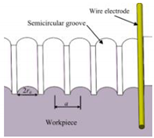 Fabrication of the wear-resistant superhydrophobic structure | Without other surface modification treatments, the maximum contact angle of the prepared micro-structure could reach 153.3° [25]. | Through the proposed one-step machining method, the corrosion resistance, and anti-icing, self-cleaning, and other properties of Al-based composites can be improved at an economic cost. | |
| Copper |  Obtaining a superhydrophobic hierarchical microgroove with nanocone structure | Compared with the flat hydrophobic copper, the CHT coefficient was increased by 82.9% [26]. | The high-efficiency CHT interface developed, using copper micro/nano micro-machining technology, can have important application scope in the next generation of high heat dissipation in small spaces. | |
| EDM | Pure copper | 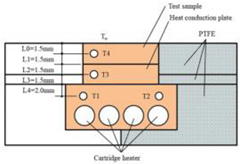 Fabrication of hierarchical micro/nano structure for heat transfer surface | The contact angle became larger as the discharge current increased [23]. | The HMNS sample machined by the EDM process is hydrophobic, which increases the frequency of air bubbles falling off, resulting in a high heat transfer coefficient of the heat transfer surface. |
| Copper sheet | 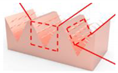 Fabricating nanowires on V-shaped micro-grooves | They proposed a simple and scalable method for preparing CuO nanowires with V-shaped micro-grooves [24]. | A low-cost manufacturing micro-slot radiator is expected to be applied in energy, micro-electronics, chemical, medical, and other fields. | |
| 6060 aluminum alloy | 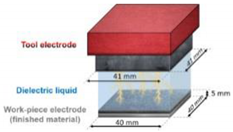 To investigate the relationship between wettability and surface micro-geometry | After EDM machining Al6060, there was a strong correlation between the surface morphology and the WCA [28]. | The increase in roughness will also lead to a larger WCA, which may be a good index of wetting performance for the process of EDM. | |
| MAE-EDM | Metal mesh |  Fabrication of a superhydrophobic mesh | The processed textured mesh had good contact angle stability, corrosion resistance and mechanical stability [27]. | The fabric mesh not only provides universal separation of various oils, but also provides high separation rate and stable performance after recycling. |
| Materials | Authors, Year | Purpose | Findings | Remarks |
|---|---|---|---|---|
| Aluminum | Feng et al., 2002 [42] | Obtain poly(vinyl alcohol) nanofibers | A superhydrophobic surface with the WCA of 171.2° could be achieved | For the first time, amphiphilic materials are used to fabricate superhydrophobic surfaces. This method can be used to fabricate superhydrophobic surfaces from a variety of materials in the future. |
| Zhang et al., 2006 [43]. | Fabricate a two-dimensional array of perfluoropolyether derivative nanopillars | The nanopillars on the lotus leaf-like topology with low contact angles could be achieved. | The surface of two-dimensional array exhibit superhydrophobicity and self-cleaning properties. | |
| Neto et al., 2009 [44]. | Obtain a surface formed by densely arranged nickel nanowires | The nickel nanowires had a very high aspect ratio. | It can be applied to the switchable wettability surface of micro-fluidic chips. | |
| Savio et al., 2021 [45]. | Fabricate raised and recessed binary microstructures | The contact angle was indeed inversely proportional to the content of surface metal aluminum. | This study can better understand the formation mechanism of its superhydrophobic surface. | |
| Silicon | Wang et al., 2007 [46]. | Produce micro/nano topography with fractal shapes | Superhydrophobic silicon surfaces with the WCA of 160° could be fabricated. | This economical processing method has potential application prospects in technical fields such as electronic chip moisture prevention. |
| Balucani et al., 2011 [47]. | Making superhydrophobic surfaces with porous silicon | Large contact angles were observed on these surfaces, which had superhydrophobic properties. | This technology can provide a cheap and effective method for reducing friction in micro-fluidic applications. | |
| Titanium | Wang et al., 2010 [48]. | Create superhydrophobic surfaces on titanium materials | The experimental results showed that the control of wettability could be achieved on these surfaces. | It can be used in the application of oil sealing and anti-leakage on the engineering surface. |
| Copper | Wu et al., 2006 [49]. | Fabricate stable superhydrophobic surfaces | The contact angle of the modified nano-needle surface was greater than 150°, and the inclination angle was less than 2°. | This simple and economical technology is expected to be applied to the walking parts of biomimetic robots with superhydrophobic sub-micron-fiber coating. |
| Others | He et al., 2010 [50]. | Fabricate a ZnO film on zinc foil with a variety of structures | The surface of the ZnO film with electro wettability changed from a hydrophilic state to a superhydrophobic state. | The method provides a simple and rapid process for large-scale synthesis of different ZnO nanostructures, and adjusts the wettability of the ZnO nanostructures through an electric field. |
| Shu et al., 2011 [51]. | Make a superhydrophobic surface with a hierarchical structure | The surface was superhydrophobic, with a WCA of up to 175°. | Since the cone diameter and tip angle of the micro-capsules can controlled by anodizing conditions, there is a good application prospect. |
