An ISFET Microarray Sensor System for Detecting the DNA Base Pairing
Abstract
:1. Introduction
2. Sensor Chip and Test System
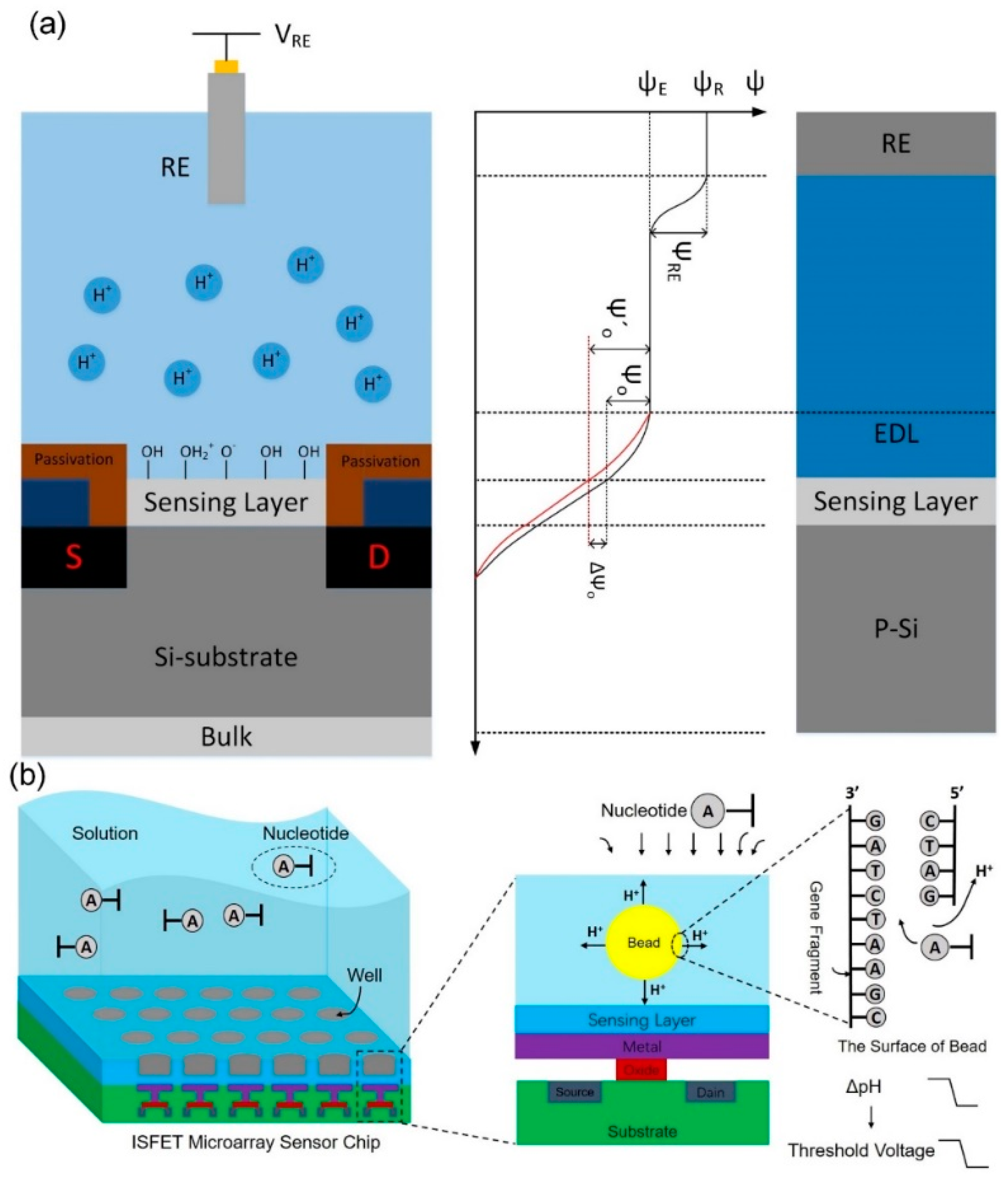
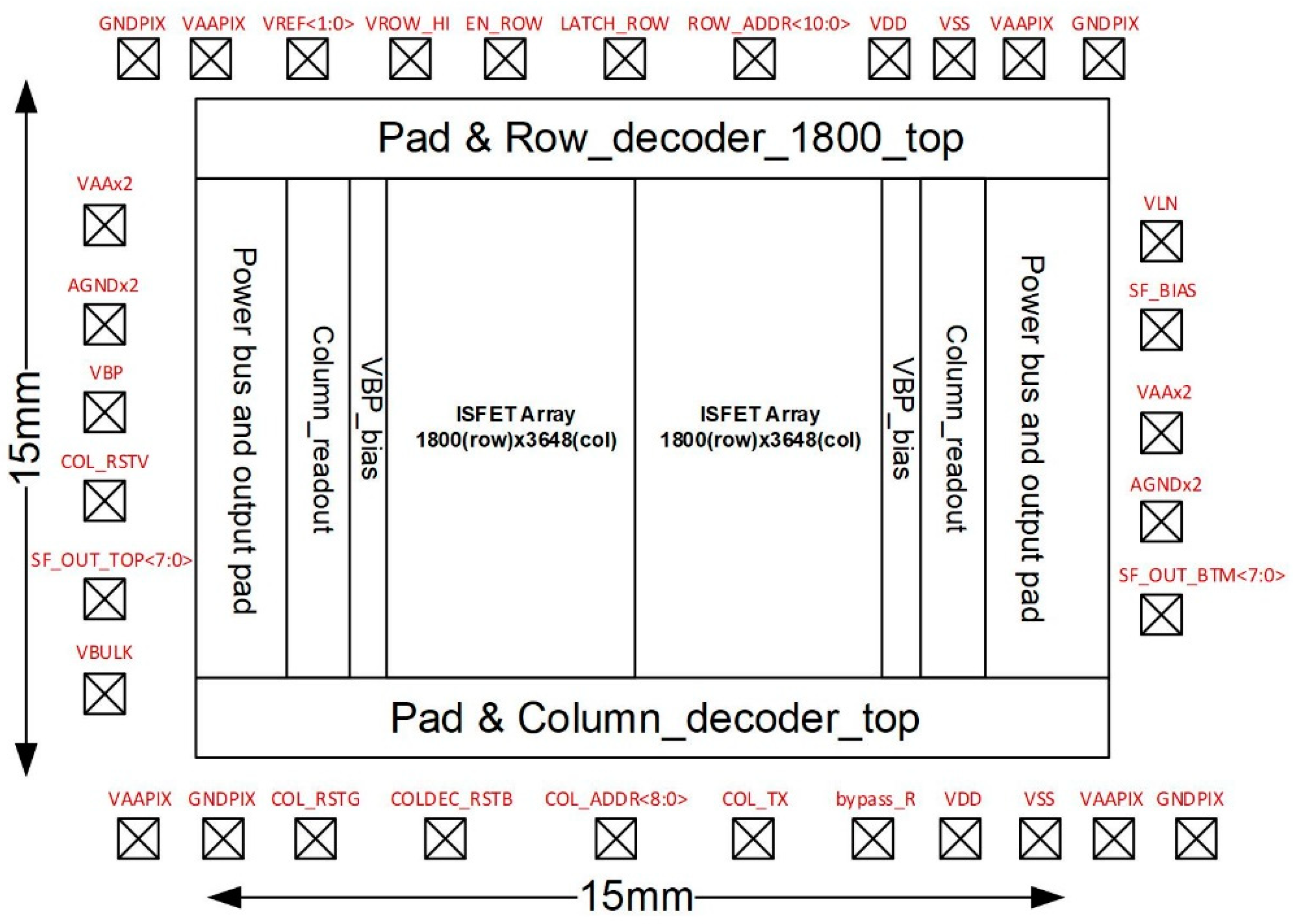
2.1. System Chip Design and Signal Readout Circuit Design
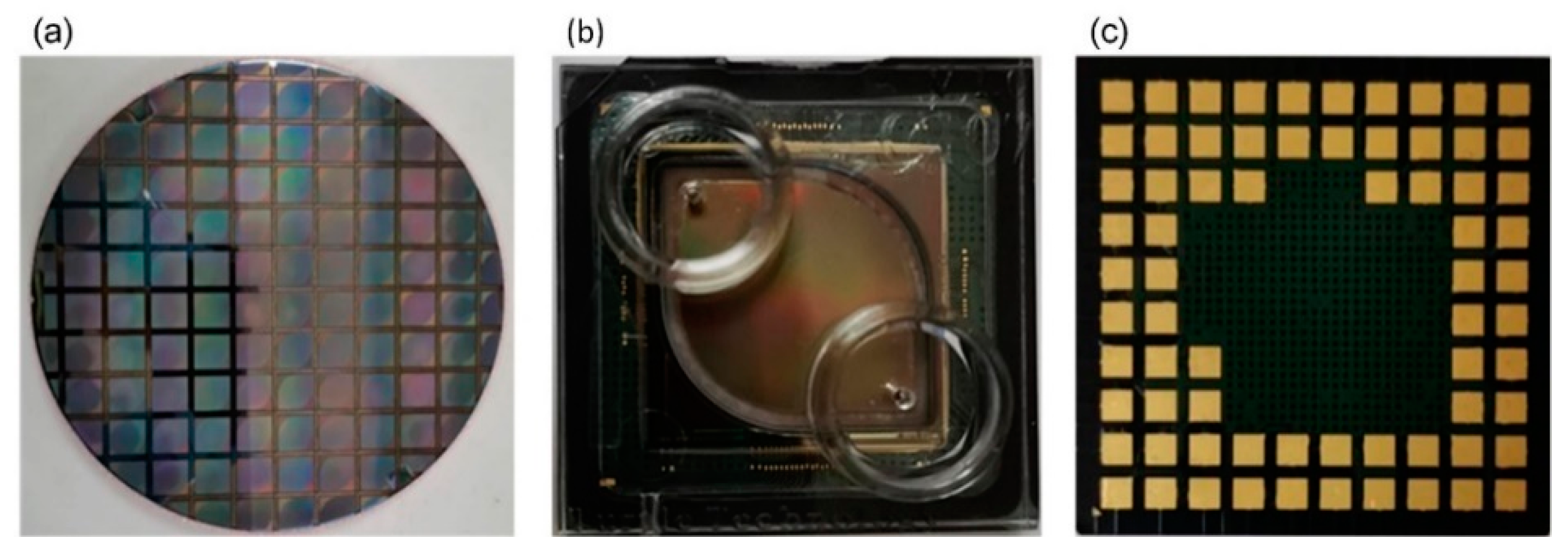
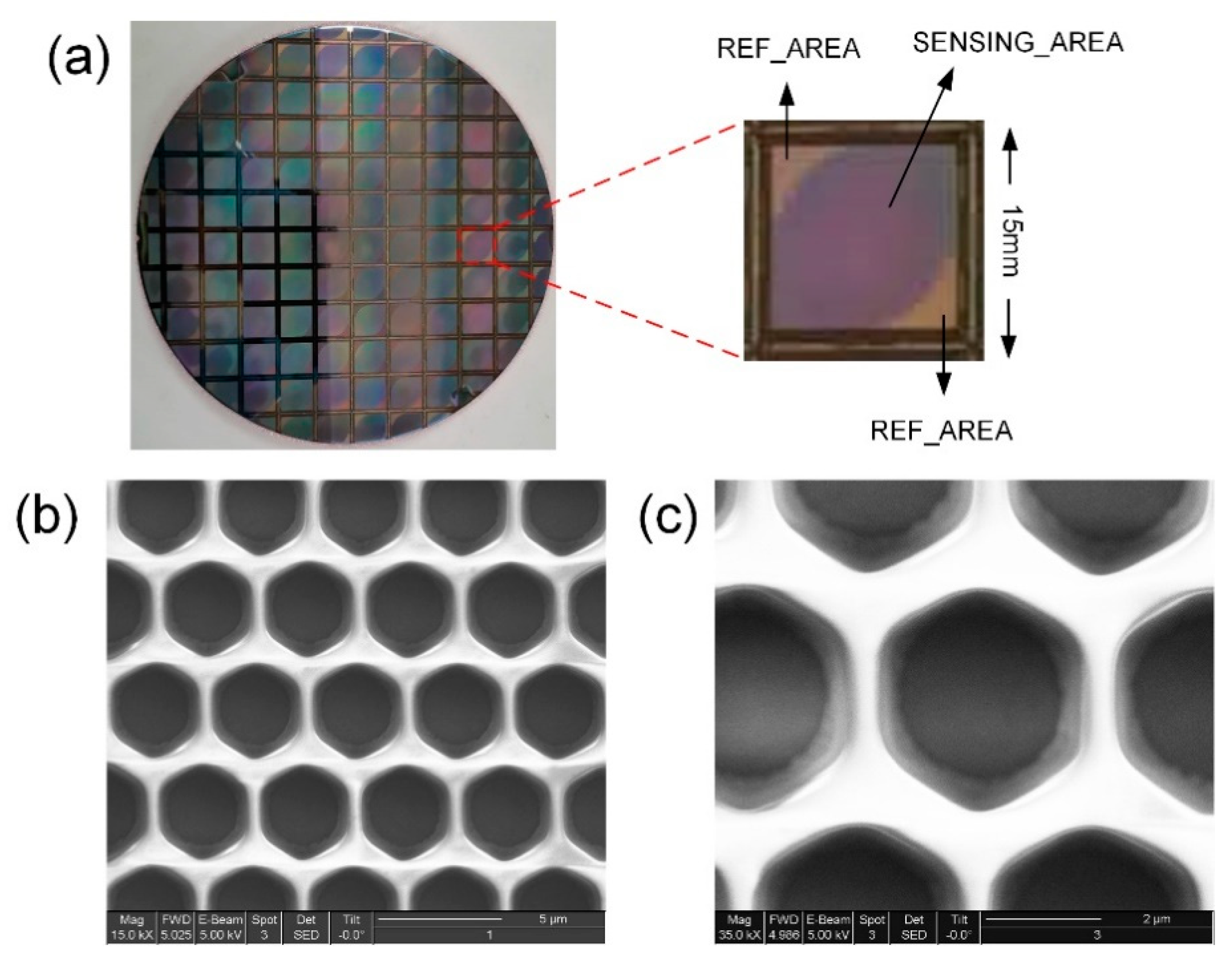
2.2. Sensor Chip Packaging
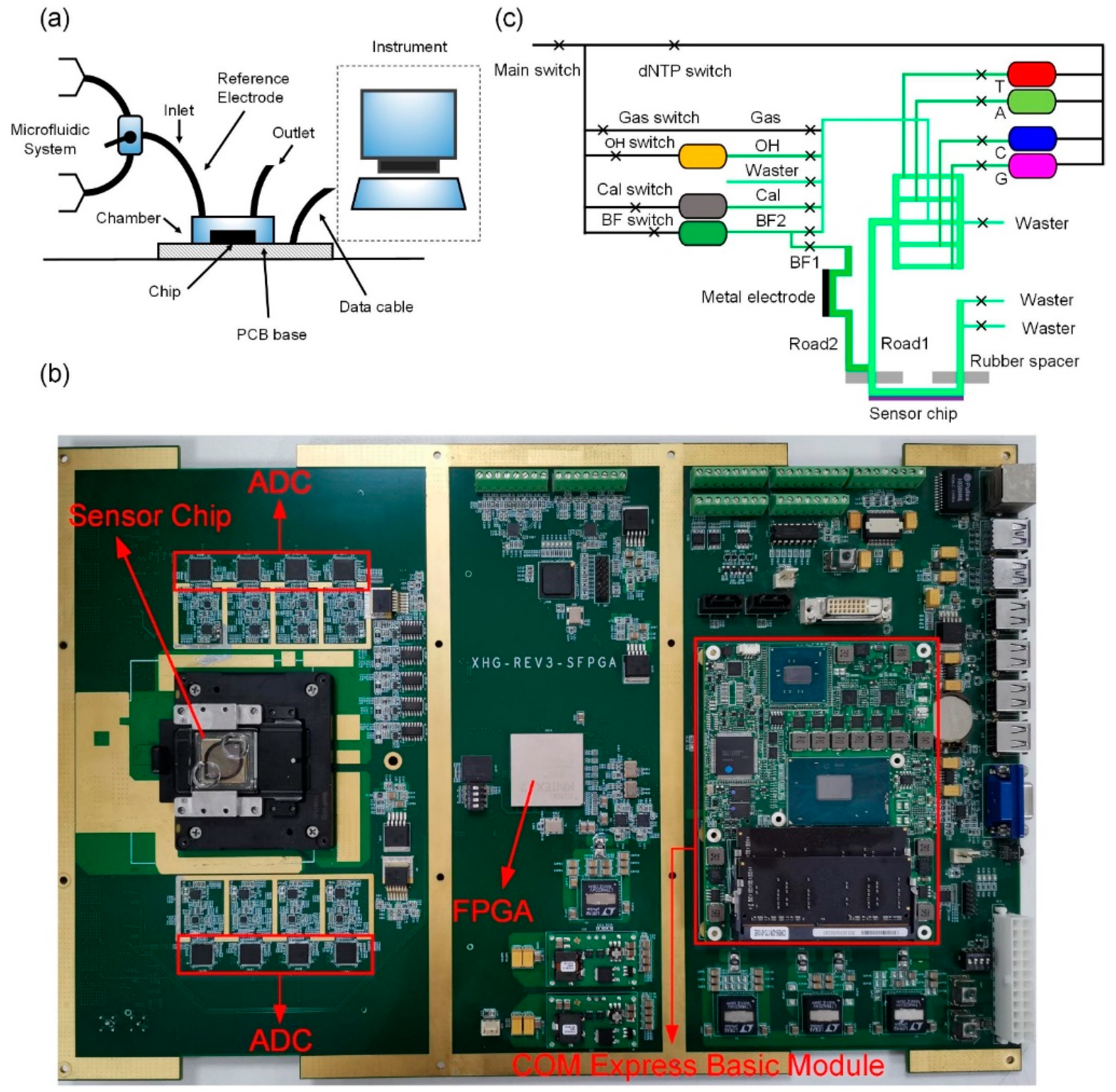
2.3. System Test Platform and Microfluidic Test System
3. Results and Discussion
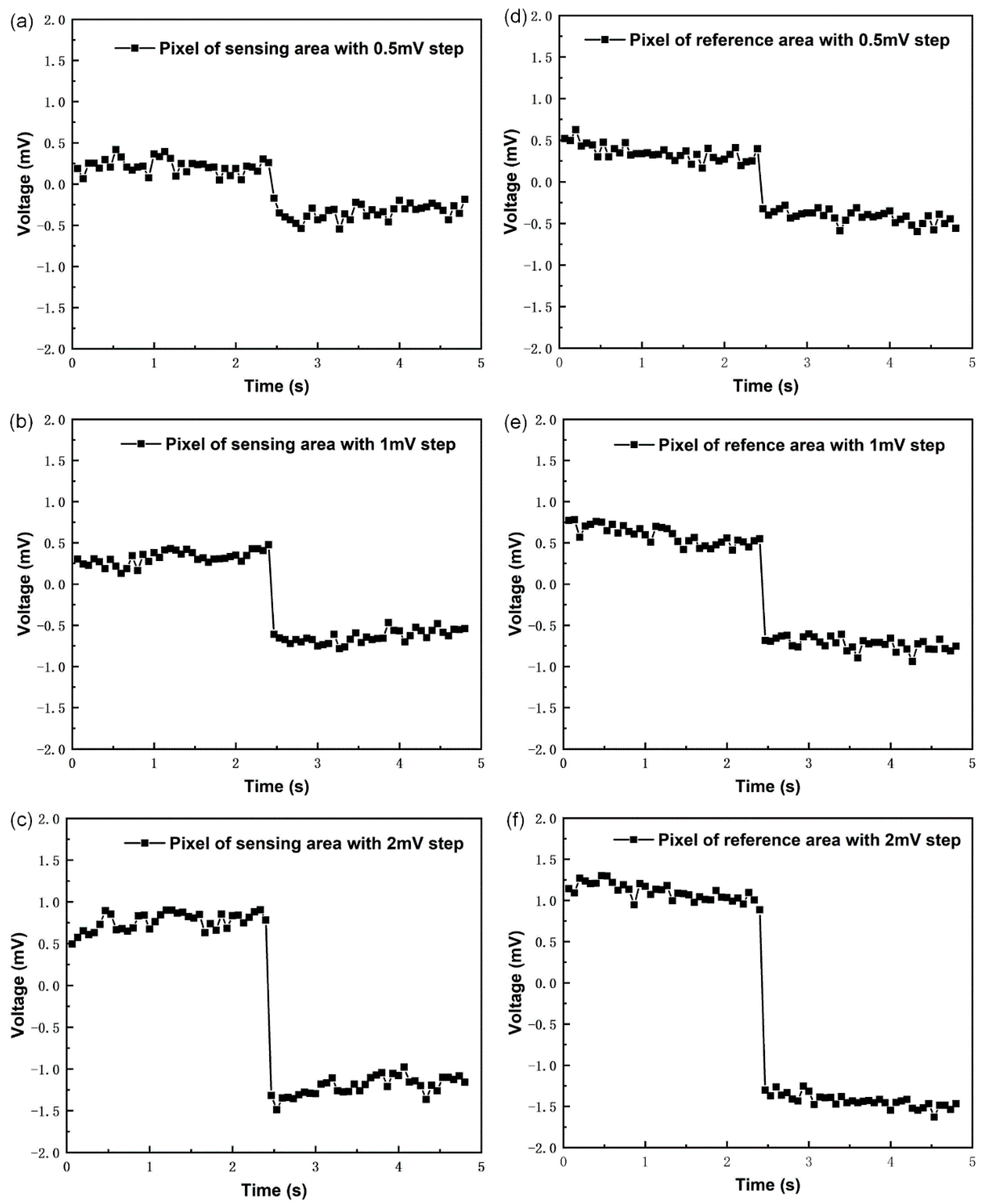
3.1. Electronic Performance
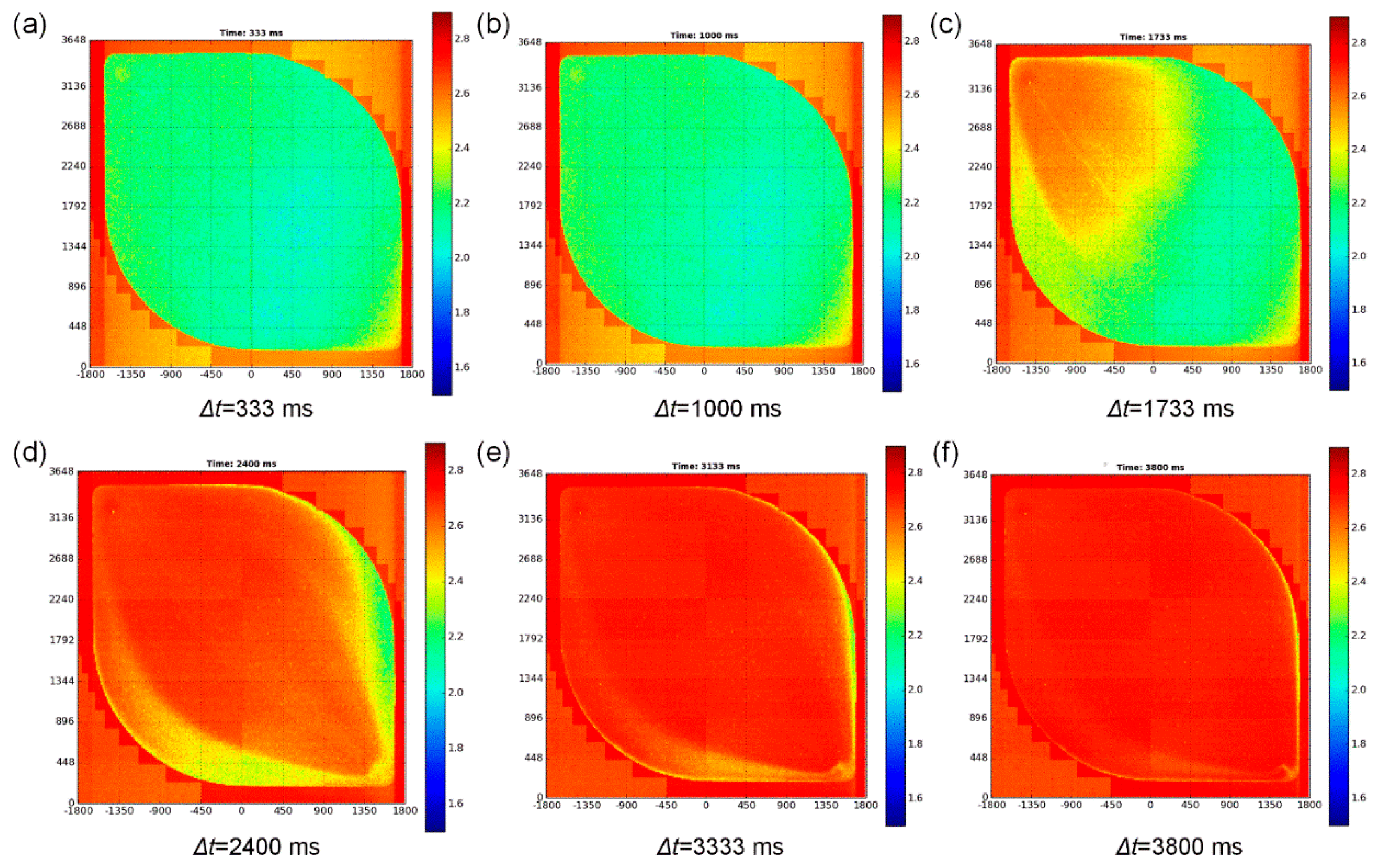
3.2. Electrolyte Flow Is Measured on the Sensor Chip Surface
3.3. PH Sensitivity Test and DNA Base Pairing Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dessouky, A.M.; Taha, T.E.; Dessouky, M.M.; Eltholth, A.A.; Hassan, E.; Abd El-Samie, F.E. Non-parametric spectral estimation techniques for DNA sequence analysis and exon region prediction. Comput. Electr. Eng. 2019, 73, 334–348. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—The next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.K.; Nickerson, E.; Simons, J.F.; Janne, P.A.; Tengs, T.; Yuza, Y.; Garraway, L.A.; LaFramboise, T.; Lee, J.C.; Shah, K.; et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat. Med. 2006, 12, 852–855. [Google Scholar] [CrossRef]
