3.1. Fluorescence Images of Mixing Caused by Droplet Rotation
As shown in
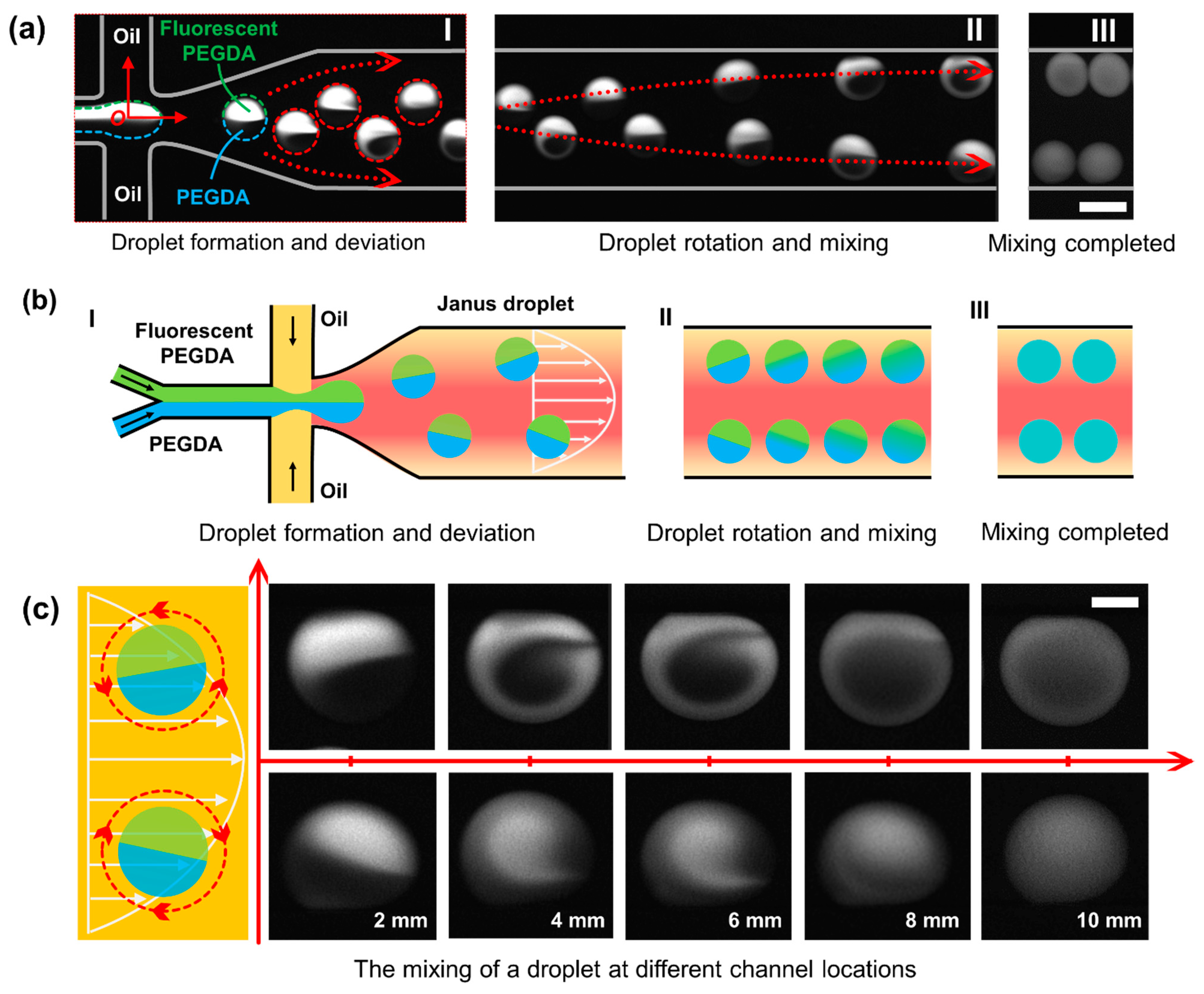
Figure 2(aI), after all the regents were introduced into the microchannel independently and met in the junction, Janus droplets were formed with the help of a combination of Y-channel and flow-focusing design. In general, the two regents from the Y-channel form the dispersed phase, and the oil served as the continuous phase (incompatible with the dispersed phase). The dispersed phase was sheared by the continuous phase and pinched off to form droplets due to the special geometric design. With the help of a microscope and CCD camera, there shows clear boundaries between the two halves inside the droplet.
