Gold–Carbon Nanocomposites for Environmental Contaminant Sensing
Abstract
:1. Introduction
2. Synthesis and Fabrication of NPs
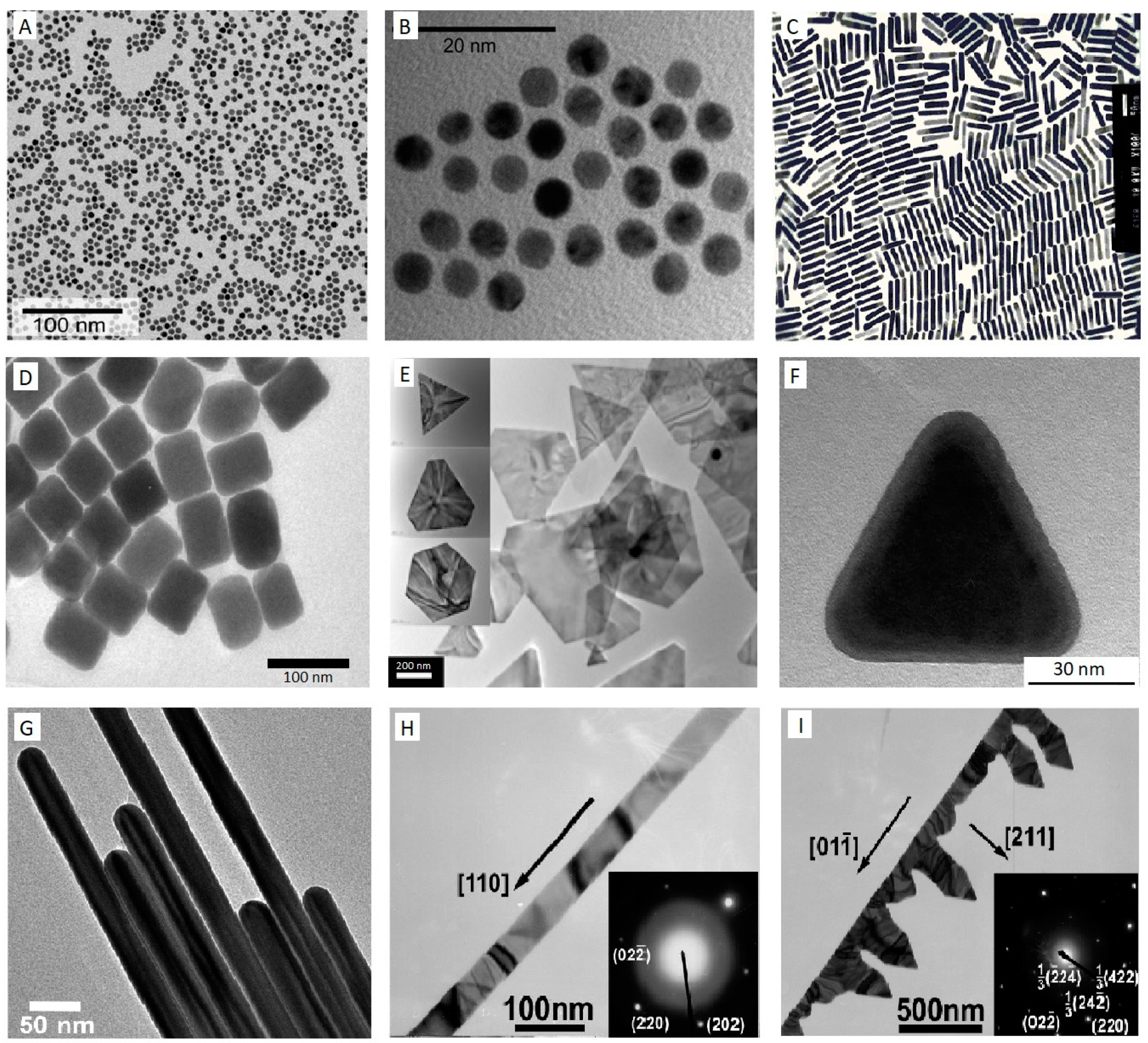
2.1. Gold Nanoparticles
2.2. Carbon Allotropes
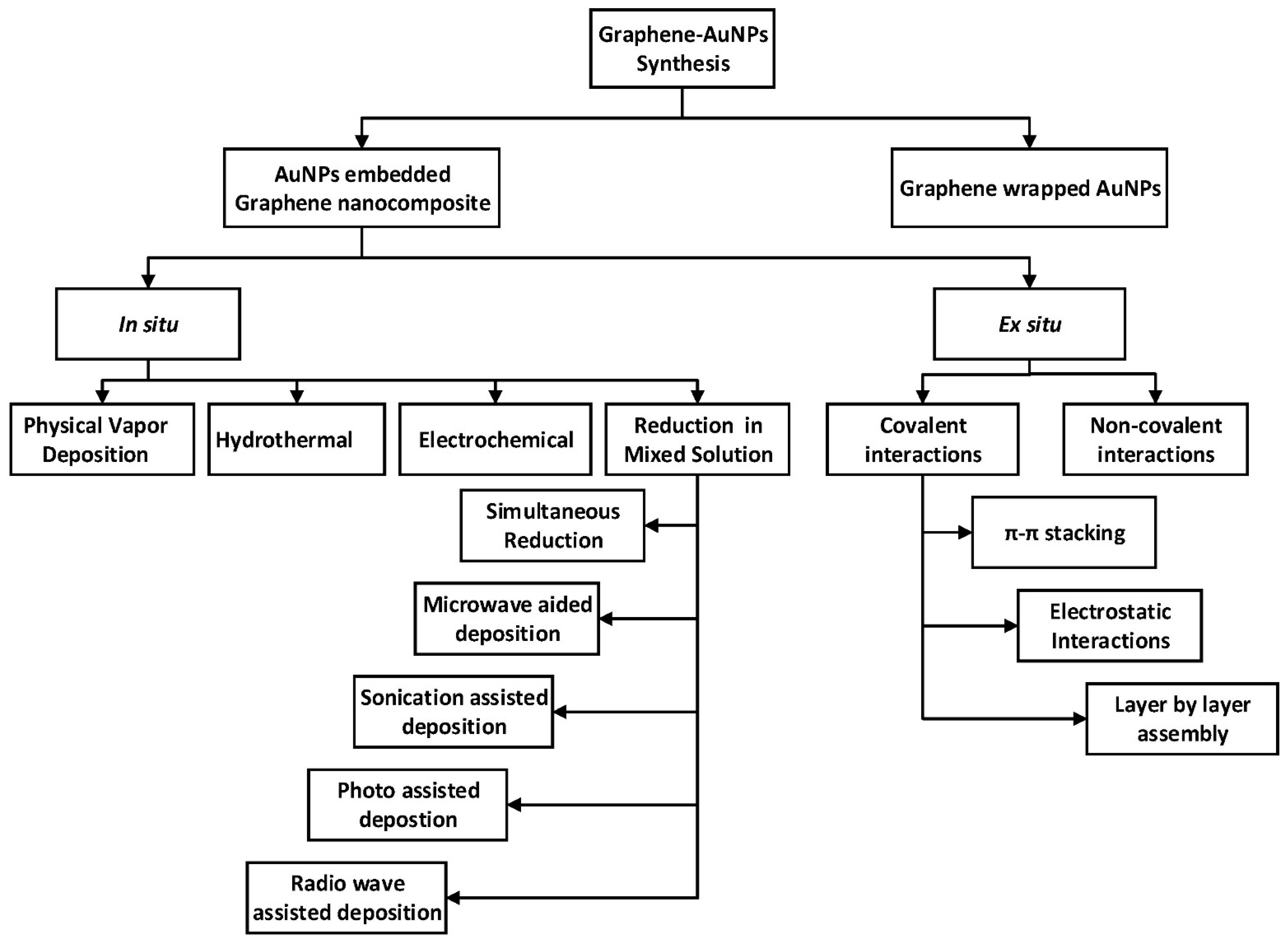
3. Gold–Carbon Nanocomposites
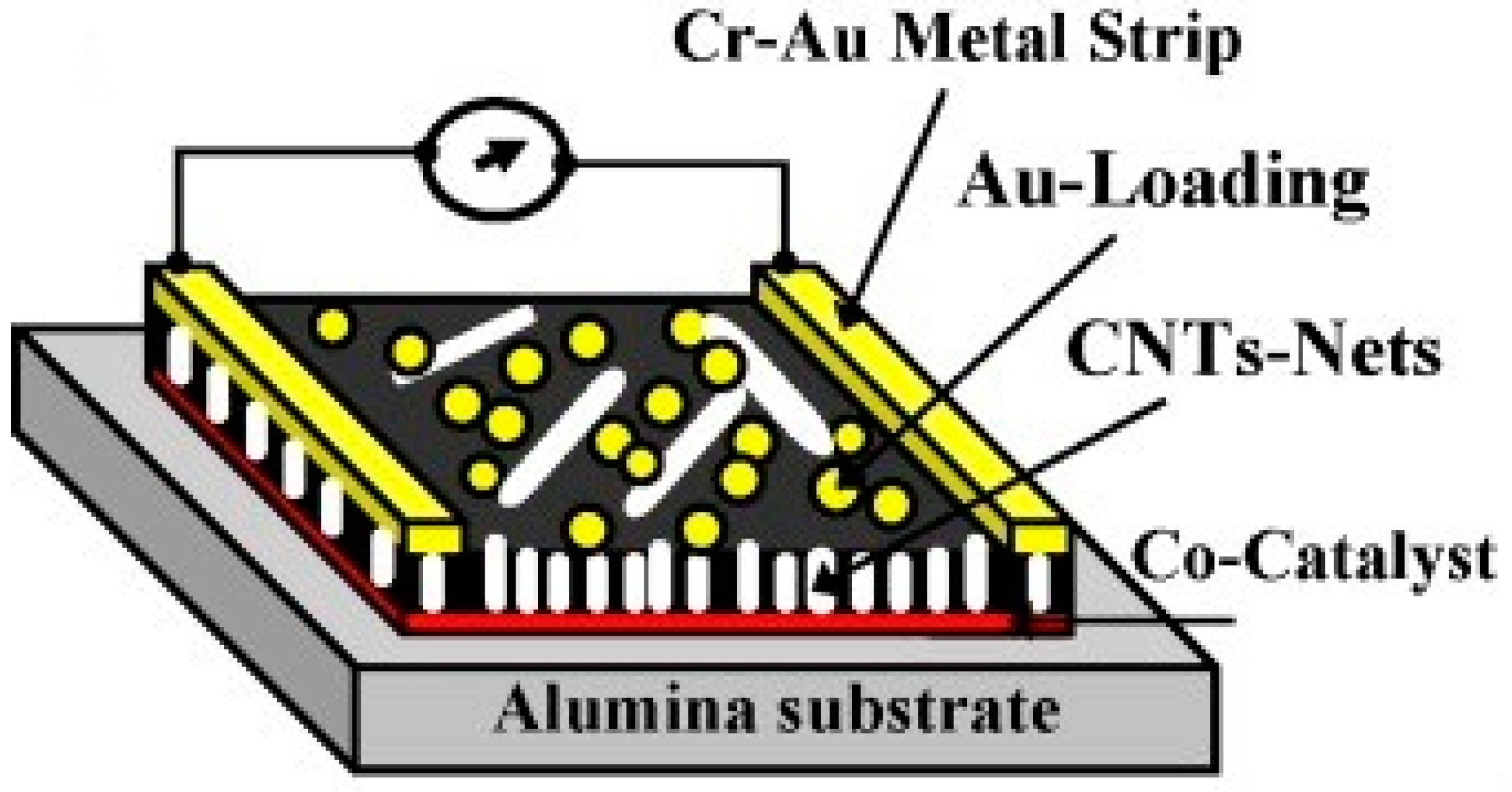
3.1. Gas Sensors
3.2. Toxicant Sensors
3.3. Pesticide Sensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, E.; Hsieh, S.; Organti, N.; Schmidt, D.; Bello, D. A high throughput in vitro analytical approach to screen for oxidative stress potential exerted by nanomaterials using a biologically relevant matrix: Human blood serum. Toxicol. Vitr. 2008, 22, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.; Miola, M.; Ferraris, S.; Ricci, G.; Rožman, K.Ž.; Kostevšek, N.; Catizone, A.; Rimondini, L.; Prat, M.; Verné, E. Tumor targeting by lentiviral vectors combined with magnetic nanoparticles in mice. Acta Biomater. 2017, 59, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Boruah, B.M.; Liang, X.-J. Gold nanoparticles: Promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J. Nanomater. 2011, 2011, 202187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yee, D.; Wang, C. Quantum dots for cancer diagnosis and therapy: Biological and clinical perspectives. Nanomedicine 2008, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Huang, C.Z.; Li, Y.F.; Zhang, L.; Chen, L.Q.; Zhen, S.J. Light-scattering signals from nanoparticles in biochemical assay, pharmaceutical analysis and biological imaging. TrAC Trends Anal. Chem. 2009, 28, 447–453. [Google Scholar] [CrossRef]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Feyziazar, M.; Hasanzadeh, M.; Farshchi, F.; Saadati, A.; Hassanpour, S. An innovative method to electrochemical branching of chitosan in the presence of copper nanocubics on the surface of glassy carbon and its electrical behaviour study: A new platform for pharmaceutical analysis using electrochemical sensors. React. Funct. Polym. 2020, 146, 104402. [Google Scholar] [CrossRef]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Cen, Y.; Kong, X.-J.; Wu, S.; Liu, C.-L.; Yu, R.-Q.; Chu, X. MnO2-nanosheet-modified upconversion nanosystem for sensitive turn-on fluorescence detection of H2O2 and glucose in blood. ACS Appl. Mater. Interfaces 2015, 7, 10548–10555. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, L.; Yang, S.; Zhou, X.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Cobalt-doped ZnO Nanoparticles Derived from Zeolite Imidazole Frameworks: Synthesis, Characterization, and Application for the Detection of an Exhaled Diabetes Biomarker. J. Colloid Interface Sci. 2020, 569, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Vaze, N.; Pyrgiotakis, G.; Mena, L.; Baumann, R.; Demokritou, A.; Ericsson, M.; Zhang, Y.; Bello, D.; Eleftheriadou, M.; Demokritou, P. A nano-carrier platform for the targeted delivery of nature-inspired antimicrobials using Engineered Water Nanostructures for food safety applications. Food Control 2019, 96, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Ma, X. Differential impacts of copper oxide nanoparticles and Copper(II) ions on the uptake and accumulation of arsenic in rice (Oryza sativa). Environ. Pollut. 