- Hahm, J.; Lieber, C.M. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004, 4, 51–54. [Google Scholar] [CrossRef]
- Park, S.J.; Taton, T.A.; Mirkin, C.A. Array-based electrical detection of DNA with nanoparticle probes. Science 2002, 295, 1503–1506. [Google Scholar]
- von Bubnoff, A. Next-generation sequencing: The race is on. Cell 2008, 132, 721–723. [Google Scholar] [CrossRef] [Green Version]
- Sage, L. Faster, cheaper DNA sequencing. Anal. Chem. 2005, 77, 415–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, T.F.; Mullikin, J.C.; Biesecker, L.G.; Progra, N.C.S. Systematic Evaluation of Sanger Validation of Next-Generation Sequencing Variants. Clin. Chem. 2016, 62, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.X.; Ruan, Q.Y.; Lin, X.Y.; Zhang, M.X.; Song, Y.L.; Zhou, L.J.; Zhu, Z.; Lin, S.C.; Wang, W.; Yang, C.J. Rapid, real-time chemiluminescent detection of DNA mutation based on digital microfluidics and pyrosequencing. Biosens. Bioelectron. 2019, 126, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Wu, H.C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009, 4, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.E.; Parthasarathy, S. Moore’s law: The future of Si microelectronics. Mater. Today 2006, 9, 20–25. [Google Scholar] [CrossRef]
- Mamoshina, P.; Vieira, A.; Putin, E.; Zhavoronkov, A. Applications of Deep Learning in Biomedicine. Mol. Pharm. 2016, 13, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.R.; Sun, J.; Zhuang, Y.; Wei, J.; Li, J.; Dong, L.X.; Yan, D.F.; Hu, A.; Zhou, X.F.; Wang, Z.K. Self-propelled droplet-based electricity generation. Nanoscale 2018, 10, 23164–23169. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.R.; Sun, J.; Li, J.; Xiang, C.H.; Che, L.F.; Wang, Z.K.; Zhou, X.F. Long-range spontaneous droplet self-propulsion on wettability gradient surfaces. Sci. Rep. 2017, 7, 7552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Bae, T.-W. VLSI Friendly ECG QRS Complex Detector for Body Sensor Networks. IEEE J. Emerg. Sel. Top. Circuits Syst. 2012, 2, 52–59. [Google Scholar] [CrossRef]
- Chen, H.J.; Rim, Y.S.; Wang, I.C.; Li, C.; Zhu, B.W.; Sun, M.; Goorsky, M.S.; He, X.M.; Yang, Y. Quasi-Two-Dimensional Metal Oxide Semiconductors Based Ultrasensitive Potentiometric Biosensors. ACS Nano 2017, 11, 4710–4718. [Google Scholar] [CrossRef]