When droplets were generated and then traveled in a straight microchannel, two symmetric counter-rotating recirculation zones were formed in each half of the droplet [
23,
24]. Thus, there will be no external disturbance, creating a barrier limiting the mixing between the two halves. Only when the two halves are agitated can rapid and complete mixing be achieved. The crucial element and main trigger of the microfluidic mixing, herein, is the rotation behavior, which can induce cross-flow and destroy the barrier. In this design, while the droplets moved along the centerline without trajectory deviation in the initial movement right-side the cross junction, they were pushed away from the centerline when entered the collapsed region and rotate due to the non-uniform distribution flow field, thereby introduce turbulence and destroy the equilibrium interface between the two fluids [
25,
26]. The disordered 3D fluid behavior inside the droplet makes the contact area between the two fluids gradually increase, thus enhance the diffusive flux. As shown in
Figure 2(aII,aIII), with the continuous rotation of the droplet, this mixing behavior continues until the mixing is complete.
Figure 2b shows the schematics of the droplet generation and droplet mixing, which is corresponding to
Figure 2a.
As shown in
Figure 2c, the distribution of the flow field is non-uniform, and the flow velocity is parabolic along the transverse direction of the microchannel. When the droplet is located in the channel center, the two halves of the droplets are balanced, and no rotation occurs. However, when the droplet deviates away from the centerline, the two halves of the droplet are not uniformly stressed, and the rotation occurs. Duo to the symmetry of the flow field, the upper droplets rotated counterclockwise