Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Silicon Surfaces
Sizes of the Designed Honeycomb Patterns
2.2. Characterization via Scanning Electron Microscopy (SEM)
2.3. Characterization via Optical Profilometry
2.4. Bacterial Strain and Culture Conditions
2.5. Cell Adhesion Experiments
2.6. Microscopy Parameters, Image Acquisition, and Analysis
3. Results
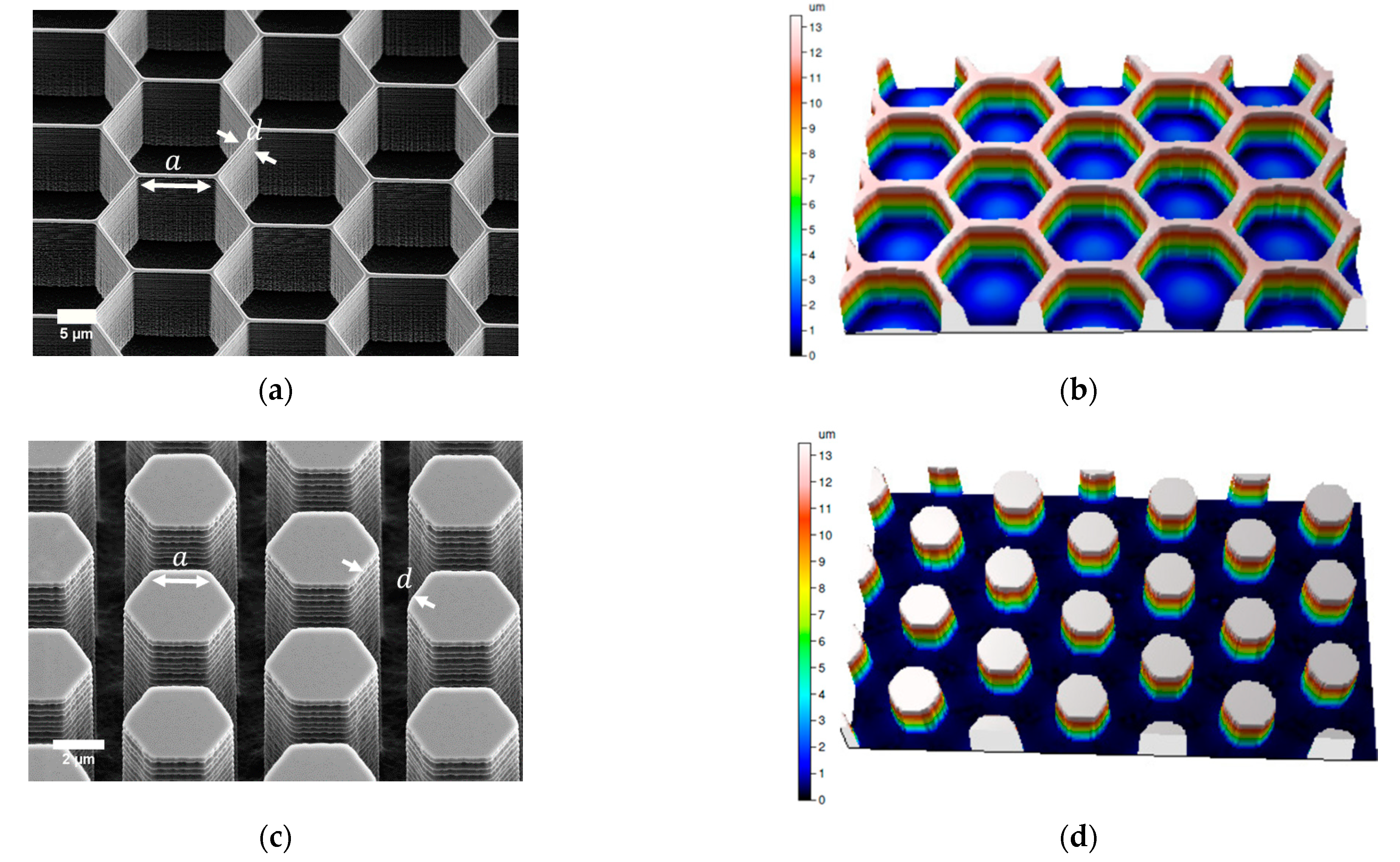
3.1. Fabricated Honeycomb Patterns
Measured Dimensions of the Patterned Surfaces
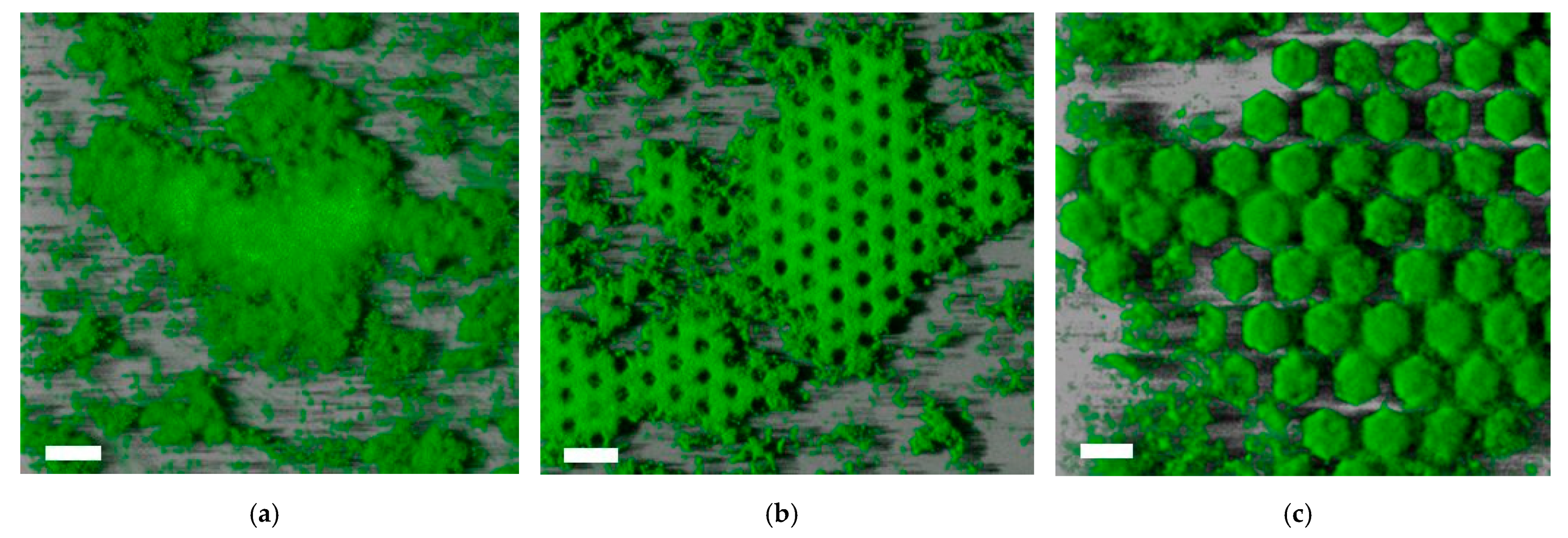
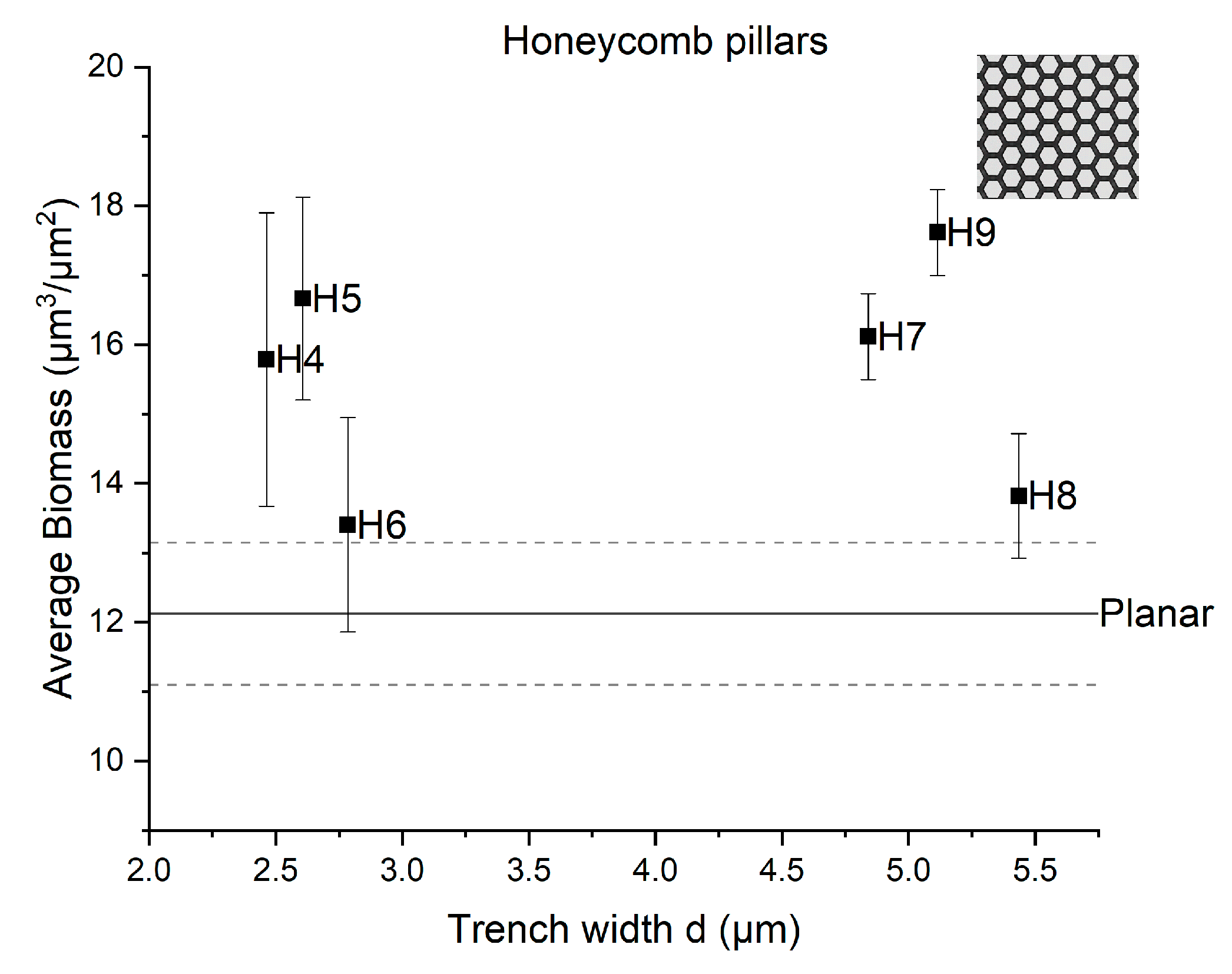
3.2. Impact of the Fabricated Patterns on Bacterial Biofilm Attachment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Bank. Fish to 2030: Prospects for Fisheries and Aquaculture; World Bank: Washington, DC, USA, 2013. [Google Scholar]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A Review of the Main Bacterial Fish Diseases in Mariculture Systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Holmstrom, K.; Graslund, S.; Wahlstrom, A.; Poungshompoo, S.; Bengtsson, B.-E.; Kautsky, N. Antibiotic Use in Shrimp Farming and Implications for Environmental Impacts and Human Health. Int. J. Food Sci. Technol. 2003, 38, 255–266. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to Antibiotics for the Control of Bacterial Disease in Aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Sonnenschein, E.C.; Phippen, C.B.W.; Nielsen, K.F.; Mateiu, R.V.; Melchiorsen, J.; Gram, L.; Overmann, J.; Freese, H.M. Phaeobacter Piscinae Sp. Nov., a Species of the Roseobacter Group and Potential Aquaculture Probiont. Int. J. Syst. Evol. Microbiol. 