- Hassibi, A.; Manickam, A.; Singh, R.; Bolouki, S.; Sinha, R.; Jirage, K.B.; McDermott, M.W.; Hassibi, B.; Vikalo, H.; Mazarei, G.; et al. Multiplexed identification, quantification and genotyping of infectious agents using a semiconductor biochip. Nat. Biotechnol. 2018, 36, 738–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potluri, S.; Pop, P.; Madsen, J. Design-for-Testability of On-Chip Control in mVLSI Biochips. IEEE Des. Test 2019, 36, 48–56. [Google Scholar] [CrossRef]
- Gao, A.R.; Lu, N.; Wang, Y.C.; Dai, P.F.; Li, T.; Gao, X.L.; Wang, Y.L.; Fan, C.H. Enhanced Sensing of Nucleic Acids with Silicon Nanowire Field Effect Transistor Biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef]
- Gao, A.R.; Yang, X.; Tong, J.; Zhou, L.; Wang, Y.L.; Zhao, J.L.; Mao, H.J.; Li, T. Multiplexed detection of lung cancer biomarkers in patients serum with CMOS-compatible silicon nanowire arrays. Biosens. Bioelectron. 2017, 91, 482–488. [Google Scholar] [CrossRef]
- Gao, A.R.; Zou, N.L.; Dai, P.F.; Lu, N.; Li, T.; Wang, Y.L.; Zhao, J.L.; Mao, H.J. Signal-to-Noise Ratio Enhancement of Silicon Nanowires Biosensor with Rolling Circle Amplification. Nano Lett. 2013, 13, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.R.; Lu, N.; Dai, P.F.; Li, T.; Pei, H.; Gao, X.L.; Gong, Y.B.; Wang, Y.L.; Fan, C.H. Silicon-Nanowire-Based CMOS-Compatible Field-Effect Transistor Nanosensors for Ultrasensitive Electrical Detection of Nucleic Acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, A.R.; Wang, Y.L.; Li, T. Wafer-level and highly controllable fabricated silicon nanowire transistor arrays on (111) silicon-on-insulator (SOI) wafers for highly sensitive detection in liquid and gaseous environments. Nano Res. 2018, 11, 1520–1529. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Fan, K.; Labunov, V.; Lazarouk, S.; Yue, X.; Liu, C.; Yang, X.; Dong, L.; Wang, G. A supersensitive silicon nanowire array biosensor for quantitating tumor marker ctDNA. Biosens. Bioelectron. 2021, 181, 113147. [Google Scholar] [CrossRef] [PubMed]
- Toumazou, C.; Shepherd, L.M.; Reed, S.C.; Chen, G.I.; Patel, A.; Garner, D.M.; Wang, C.J.A.; Ou, C.P.; Amin-Desai, K.; Athanasiou, P.; et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 2013, 10, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Merriman, B.; Rothberg, J.M.; Ion Torrent R&D Team. Progress in Ion Torrent semiconductor chip based sequencing. Electrophoresis 2012, 33, 3397–3417. [Google Scholar] [CrossRef]