while the lower droplets rotate clockwise. The rotation introduced turbulence and destroyed the barrier between the two inner liquids, which increased the contact area and increased the diffusion flux. The series of pictures right-side shows the mixing state of the droplet at different positions (a corresponding video is provided in
Supplementary Materials Video S1).
In fact, the rotating mechanism is a complex phenomenon involving the understanding of non-uniform flow field, non-uniform mass distribution between the two halves within the droplet, and their strong dynamic coupling, which is beyond the scope of this study. For example, the subsidence of the channel roof will affect the distribution of the flow field, transforming it from a two-dimensional parabolic distribution to a three-dimensional complex distribution, resulting in the 3D rotation of the droplet. Other disturbances seemingly weak can also affect the direction and speed of rotation, such as the small difference in density or viscosity between the two halves of the droplet.
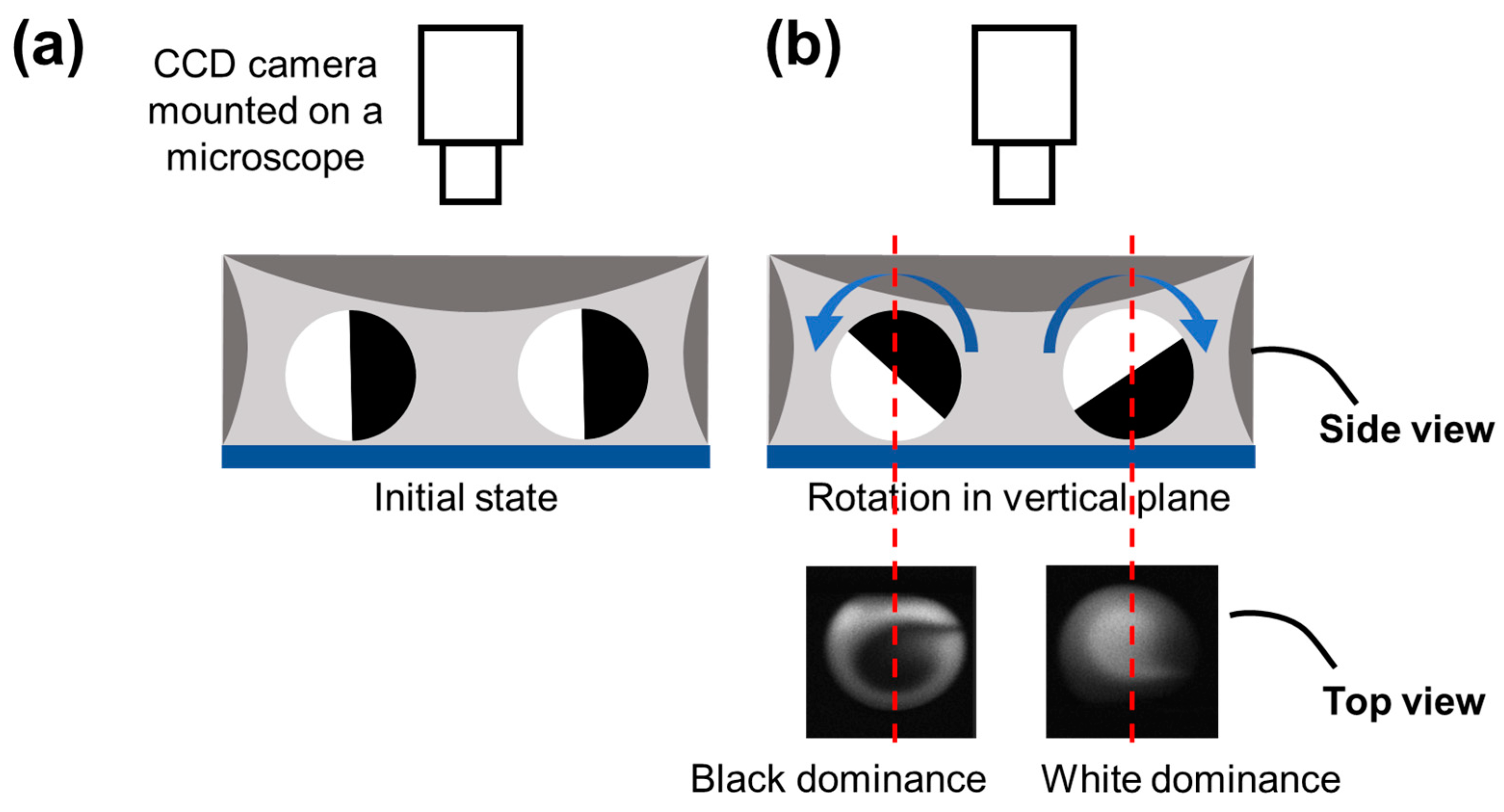
When the droplet rotation occurs only in the horizontal plane, each of the two fluids (characterized by black and white) occupies half of the droplet from the top view. However, as shown in
Figure 2c, from the real-time pictures taken from the top view, the half-half status of the two fluids in a rotating droplet changed gradually to form a Taiji pattern. Thus, there must be a rotation behavior that occurred perpendicular to the horizontal plane. As shown in
Figure 3, the rotation in the vertical plane will result in black or white dominance in the droplet from the top view, exactly matching the real-time fluorescent pictures.
In order to shed light on the mixing mechanism, the study only focused on the droplet rotation on two-dimensional as discussed in