2019, 252, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Cittadini, M.; Bersani, M.; Perrozzi, F.; Ottaviano, L.; Wlodarski, W.; Martucci, A. Graphene oxide coupled with gold nanoparticles for localized surface plasmon resonance based gas sensor. Carbon 2014, 69, 452–459. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Abdelghafour, M.M.; Deák, Á.; Mérai, L.; Ágoston, Á.; Bélteki, R.; Sebők, D.; Dékány, I.; Janovák, L. Photocatalytic elimination of interfacial water pollutants by floatable photoreactive composite nanoparticles. Environ. Pollut. 2020, 266, 115285. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today 2010, 5, 128–142. [Google Scholar] [CrossRef]
- Thatai, S.; Khurana, P.; Kumar, D. Role of Advanced Materials as Nanosensors in Water Treatment. Biosens. Nanotechnol. 2014, 315–343. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Matbouie, Z.; Asgharinezhad, A.A.; Dehghani, A. Solid phase extraction of Cd (II) and Pb (II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim. Acta 2013, 180, 589–597. [Google Scholar] [CrossRef]
- Da Silva Medeiros, D.C.C.; Piechontcoski, F.; da Rocha Watanabe, E.R.L.; Chaves, E.S.; Inglez, S.D. Fast and effective simultaneous determination of metals in soil samples by ultrasound-assisted extraction and flame atomic absorption spectrometry: Assessment of trace elements contamination in agricultural and native forest soils from Paraná-Brazil. Environ. Monit. Assess. 2020, 192, 111. [Google Scholar] [CrossRef]
- Radu, T.; Diamond, D. Comparison of soil pollution concentrations determined using AAS and portable XRF techniques. J. Hazard. Mater. 2009, 171, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.P.; Karthikeyan, S.; Vijayalekshmy, B.; Iyer, C. Speciative determination of chromium (VI) and chromium (III) using flow-injection on-line preconcentration and flame atomic-absorption spectrometric detection. Anal. Chim. Acta 1998, 369, 69–77. [Google Scholar] [CrossRef]
- De Almeida Azevedo, D.; Lacorte, S.l.; Vinhas, T.; Viana, P.; Barceló, D. Monitoring of priority pesticides and other organic pollutants in river water from Portugal by gas chromatography–mass spectrometry and liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2000, 879, 13–26. [Google Scholar] [CrossRef]
- Reemtsma, T. Liquid chromatography–mass spectrometry and strategies for trace-level analysis of polar organic pollutants. J. Chromatogr. A 2003, 1000, 477–501. [Google Scholar] [CrossRef]
- Sanz-Medel, A.; Montes-Bayón, M.; Sánchez, M.L.F. Trace element speciation by ICP-MS in large biomolecules and its potential for proteomics. Anal. Bioanal. Chem. 2003, 377, 236–247. [Google Scholar] [CrossRef]
- Fitzpatrick, S.; Ebdon, L.; Foulkes, M.E. Separation and detection of arsenic and selenium species in environmental samples by HPLC-ICP-MS. Int. J. Environ. Anal. Chem. 2002, 82, 835–841. [Google Scholar] [CrossRef]
- Moor, C.; Lymberopoulou, T.; Dietrich, V.J. Determination of heavy metals in soils, sediments and geological materials by ICP-AES and ICP-MS. Microchim. Acta 2001, 136, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Boevski, I.; Daskalova, N.; Havezov, I. Determination of barium, chromium, cadmium, manganese, lead and zinc in atmospheric particulate matter by inductively coupled plasma atomic emission spectrometry (ICP-AES). Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 1643–1657. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparza, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537. [Google Scholar] [CrossRef]