- Toumazou, C.; Thay, T.S.L.K.; Georgiou, P. A new era of semiconductor genetics using ion-sensitive field-effect transistors: The gene-sensitive integrated cell. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130112. [Google Scholar] [CrossRef]
- Douthwaite, M.; Koutsos, E.; Yates, D.C.; Mitcheson, P.D.; Georgiou, P. A Thermally Powered ISFET Array for On-Body pH Measurement. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1324–1334. [Google Scholar] [CrossRef]
- Livi, P.; Shadmani, A.; Wipf, M.; Stoop, R.L.; Rothe, J.; Chen, Y.H.; Calame, M.; Schonenberger, C.; Hierlemann, A. Sensor system including silicon nanowire ion sensitive FET arrays and CMOS readout. Sens. Actuator B Chem. 2014, 204, 568–577. [Google Scholar] [CrossRef]
- Moser, N.; Rodriguez-Manzano, J.; Lande, T.S.; Georgiou, P. A Scalable ISFET Sensing and Memory Array With Sensor Auto-Calibration for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Miscourides, N.; Georgiou, P. Impact of Technology Scaling on ISFET Performance for Genetic Sequencing. IEEE Sens. J. 2015, 15, 2219–2226. [Google Scholar] [CrossRef]
- Huang, X.W.; Yu, H.; Liu, X.; Jiang, Y.; Yan, M.; Wu, D.P. A Dual-Mode Large-Arrayed CMOS ISFET Sensor for Accurate and High-Throughput pH Sensing in Biomedical Diagnosis. IEEE Trans. Biomed. Eng. 2015, 62, 2224–2233. [Google Scholar] [CrossRef]
- Miscourides, N.; Yu, L.S.; Rodriguez-Manzano, J.; Georgiou, P. A 12.8 k Current-Mode Velocity-Saturation ISFET Array for On-Chip Real-Time DNA Detection. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.X.; Zhang, J.K.; Sun, C.L.; Xu, M.; Zhang, S.L.; Wu, D.P. A reference-less semiconductor ion sensor. Sens. Actuator B Chem. 2018, 254, 102–109. [Google Scholar] [CrossRef]
- Yeow, T.C.W.; Haskard, M.R.; Mulcahy, D.E.; Seo, H.I.; Kwon, D.H. A very large integrated pH-ISFET sensor array chip compatible with standard CMOS processes. Sens. Actuator B Chem. 1997, 44, 434–440. [Google Scholar] [CrossRef]
- Moser, N.; Panteli, C.; Fobelets, K.; Georgiou, P. Mechanisms for enhancement of sensing performance in CMOS ISFET arrays using reactive ion etching. Sens. Actuator B Chem. 2019, 292, 297–307. [Google Scholar] [CrossRef]
- Bausells, J.; Carrabina, J.; Errachid, A.; Merlos, A. Ion-sensitive field-effect transistors fabricated in a commercial CMOS technology. Sens. Actuator B Chem. 1999, 57, 56–62. [Google Scholar] [CrossRef] [Green Version]