Section 3.3, which is the largest component in the 3D rotation of the droplet.
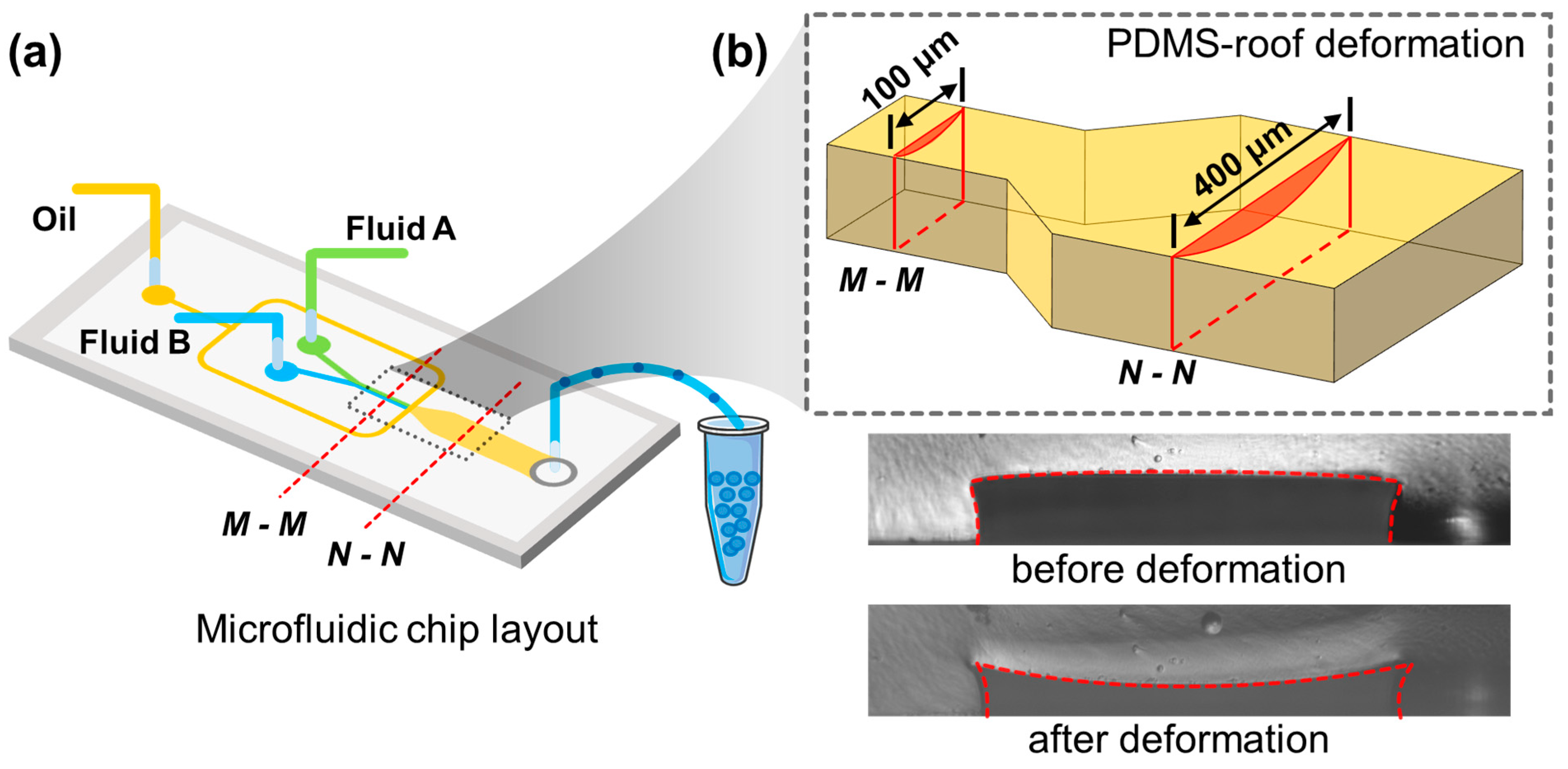
3.2. Droplet Rotation Caused by PDMS Deformations
The compatibility of PDMS channels with certain solvents is bad due to the porous nature of PDMS. Studies have shown the secret to realize the PDMS deformation is the use of organic solvents such as hexadecane, and silicone oil, which can penetrate the PDMS and result in a rubber-like expansion. Here in this work, solvent hexadecane was encapsulated in a microchannel for 10 min to obtain subsidence on the roof of the microchannel.
The cross-linked PDMS consists of high-molecular-polymers; when PDMS is immersed in the organic solution, the small solvent molecules continuously diffuse into the polymer chains and make it stretched. As the network continues to stretch, there is also a tendency to restore its original state due to the entropy elasticity. Thus, when the solvation and entropy elasticity keep balance, the swelling process can reach equilibrium, and the PDMS deformation is completed. The “PDMS polymer-solvent” system satisfies the following equation [
27,
28]:
where
is polymer density,
is the molar volume of the solvent,
is the average molecular weight between two crosslink points of PDMS,
is the interaction parameter between solute and solvent molecules,
is the volume fraction of solute, while it is also meaningful in terms of geometry. The solvents in the solution will penetrate the PDMS polymer; thus, the original dry PDMS polymer network will swell, resulting in an arched deformation.
can also be described by the following equation:
where
is the depth of penetration,
is the subsidence of the channel roof,
w is the channel width,
l is the channel length.