| Materials | Authors, Year | Purpose | Findings | Remarks |
|---|---|---|---|---|
| Gold | Ye et al., 2010 [52] | Deposit gold microstructures on the ITO substrate | It had significant superhydrophobicity even in corrosive solutions with a wide pH range. | The fabricated surface has higher electro catalytic activity and stability to the electro oxidation of ethanol. |
| Shi et al., 2005 [53]. | Produce superhydrophobic coatings on gold wires | A small amount of micro/nano gold aggregates were formed on the surface of gold wire. The superhydrophobic coating with micro/nano gold aggregates could provide more bending force. | This understanding opens up new application prospects for bionic drag reduction and rapid advancement technology. | |
| Ren et al., 2009 [54]. | Obtain hierarchical cauliflower-like gold structures | The surface of the cauliflower-like structure presented a high contact angle (WCA = 161.9°) and a low sliding angle. | The proposed two-step method for supporting electrochemical structures of gold micro/nano structures could be used in the ITO/glass substrates. | |
| Shepherdet al., 2020 [55]. | Obtain heterogeneously mixed monolayers on the surface of polycrystalline gold | A local hydrophobic environment was formed near the molecular membrane. | It is expected to be used in the fields of anti-oxidation or anti-corrosion, chemical/biochemical sensors, etc. in the future. | |
| Silver | Zhao et al., 2005 [56]. | Making Ag aggregate dendritic structure on the polyelectrolyte multilayer film substrate | The prepared WCA was as high as 154°, and it became superhydrophobic. | The electrochemical deposition technology is used to control the density and morphology of the silver aggregates deposited on the multilayer film, which provides a possible new method for manufacturing self-cleaning surfaces. |
| Gu et al., 2008 [57]. | Grow single crystal Ag dendrites on Ni/Cu substrates | A superhydrophobic surface with a WCA of 154.5°+/−1.0° and an inclination angle of less than 2° could be obtained. | This method does not require a template and is simple and practical. Therefore, This self-cleaning surface has potential applications in nanotechnology. | |
| Copper and copper oxides | Huang et al., 2011 [58]. | Fabricate a particulate superhydrophobic aluminum surface | The aluminum substrate had a superhydrophobic surface with a roughness of 6–7 μm (WCA = 157°). | Nanostructured superhydrophobic aluminum surfaces can be prepared by two step processes: electrochemical deposition and electrochemical modification. |
| Si and Ag + | Yang et al., 2011 [59]. | Fabricate a silicon micro/nano layered structure | By adjusting the process parameters, the morphology of the nanostructures could be partially controlled. | The superhydrophobic silicon surface produced by the ECM method has broad application prospects in micro/nano electromechanical systems (MEMS/NEMS). |
| Others | Huang et al., 2011 [60]. | Deposit composite coating with a thorn-like hierarchical structure with high roughness | The geometry of this hierarchical structure could be controlled to make the contact angle as high as 174.9°. | Because this method saves time and money, it has broad application prospects in the industrial field. |
| Xue et al., 2019 [61]. | Fabricate a bimetallic NiCo coating with a layered micro-sphere structure on a carbon steel substrate | The layered micro-sphere structure of NiCo coating had a very high contact angle (about 165°) and exhibited superhydrophobic properties. | It has good anti-corrosion performance for bare carbon steel. | |
| Wang et al., 2020 [62]. | Fabricate superhydrophobic cobalt-nickel coatings reinforced by micro/nano tungsten carbide (WC) particles | The prepared superhydrophobic Co-Ni/WC composite coating (with a WC content of 9.8 wt %) had excellent wear resistance. | The prepared Co-Ni/WC superhydrophobic coating with good mechanical durability is a promising alternative technology for corrosion protection. |
| Machining Process | Economy and Performances | |||||
|---|---|---|---|---|---|---|
| Capital Investment | Tooling/Fixtures | Power Requirements | Tool Wear | Machining Efficiency | Machining Quality | |
| LBM | Medium /High | Low | Middle | Very low | Low/Medium /High | Low/Medium/High |
| EDM | Medium | High | Low | High | Medium | Medium |
| ECM | Very high | Medium | High | Very low | Medium | Medium |
| USM | Low | Low | Low | Low | / | / |
| WJM | Medium | Medium | Middle | Low | / | / |
| PSM | High | Medium | High | Low | High | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Ming, W.; Ren, X.; Xie, Z.; Liu, X. Progress in Non-Traditional Processing for Fabricating Superhydrophobic Surfaces. Micromachines 2021, 12, 1003. https://doi.org/10.3390/mi12091003
Shen D, Ming W, Ren X, Xie Z, Liu X. Progress in Non-Traditional Processing for Fabricating Superhydrophobic Surfaces. Micromachines. 2021; 12(9):1003. https://doi.org/10.3390/mi12091003
Chicago/Turabian StyleShen, Dili, Wuyi Ming, Xinggui Ren, Zhuobin Xie, and Xuewen Liu. 2021. "Progress in Non-Traditional Processing for Fabricating Superhydrophobic Surfaces" Micromachines 12, no. 9: 1003. https://doi.org/10.3390/mi12091003
APA StyleShen, D., Ming, W., Ren, X., Xie, Z., & Liu, X. (2021). Progress in Non-Traditional Processing for Fabricating Superhydrophobic Surfaces. Micromachines, 12(9), 1003. https://doi.org/10.3390/mi12091003