2017, 67, 4559–4564. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Erner, K.E.; Bentzon-Tilia, M.; Gram, L. Effect of TDA-Producing Phaeobacter Inhibens on the Fish Pathogen Vibrio Anguillarum in Non-Axenic Algae and Copepod Systems. Microb. Biotechnol. 2018, 11, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- D’Alvise, P.W.; Lillebø, S.; Prol-Garcia, M.J.; Wergeland, H.I.; Nielsen, K.F.; Bergh, Ø.; Gram, L. Phaeobacter Gallaeciensis Reduces Vibrio Anguillarum in Cultures of Microalgae and Rotifers, and Prevents Vibriosis in Cod Larvae. PLoS ONE 2012, 7, e43996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alvise, P.W.; Melchiorsen, J.; Porsby, C.H.; Nielsen, K.F.; Gram, L. Inactivation of Vibrio Anguillarum by Attached and Planktonic Roseobacter Cells. Appl. Environ. Microbiol. 2010, 76, 2366–2370. [Google Scholar] [CrossRef] [Green Version]
- Segev, E.; Tellez, A.; Vlamakis, H.; Kolter, R. Morphological Heterogeneity and Attachment of Phaeobacter Inhibens. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.B.; Gram, L.; Belas, R. Production of Antibacterial Compounds and Biofilm Formation by Roseobacter Species Are Influenced by Culture Conditions. Appl. Environ. Microbiol. 2007, 73, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Gram, L.; Rasmussen, B.B.; Wemheuer, B.; Bernbom, N.; Ng, Y.Y.; Porsby, C.H.; Breider, S.; Brinkhoff, T. Phaeobacter Inhibens from the Roseobacter Clade Has an Environmental Niche as a Surface Colonizer in Harbors. Syst. Appl. Microbiol. 