Based on the Equations (2)–(5), ∆
h can be calculated by the following equation:
Only if the
is large enough can the droplets be pushed away from the centerline; thus, it is crucial to create enough roof subsidence. Among the several parameters that affect
as shown in Equation (6),
is the easiest to change by adjusting the ratio of PDMS base to curing agents. When the ratio increased, the average molecular weight between two crosslink points increased; thus, the organic solvent could penetrate PDMS more easily and make
increase. From a macro view, by reducing the proportion of curing agents, the resulting elastomer is softer and less rigid, which is more prone to deformation. While the higher ratio between the prepolymer to the curing agent enhances the roof subsidence, it may also result in prolonged curing time (when the ratio is 15:1, it takes 5 h to be cured completely at 80 °C). Moreover, a higher ratio may lead to unexpected and excessive subsidence of PDMS and increase the flow resistance significantly, even block the microchannels. As a result, it may fail to produce or deliver droplets within the microchannel. In our conditions, we optimized the PDMS/curing agent ratios as 12:1 to achieve excellent performance for droplet rotation and mixing with a reasonable curing time and a proper size of roof subsidence. As shown in
Figure 1b, the deformations of the roof of PDMS microchannels can be experimentally measured by the optical method, which was 19.6 μm at the middle position from measurement. The deformation on the roof is much greater than that of the sidewall. The deformation of the two sidewalls is symmetrical; thus, the impacts on mixing caused by sidewall deformation were offset with each other; thus, it can be ignored herein. There is no deformation at the glass substrate, while the PDMS roof shows large deformation. That is to say, the roof subsidence is the most crucial factor for droplet rotation and the mixing performance within the droplets. Moreover, the mixing experiment carried out in this work also demonstrates that we can achieve droplet rotation and mixing by controlling the roof subsidence only. From these two points above, it is not necessary to pay attention to the effects of the deformation of sidewalls on the droplet rotations.
3.3. Effects of Contact Areas on Mixing Efficiency
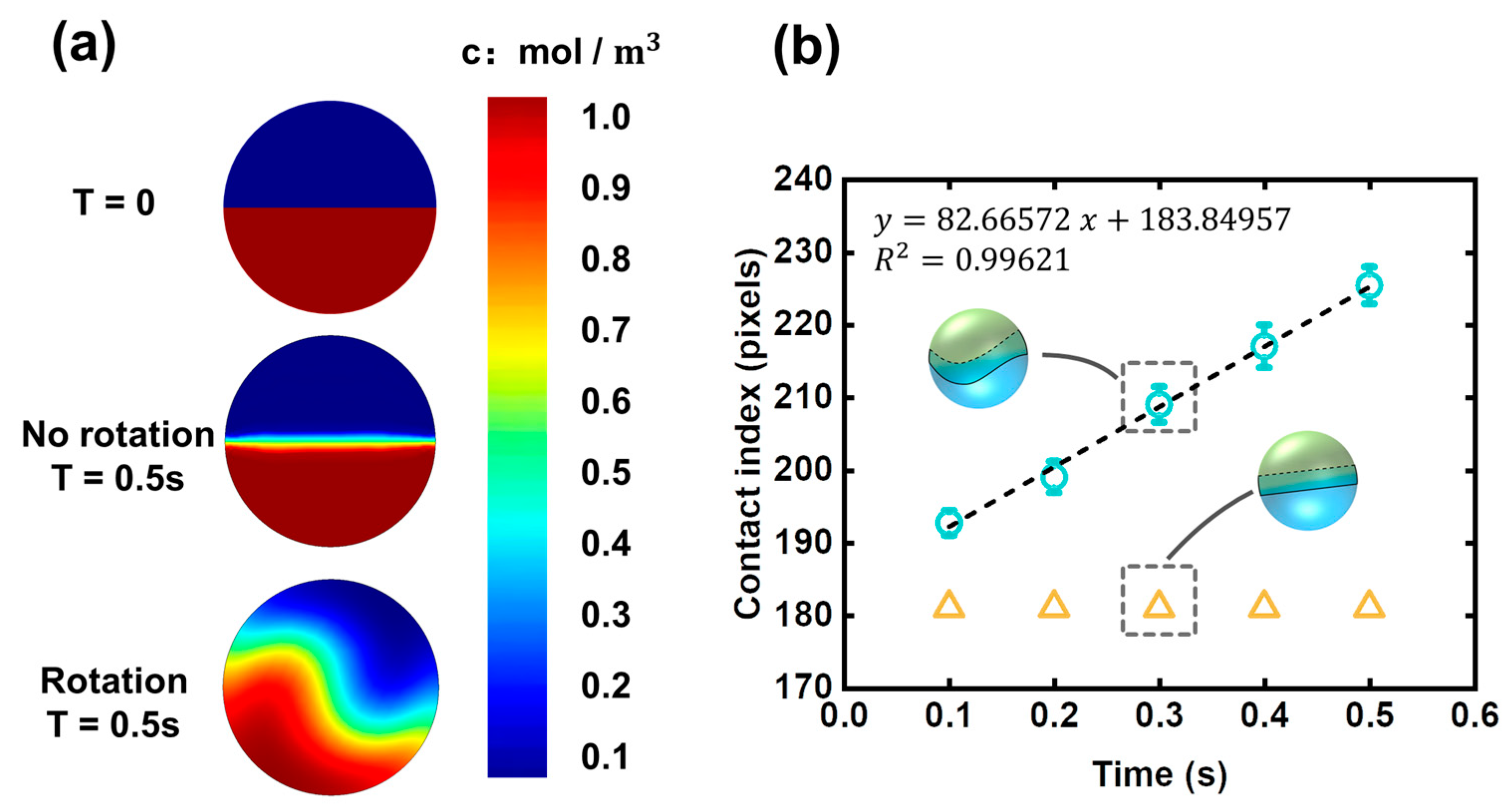
The mixing state of the two halves of a droplet was shown in
Figure 4a, which is a numerical prediction using commercial software, COMSOL Multiphysics. The multi-physical field is a combination of laminar flow (spf) and transport of diluted species (tds). The frozen rotor method was used the simulate the mixing process inside the droplet. The droplet diameter was set at 100 μm.