- Li, G.-J.; Wu, H.-J.; Wang, Y.; Hung, W.-L.; Rouseff, R.L. Determination of citrus juice coumarins, furanocoumarins and methoxylated flavones using solid phase extraction and HPLC with photodiode array and fluorescence detection. Food Chem. 2019, 271, 29–38. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Foss, D.; Cho, J.; Yoon, Y.; Kosenka, P. Optimization of method for detecting and characterizing NOM by HPLC− size exclusion chromatography with UV and on-line DOC detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, L.; Sun, X.; Du, X.; Yue, Y.; He, D.; Xu, H.; Zeng, Q.; Wang, H.; Ding, L. Analysis of sulfonamides in environmental water samples based on magnetic mixed hemimicelles solid-phase extraction coupled with HPLC–UV detection. Chemosphere 2009, 77, 1306–1312. [Google Scholar] [CrossRef]
- Jin, W.; Maduraiveeran, G. Electrochemical detection of chemical pollutants based on gold nanomaterials. Trends Environ. Anal. Chem. 2017, 14, 28–36. [Google Scholar] [CrossRef]
- Cheng, Y.; Ekker, M.; Chan, H.M. Relative developmental toxicities of pentachloroanisole and pentachlorophenol in a zebrafish model (Danio rerio). Ecotoxicol. Environ. Saf. 2015, 112, 7–14. [Google Scholar] [CrossRef]
- Bernalte, E.; Sánchez, C.M.; Gil, E.P. Gold nanoparticles-modified screen-printed carbon electrodes for anodic stripping voltammetric determination of mercury in ambient water samples. Sens. Actuators B Chem. 2012, 161, 669–674. [Google Scholar] [CrossRef]
- Pho, Q.H.; Losic, D.; Ostrikov, K.; Tran, N.N.; Hessel, V. Perspectives on plasma-assisted synthesis of N-doped nanoparticles as nanopesticides for pest control in crops. React. Chem. Eng. 2020, 5, 1374–1396. [Google Scholar] [CrossRef]
- Hrapovic, S.; Majid, E.; Liu, Y.; Male, K.; Luong, J.H. Metallic nanoparticle—Carbon nanotube composites for electrochemical determination of explosive nitroaromatic compounds. Anal. Chem. 2006, 78, 5504–5512. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Lee, H.K. Simultaneous electrochemical detection of carcinogenic polycyclic aromatic amines in environmental samples using single-walled carbon nanotube-gold nanoparticle composite. Anal. Methods 2010, 2, 326–334. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H. Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with gold nanoparticles for electro-oxidation and sensitive determination of nitrite. Biosens. Bioelectron. 2014, 51, 379–385. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Olin, H. Gold-carbon nanotube nanocomposites: Synthesis and applications. Int. J. Biomed. Nanosci. Nanotechnol. 2011, 2, 112–135. [Google Scholar] [CrossRef]
- Ishida, H.; Campbell, S.; Blackwell, J. General Approach to Nanocomposite Preparation. Chem. Mater. 2000, 12, 1260–1267. [Google Scholar] [CrossRef]
- Haider, W.; Hayat, A.; Raza, Y.; Chaudhry, A.A.; Marty, J.L. Gold nanoparticle decorated single walled carbon nanotube nanocomposite with synergistic peroxidase like activity for d-alanine detection. RSC Adv. 2015, 5, 24853–24858. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, K.; Nam, H.; Shin, K.; Choi, W.; Shin, J.H.; Lim, G. CNT-Au nanocomposite deposition on gold microelectrodes for improved neural recordings. Sens. Actuators B Chem. 2017, 252, 152–158. [Google Scholar] [CrossRef]