2015, 38, 483–493. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and Managing Microbes in Aquaculture—Towards a Sustainable Industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of Surface Topography on Bacterial Adhesion: A Review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, Y.; Liu, S.; Pihl, M.; van der Mei, H.C.; Liu, J.; Hizal, F.; Choi, C.H.; Chen, H.; Ren, Y.; Busscher, H.J. Bacterial Interactions with Nanostructured Surfaces. Curr. Opin. Colloid Interface Sci. 2018, 38, 170–189. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro-and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghilini, F.; Pissinis, D.E.; Miñán, A.; Schilardi, P.L.; Diaz, C. How Functionalized Surfaces Can Inhibit Bacterial Adhesion and Viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The Interaction of Cells and Bacteria with Surfaces Structured at the Nanometre Scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Hsu, L.; Fang, J.; Borca-Tasciuc, D.; Worobo, R.; Moraru, C.I. The Effect of Micro- and Nanoscale Topography on the Adhesion of Bacterial Cells to Solid Surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial Flagella Explore Microscale Hummocks and Hollows to Increase Adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [Green Version]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rößler, F.; Lasagni, A.; Werner, C. The Impact of Structure Dimensions on Initial Bacterial Adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanzi, S.; Ostergaard, P.F.; Matteucci, M.; Christiansen, T.L.; Cech, J.; Marie, R.; Taboryski, R. Fabrication of Combined-Scale Nano- and Microfluidic Polymer Systems Using a Multilevel Dry Etching, Electroplating and Molding Process. J. Micromechanics Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Søgaard, E.; Andersen, N.K.; Smistrup, K.; Larsen, S.T.; Sun, L.; Taboryski, R. Study of Transitions between Wetting States on Microcavity Arrays by Optical Transmission Microscopy. Langmuir 2014, 30, 12960–12968. [Google Scholar] [CrossRef]
- Dill, F.H. Optical Lithography. IEEE Trans. Electron. Devices 1975, 22, 440–444. [Google Scholar] [CrossRef]
- Laermer, F.; Schilp, A. Method of Anisotropically Etching Silicon. US5501893A Patent 26 March 1996. [Google Scholar]
- Li, D. Aspect Ratio Dependent Etching. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2008; p. 56. [Google Scholar] [CrossRef]
- Lai, S.L.; Johnson, D.; Westerman, R. Aspect Ratio Dependent Etching Lag Reduction in Deep Silicon Etch Processes. J. Vac. Sci. Technol. A Vac. Surf. Film 2006, 24, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Jansen, H.; de Boer, M.; Wiegerink, R.; Tas, N.; Smulders, E.; Neagu, C.; Elwenspoek, M. BSM 7: RIE Lag in High Aspect Ratio Trench Etching of Silicon. Microelectron. Eng. 1997, 35, 45–50. [Google Scholar] [CrossRef] [Green Version]
- SensoMAP. Available online: https://www.sensofar.com/wp-content/uploads/2015/07/Brochure_SensoMAP_v7-EN.pdf (accessed on 15 May 2021).