In the laminar flow part, rotation speed was set at 2π rad/s. The following equation was applied to the model.
In the transport of diluted species part, the concentrations of the two fluids were set at 0 and 1 mol/m
3 the droplet diffusion coefficient was set at
[m
2/s]. The following equation was applied to the model.
It can be seen that the diffusion flux is high in the rotating droplet while weak in the non-rotating droplet. Although the simulation here is two-dimensional, it can still reflect how the rotation affects the two halves of a droplet. The working principle of the droplet rotation is “increasing contact area”. Rotation behavior induces disturbance to the flow field inside the droplet, which can enhance cross-stream mixing, thus resulting in the increasing of contact area between the two halves of the droplet. Contact area has a crucial influence on the diffusion flux; herein, the contact area is indicated by the contact index, which can be obtained by measuring the length of the two-half boundary. Time dependence of contact index in rotating and non-rotating droplets was shown in
Figure 4b. The contact index in a rotating droplet is greater than that of the non-rotating droplet, indicating there are more complicated entanglements between the two inner fluids when rotation behavior occurs.
The diffusion coefficient of fluorescent molecules in high-viscosity solution (such as PEGDA solution used here) is smaller compared with low-viscosity solution (such as pure water). Moreover, low-viscosity fluids are easier to be driven and merge with each other; thus, it can be inferred that there will be a better mixing effect for the experiments involving low-viscosity fluids.
In order to study the mixing efficiencies at different positions, fluorescent images were taken from seven locations (0, 2, 4, 6, 8, 10, 12 mm) were used for fluorescent intensity analysis. As shown in
Figure 5a, we took 21 points in each microscopic to calculate the mixing index. The corresponding data graph showed the continuous improvement of the mixing efficiency as the droplet traveling on. The X-axis is the distance that the droplet traveled after entering the rotation region (the center of the junction where generates the droplet is marked as x = 0 as shown in
Figure 2a).
In order to evaluate the mixing effect in general, we studied the mixing state of the droplet at the starting point and the endpoint. As shown in
Figure 5b, the initial state and mixed state of a droplet were presented. When the droplet rotated for a period of time, the clear boundaries between the black and white area were gone, and the two halves were thoroughly mixed. Fluorescent intensity profiles were measured from the microscopic images. Two dash line scans across the droplet demonstrate the intensity profiles before and after mixing. From the right-side graph, it can be seen that the fluorescence intensity of the mixed droplet is relatively homogeneous compared with the unmixed droplet, indicating that the mixing based on droplet rotation is achievable and effective.
3.4. Effects of Flow Rates on Mixing Efficiency
In general, when the generated droplets move forward inside a straight microchannel without any perturbation, the cross-stream mixing is suppressed due to the barrier between the two halves of the droplet, which can prevent the cross-stream mixing. At the same time, the chaotic advection inside the two halves cannot guarantee a full mixing. However, when placed in a non-uniform distributed flow field, the droplet can rotate, and the barrier between the two halves is broken. In fact, there are still many factors that affect the mixing efficiency, including the fluids density, fluids viscosity, diffusion coefficient, contact area, concentration gradient, and so on.