- Shi, P.; Xue, R.; Wei, Y.; Lei, X.; Ai, J.; Wang, T.; Shi, Z.; Wang, X.; Wang, Q.; Mohammed Soliman, F.; et al. Gold nanoparticles/tetraaminophenyl porphyrin functionalized multiwalled carbon nanotubes nanocomposites modified glassy carbon electrode for the simultaneous determination of p-acetaminophen and p-aminophenol. Arab. J. Chem. 2020, 13, 1040–1051. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Sun, D.; Zhang, R.; Lin, W.-F.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold nanoparticle-polymer nanocomposites synthesized by room temperature atmospheric pressure plasma and their potential for fuel cell electrocatalytic application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.; Xu, Y.; Song, W. One-step synthesis of 3D dendritic gold@ polypyrrole nanocomposites via a simple self-assembly method and their electrocatalysis for H2O2. Electrochim. Acta 2012, 60, 71–77. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. Developing a novel sensor based on ionic liquid molecularly imprinted polymer/gold nanoparticles/graphene oxide for the selective determination of an anti-cancer drug imiquimod. Biosens. Bioelectron. 2019, 143, 111620. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.E.; Sanghavi, B.J.; Farmehini, V.; Chávez, J.L.; Hagen, J.; Kelley-Loughnane, N.; Chou, C.-F.; Swami, N.S. Aptamer-functionalized graphene-gold nanocomposites for label-free detection of dielectrophoretic-enriched neuropeptide Y. Electrochem. Commun. 2016, 72, 144–147. [Google Scholar] [CrossRef]
- Lai, G.; Cheng, H.; Yin, C.; Fu, L.; Yu, A. One-Pot Preparation of Graphene/Gold Nanocomposites for Ultrasensitive Nonenzymatic Electrochemical Immunoassay. Electroanalysis 2016, 28, 69–75. [Google Scholar] [CrossRef]
- Yu, Y.; Si, J.; Yan, L.; Li, M.; Hou, X. Enhanced nonlinear absorption and ultrafast carrier dynamics in graphene/gold nanoparticles nanocomposites. Carbon 2019, 148, 72–79. [Google Scholar] [CrossRef]
- Odaci, D.; Kahveci, M.U.; Sahkulubey, E.L.; Ozdemir, C.; Uyar, T.; Timur, S.; Yagci, Y. In situ synthesis of biomolecule encapsulated gold-cross-linked poly (ethylene glycol) nanocomposite as biosensing platform: A model study. Bioelectrochemistry 2010, 79, 211–217. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, F.; Liu, B.; Ding, J.; Xu, X.; Kong, J. A sensitive impedance immunosensor based on functionalized gold nanoparticle–protein composite films for probing apolipoprotein AI. Talanta 2007, 71, 874–881. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Hwang, S.; Lee, J.S.; Kim, S. Self-assembled gold nanoparticle–mixed metal oxide nanocomposites for self-sensitized dye degradation under visible light irradiation. Langmuir 2012, 28, 17530–17536. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Miao, Z.; Ma, M.; Du, X.; Lin, J.; Han, B.; Takahashi, S.; Anzai, J.-i.; Chen, Q. Dual-function amperometric sensors based on poly (diallydimethylammoniun chloride)-functionalized reduced graphene oxide/manganese dioxide/gold nanoparticles nanocomposite. Sens. Actuators B Chem. 2016, 222, 663–673. [Google Scholar] [CrossRef]