- Zech, H.; Thole, S.; Schreiber, K.; Kalhöfer, D.; Voget, S.; Brinkhoff, T.; Simon, M.; Schomburg, D.; Rabus, R. Growth Phase-Dependent Global Protein and Metabolite Profiles of Phaeobacter Gallaeciensis Strain DSM 17395, a Member of the Marine Roseobacter-Clade. Proteomics 2009, 9, 3677–3697. [Google Scholar] [CrossRef]
- Vorregaard, M. Comstat2—A Modern 3D Image Analysis Environment for Biofilms. 2008. Available online: http://www2.imm.dtu.dk/pubdb/edoc/imm5628.pdf (accessed on 15 May 2021).
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of Biofilm Structures by the Novel Computer Program Comstat. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [Green Version]
- An, Y.H.; Friedman, R.J. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterial Surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Epstein, A.K.; Hochbaum, A.I.; Kim, P.; Aizenberg, J. Control of Bacterial Biofilm Growth on Surfaces by Nanostructural Mechanics and Geometry. Nanotechnology 2011, 22, 494007. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial Attachment and Biofilm Formation on Surfaces Are Reduced by Small-Diameter Nanoscale Pores: How Small Is Small Enough? Npj Biofilms Microbiomes 2015, 1, 15022. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.; Han, B.; Zhang, X.; Jin, F.; Xu, F.; et al. Bioinspired Engineering of Honeycomb Structure–Using Nature to Inspire Human Innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Franssila, S. Deep Reactive Ion Etching. In Introduction to Microfabrication; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 255–270. [Google Scholar]
- Butt, H.; Vollmer, D. Submicrometer-Sized Roughness Suppresses Bacteria Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Hadjiev, D.; Dimitrov, D.; Martinov, M.; Sire, O. Enhancement of the Biofilm Formation on Polymeric Supports by Surface Conditioning. Enzym. Microb. Technol. 2007, 40, 840–848. [Google Scholar] [CrossRef]
- Senesi, G.S.; D’Aloia, E.; Gristina, R.; Favia, P.; d’Agostino, R. Surface Characterization of Plasma Deposited Nano-Structured Fluorocarbon Coatings for Promoting in Vitro Cell Growth. Surf. Sci. 2007, 601, 1019–1025. [Google Scholar] [CrossRef]
- Telecka, A.; Murthy, S.; Schneider, L.; Pranov, H.; Taboryski, R. Superhydrophobic Properties of Nanotextured Polypropylene Foils Fabricated by Roll-to-Roll Extrusion Coating. ACS Macro Lett. 2016, 5, 1034–1038. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Laustsen, M.; Mandsberg, N.; Taboryski, R. The Influence of Structure Heights and Opening Angles of Micro- and Nanocones on the Macroscopic Surface Wetting Properties. Sci. Rep. 2016, 6, 21400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telecka, A.; Mandsberg, N.K.; Li, T.; Ludvigsen, E.; Ndoni, S.; di Mundo, R.; Palumbo, F.; Fiutowski, J.; Chiriaev, S.; Taboryski, R. Mapping the Transition to Superwetting State for Nanotextured Surfaces Templated from Block-Copolymer Self-Assembly. Nanoscale 2018, 10, 20652–20663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Zhang, P.; Wang, S. Recent Progress in Biointerfaces with Controlled Bacterial Adhesion by Using Chemical and Physical Methods. Chem. Asian J. 2014, 9, 2004–2016. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droumpali, A.; Hübner, J.; Gram, L.; Taboryski, R. Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm. Micromachines 2021, 12, 926. https://doi.org/10.3390/mi12080926
Droumpali A, Hübner J, Gram L, Taboryski R. Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm. Micromachines. 2021; 12(8):926. https://doi.org/10.3390/mi12080926
Chicago/Turabian StyleDroumpali, Ariadni, Jörg Hübner, Lone Gram, and Rafael Taboryski. 2021. "Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm" Micromachines 12, no. 8: 926. https://doi.org/10.3390/mi12080926
APA StyleDroumpali, A., Hübner, J., Gram, L., & Taboryski, R. (2021). Fabrication of Microstructured Surface Topologies for the Promotion of Marine Bacteria Biofilm. Micromachines, 12(8), 926. https://doi.org/10.3390/mi12080926