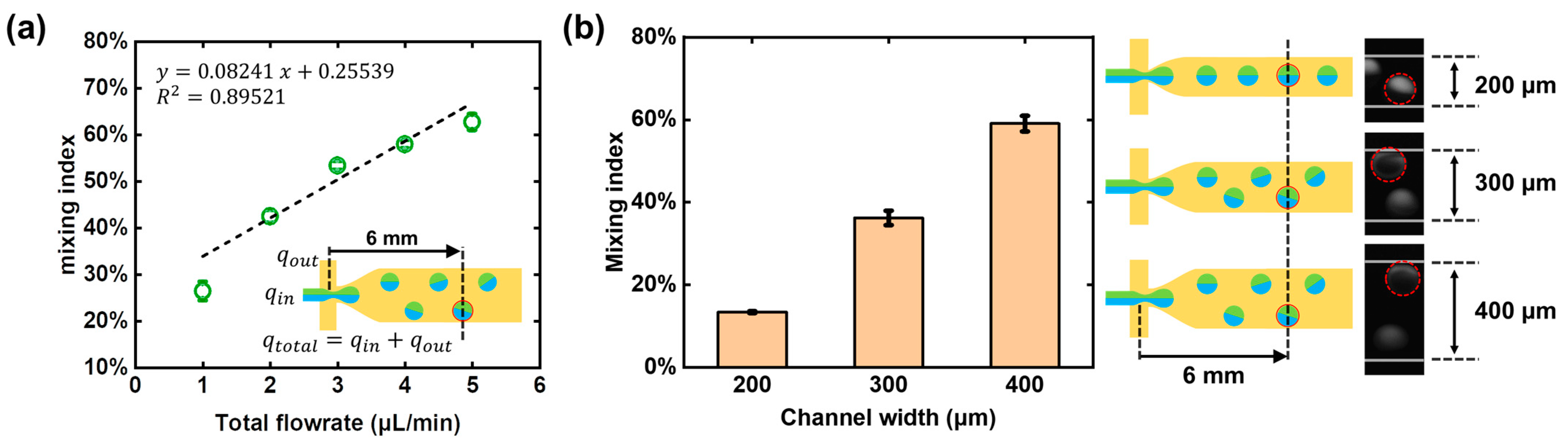
We have also studied the effect of the fluid flow rate on the mixing index. Since the rotation of droplets are affected by the non-uniform distribution of the flow field, the total flowrate
consisting of
(water phase) and
(oil phase) is also discussed. As shown in
Figure 6a, the mixing efficiency of the droplets at the 6 mm position of the microchannel under different flowrate conditions were studied, respectively. The
was kept at 1 μL/min (
), 2 μL/min (
), 3 μL/min (
), 4 μL/min (
), and 5 μL/min (
), so as to generate droplets of uniform size (CV < 5%). As the fluid flow rate increased, the flow field changed more drastically along the transverse direction of the microchannel, the force acting on the upper and lower parts of the droplet was more unbalanced, so the torsional moment increased, thus resulting in the more intense rotation and larger diffusion flux.
3.5. Effects of Channel Widths on Mixing Efficiency
The PDMS deformation has a crucial effect on the droplet rotation-based mixing. The subsidence of the channel roof is not enough when the PDMS deformation is insufficient; thus, the droplets will not be pushed away from the centerline, and there will be no rotation, resulting in low diffusion flux. From the above analysis in
Section 3.2, it has been demonstrated that the ratio of PDMS base and curing regents can affect the PDMS deformation. By reducing the amount of curing agent used, a softer PDMS elastomer can be obtained, and more deformation can be achieved. At the same time, the roof subsidence can also be effectively controlled by the microchannel design. As shown in
Figure 6b, three microchannels with the width of 200, 300, and 400 μm were used in the droplet rotation-based mixing experiments, and the fluorescent images at the same travel distance (6 mm) were taken for the intensity analysis. When the channel width reduced from 400 μm to 200 μm, the mixing index changed from 59% to 13%, showing a drop in the mixing efficiency. This phenomenon could be explained in geometrics. The roof subsidence in a narrow microchannel is so small that the flow resistance cannot increase significantly; thus, the droplet cannot be placed in a sufficiently non-uniform distributed flow field, and the chaotic advection in a rotating droplet will not happen. In general, the wider the microchannel, the greater the subsidence and the more impact on the droplet trajectory.