- Er, E.; Çelikkan, H.; Erk, N.; Aksu, M.L. A new generation electrochemical sensor based on Graphene nanosheets/Gold nanoparticles/Nafion nanocomposite for determination of Silodosin. Electrochim. Acta 2015, 157, 252–257. [Google Scholar] [CrossRef]
- Mathew, M.; Sureshkumar, S.; Sandhyarani, N. Synthesis and characterization of gold–chitosan nanocomposite and application of resultant nanocomposite in sensors. Colloids Surf. B Biointerfaces 2012, 93, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Drmosh, Q.A.; Hendi, A.H.; Hossain, M.K.; Yamani, Z.H.; Moqbel, R.A.; Hezam, A.; Gondal, M.A. UV-activated gold decorated rGO/ZnO heterostructured nanocomposite sensor for efficient room temperature H2 detection. Sens. Actuators B Chem. 2019, 290, 666–675. [Google Scholar] [CrossRef]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Guo, Y.; Sun, X.; Zheng, Y.; Wang, X. An acetylcholinesterase biosensor based on graphene–gold nanocomposite and calcined layered double hydroxide. Enzym. Microb. Technol. 2014, 58, 8–13. [Google Scholar] [CrossRef]
- Hatami, Z.; Ragheb, E.; Jalali, F.; Tabrizi, M.A.; Shamsipur, M. Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry 2020, 133, 107458. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.S.; Costa, D.; Domingues, R.; Apreutesei, M.; Pedrosa, P.; Martin, N.; Correlo, V.; Reis, R.; Alves, E.; Barradas, N. Optimization of nanocomposite Au/TiO2 thin films towards LSPR optical-sensing. Appl. Surf. Sci. 2018, 438, 74–83. [Google Scholar] [CrossRef]
- Mohan, P.; Shinta, R.; Fujiwara, J.; Takahashi, H.; Mott, D.; Matsumura, Y.; Mizutani, G.; Iwami, K.; Umeda, N.; Maenosono, S. Boehmite nanorod/gold nanoparticle nanocomposite film for an easy-to-use optical humidity sensor. Sens. Actuators B Chem. 2012, 168, 429–435. [Google Scholar] [CrossRef]
- Massaro, A.; Spano, F.; Cazzato, P.; Cingolani, R.; Athanassiou, A. Real time optical pressure sensing for tactile detection using gold nanocomposite material. Microelectron. Eng. 2011, 88, 2767–2770. [Google Scholar] [CrossRef]
- Yuan, X.; He, Y.; Zhou, G.; Li, X.; Feng, A.; Zheng, W. Target challenging-cancer drug delivery to gastric cancer tissues with a fucose graft epigallocatechin-3-gallate-gold particles nanocomposite approach. J. Photochem. Photobiol. B Biol. 2018, 183, 147–153. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M. Gold nanorods–graphene oxide nanocomposite incorporated carbon nanotube paste modified glassy carbon electrode for voltammetric determination of indomethacin. Sens. Actuators B Chem. 2013, 186, 622–632. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Z.; Fang, J.; Wang, B.; Lin, Z.; Chen, Z.-S.; Chen, Y.; Zhang, N.; Yang, X.; Gao, W. A multi-functionalized nanocomposite constructed by gold nanorod core with triple-layer coating to combat multidrug resistant colorectal cancer. Mater. Sci. Eng. C 2020, 107, 110224. [Google Scholar] [CrossRef]