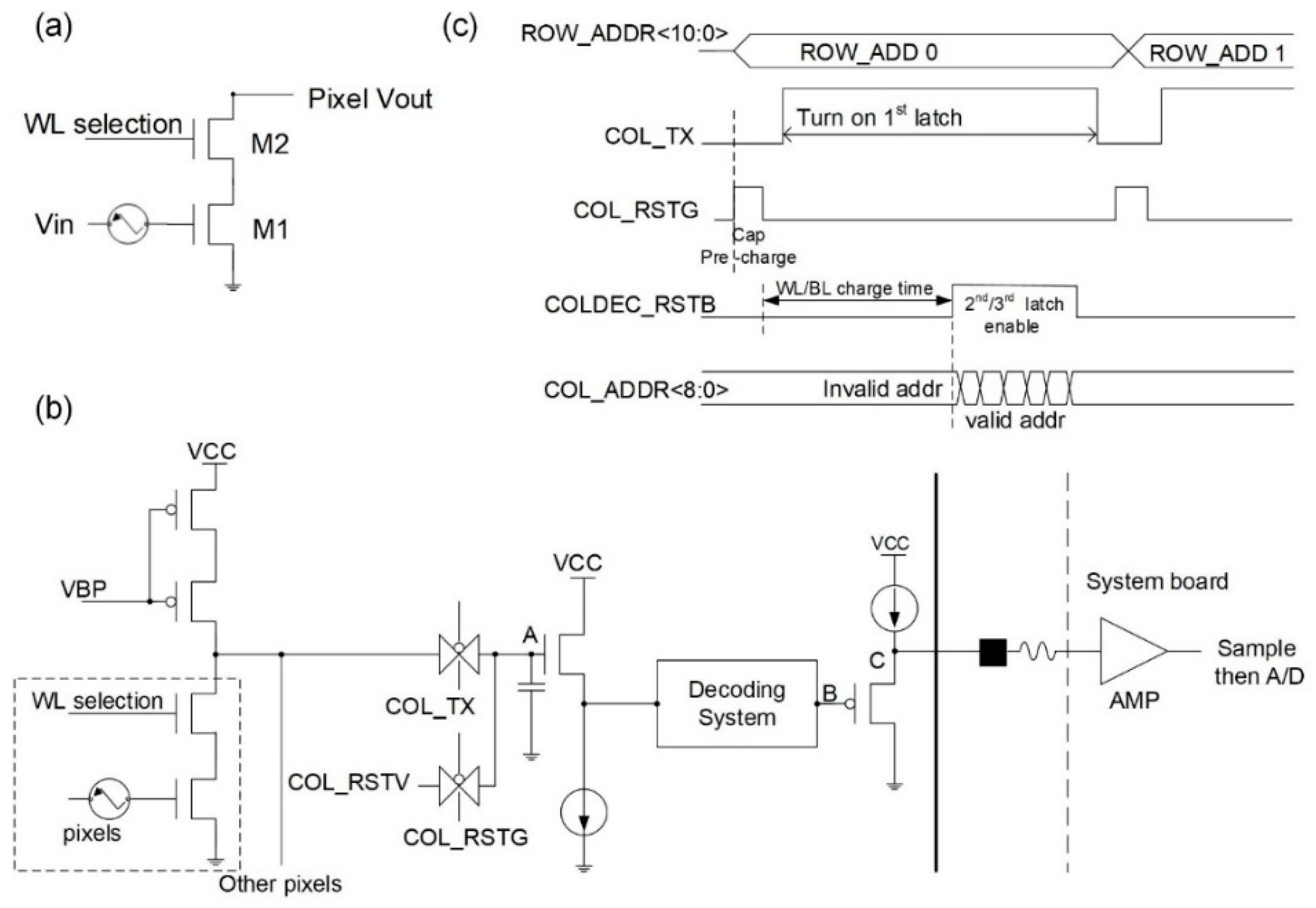


| Signal Name | Description |
|---|---|
| WL selection | word line selection |
| VBP bias | constant voltage bias |
| ROW_ADDR | row address |
| COL_TX | column enable |
| COL_RSTG | pre-charge pulse |
| COL_RSTV | pre-charge voltage |
| COLDEC_RSTB | column reset |
| COL_ADDR | column address |
| AMP | amplifier |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Cong, Y.; Xu, M.; Si, H.; Zhao, D.; Wu, D. An ISFET Microarray Sensor System for Detecting the DNA Base Pairing. Micromachines 2021, 12, 731. https://doi.org/10.3390/mi12070731
Sun P, Cong Y, Xu M, Si H, Zhao D, Wu D. An ISFET Microarray Sensor System for Detecting the DNA Base Pairing. Micromachines. 2021; 12(7):731. https://doi.org/10.3390/mi12070731
Chicago/Turabian StyleSun, Peng, Yongxin Cong, Ming Xu, Huaqing Si, Dan Zhao, and Dongping Wu. 2021. "An ISFET Microarray Sensor System for Detecting the DNA Base Pairing" Micromachines 12, no. 7: 731. https://doi.org/10.3390/mi12070731
APA StyleSun, P., Cong, Y., Xu, M., Si, H., Zhao, D., & Wu, D. (2021). An ISFET Microarray Sensor System for Detecting the DNA Base Pairing. Micromachines, 12(7), 731. https://doi.org/10.3390/mi12070731