- Singh, R.; Premkumar, T.; Shin, J.Y.; Geckeler, K.E. Carbon Nanotube and Gold-Based Materials: A Symbiosis. Chem. Eur. J. 2010, 16, 1728–1743. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Xu, L.; Chen, W.; Zhu, Y.; Xu, C.; Kotov, N.A. Nanoparticle-based environmental sensors. Mater. Sci. Eng. R Rep. 2010, 70, 265–274. [Google Scholar] [CrossRef]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.K.; Modi, T.; Aguilar, Z.P.; Rodgers, H.; Hamilton, W.; Marutharaj, T.; Webb, C. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. (Landmark Ed.) 2014, 19, 1320–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.S.; Sachar, S.; Kaur, G.; Bhandari, P.; Kaur, G.; Biesinger, M.C.; Possmayer, F.; Petersen, N.O. Dependence of crystal growth of gold nanoparticles on the capping behavior of surfactant at ambient conditions. Cryst. Growth Des. 2008, 8, 1713–1719. [Google Scholar] [CrossRef]
- Kambayashi, M.; Zhang, J.; Oyama, M. Crystal growth of gold nanoparticles on indium tin oxides in the absence and presence of 3-mercaptopropyl-trimethoxysilane. Cryst. Growth Des. 2005, 5, 81–84. [Google Scholar] [CrossRef]
- González-Rubio, G.; Scarabelli, L.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Surfactant-Assisted Symmetry Breaking in Colloidal Gold Nanocrystal Growth. ChemNanoMat 2020, 6, 698–707. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Lam, E.; Hrapovic, S.; Majid, E.; Chong, J.H.; Luong, J.H. Catalysis using gold nanoparticles decorated on nanocrystalline cellulose. Nanoscale 2012, 4, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Pandikumar, A.; Ramaraj, R. Titanium dioxide–gold nanocomposite materials embedded in silicate sol–gel film catalyst for simultaneous photodegradation of hexavalent chromium and methylene blue. J. Hazard. Mater. 2012, 203–204, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Carregal-Romero, S.; Buurma, N.J.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hervés, P. Catalysis by Au@pNIPAM Nanocomposites: Effect of the Cross-Linking Density. Chem. Mater. 2010, 22, 3051–3059. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, M.; Uhlmann, P.; Simon, F.; Oertel, U.; Stamm, M. Gold nanoparticles immobilized on stimuli responsive polymer brushes as nanosensors. Macromolecules 2008, 41, 8152–8158. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Daniel, W.L.; Xia, Z.; Xie, H.; Mirkin, C.A.; Rao, J. Bioluminescent nanosensors for protease detection based upon gold nanoparticle–luciferase conjugates. Chem. Commun. 2010, 46, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Shumyantseva, V.V.; Carrara, S.; Bavastrello, V.; Riley, D.J.; Bulko, T.V.; Skryabin, K.G.; Archakov, A.I.; Nicolini, C. Direct electron transfer between cytochrome P450scc and gold nanoparticles on screen-printed rhodium–graphite electrodes. Biosens. Bioelectron. 2005, 21, 217–222. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, X.; Zhang, J.; Zhu, J.; Wong, D.K. Enhancing direct electron transfer of glucose oxidase using a gold nanoparticle| titanate nanotube nanocomposite on a biosensor. Electrochim. Acta 2015, 163, 64–70. [Google Scholar] [CrossRef]
- Zhong, D.; Yang, K.; Wang, Y.; Yang, X. Dual-channel sensing strategy based on gold nanoparticles cooperating with carbon dots and hairpin structure for assaying RNA and DNA. Talanta 2017, 175, 217–223. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Liang, S.; Zhang, J. Detection of Hg(II) in adsorption experiment by a lateral flow biosensor based on streptavidin-biotinylated DNA probes modified gold nanoparticles and smartphone reader. Environ. Pollut. 2020, 266, 115389. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Julkapli, N.M.; Rahmati, S.; Sina, A.A.I.; Basirun, W.J.; Johan, M.R. Graphene oxide and gold nanoparticle based dual platform with short DNA probe for the PCR free DNA biosensing using surface-enhanced Raman scattering. Biosens. Bioelectron. 2019, 131, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.-L.; Xu, J.-J.; Zhang, Q.; Yang, G.-J.; Chen, H.-Y. Electrochemically deposited chitosan hydrogel for horseradish peroxidase immobilization through gold nanoparticles self-assembly. Biosens. Bioelectron. 2005, 21, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, M.; Dujardin, E.; Girard, C.; Mann, S. One-dimensional plasmon coupling by facile self-assembly of gold nanoparticles into branched chain networks. Adv. Mater. 2005, 17, 2553–2559. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20. [Google Scholar] [CrossRef]
- Calandra, P.; Calogero, G.; Sinopoli, A.; Gucciardi, P.G. Metal nanoparticles and carbon-based nanostructures as advanced materials for cathode application in dye-sensitized solar cells. Int. J. Photoenergy 2010, 2010. [Google Scholar] [CrossRef]
- Mazid, R.R.; Si, K.J.; Cheng, W. DNA based strategy to nanoparticle superlattices. Methods 2014, 67, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Chang, S.-S.; Shih, C.-W.; Chen, C.-D.; Lai, W.-C.; Wang, C.C. The shape transition of gold nanorods. Langmuir 1999, 15, 701–709. [Google Scholar] [CrossRef]
- Niu, W.; Zheng, S.; Wang, D.; Liu, X.; Li, H.; Han, S.; Chen, J.; Tang, Z.; Xu, G. Selective synthesis of single-crystalline rhombic dodecahedral, octahedral, and cubic gold nanocrystals. J. Am. Chem. Soc. 2008, 131, 697–703. [Google Scholar] [CrossRef]
- Wu, H.-L.; Kuo, C.-H.; Huang, M.H. Seed-mediated synthesis of gold nanocrystals with systematic shape evolution from cubic to trisoctahedral and rhombic dodecahedral structures. Langmuir 2010, 26, 12307–12313. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Liang, H.; Xu, H. Surfactant-promoted reductive synthesis of shape-controlled gold nanostructures. Cryst. Growth Des. 2008, 9, 858–862. [Google Scholar] [CrossRef]
- Liu, B.; Xie, J.; Lee, J.; Ting, Y.; Chen, J.P. Optimization of high-yield biological synthesis of single-crystalline gold nanoplates. J. Phys. Chem. B 2005, 109, 15256–15263. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Shape-controlled synthesis of gold nanoprism and nanoperiwinkles with pronounced electrocatalytic activity. J. Phys. Chem. C 2007, 111, 15146–15153. [Google Scholar] [CrossRef]
- Kim, F.; Sohn, K.; Wu, J.; Huang, J. Chemical synthesis of gold nanowires in acidic solutions. J. Am. Chem. Soc. 2008, 130, 14442–14443. [Google Scholar] [CrossRef]
- Karim, S.; Toimil-Molares, M.; Maurer, F.; Miehe, G.; Ensinger, W.; Liu, J.; Cornelius, T.; Neumann, R. Synthesis of gold nanowires with controlled crystallographic characteristics. Applied Phys. A 2006, 84, 403–407. [Google Scholar] [CrossRef]
- Zhao, N.; Wei, Y.; Sun, N.; Chen, Q.; Bai, J.; Zhou, L.; Qin, Y.; Li, M.; Qi, L. Controlled synthesis of gold nanobelts and nanocombs in aqueous mixed surfactant solutions. Langmuir 2008, 24, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, J.; Han, B.; Liu, Z.; Jiang, T.; Zhang, Z. Sonochemical formation of single-crystalline gold nanobelts. Angew. Chem. Int. Ed. 2006, 45, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Raliya, R.; Saha, D.; Chadha, T.S.; Raman, B.; Biswas, P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci. Rep. 2017, 7, 44718. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.; Govindaraj, A. Nanotubes and Nanowires; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Qingkun, L.; Yi, S.; Zhiyuan, L.; Yu, Z. Lonsdaleite—A material stronger and stiffer than diamond. Scr. Mater. 2011, 65, 229–232. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639. [Google Scholar]
- Silva, R.; Silva, S. Properties of Amorphous Carbon; Institution of Engineering and Technology: Hertfordshire, UK, 2003. [Google Scholar]
- Nayfeh, M. (Ed.) Chapter 10—Nanocarbon. In Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines; Elsevier: Amsterdam, The Netherlands, 2008; pp. 287–309. [Google Scholar]
- Saba, N.; Jawaid, M.; Fouad, H.; Alothman, O.Y. 9—Nanocarbon: Preparation, properties, and applications. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin, Asiri, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 327–354. [Google Scholar]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Joseph Botlhoko, O.; Setshedi, K.; Scriba, M.; Masukume, M.; Sinha Ray, S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Ji, R.; Teng, K.S.; Tai, G.; Ye, J.; Wei, C.; Lau, S.P. Bottom-up synthesis of large-scale graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 5676–5683. [Google Scholar] [CrossRef]
- Riedl, C.; Coletti, C.; Starke, U. Structural and electronic properties of epitaxial graphene on SiC(0 0 0 1): A review of growth, characterization, transfer doping and hydrogen intercalation. J. Phys. D Appl. Phys. 2010, 43, 374009. [Google Scholar] [CrossRef] [Green Version]
- Emtsev, K.V.; Speck, F.; Seyller, T.; Ley, L.; Riley, J.D. Interaction, growth, and ordering of epitaxial graphene on SiC{0001} surfaces: A comparative photoelectron spectroscopy study. Phys. Rev. B 2008, 77, 155303. [Google Scholar] [CrossRef] [Green Version]
- Fogarassy, Z.; Rümmeli, M.H.; Gorantla, S.; Bachmatiuk, A.; Dobrik, G.; Kamarás, K.; Biró, L.P.; Havancsák, K.; Lábár, J.L. Dominantly epitaxial growth of graphene on Ni (111) substrate. Appl. Surf. Sci. 2014, 314, 490–499. [Google Scholar] [CrossRef]
- Gao, M.; Pan, Y.; Huang, L.; Hu, H.; Zhang, L.Z.; Guo, H.M.; Du, S.X.; Gao, H.J. Epitaxial growth and structural property of graphene on Pt(111). Appl. Phys. Lett. 2011, 98, 033101. [Google Scholar] [CrossRef]
- Van Wesep, R.G.; Chen, H.; Zhu, W.; Zhang, Z. Communication: Stable carbon nanoarches in the initial stages of epitaxial growth of graphene on Cu(111). J. Chem. Phys. 2011, 134, 171105. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.; Grandthyll, S.; Zeitz, C.; Jacobs, K.; Hüfner, S.; Gsell, S.; Schreck, M. Epitaxial growth of graphene on Ir(111) by liquid precursor deposition. Phys. Rev. B 2011, 84, 075472. [Google Scholar] [CrossRef]
- Varykhalov, A.; Rader, O. Graphene grown on Co(0001) films and islands: Electronic structure and its precise magnetization dependence. Phys. Rev. B 2009, 80, 035437. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Q.-Y.; Han, B.-H. Solvothermal synthesis of homogeneous graphene dispersion with high concentration. Carbon 2011, 49, 3920–3927. [Google Scholar] [CrossRef]
- Al-Hazmi, F.S.; Al-Harbi, G.H.; Beall, G.W.; Al-Ghamdi, A.A.; Obaid, A.Y.; Mahmoud, W.E. One pot synthesis of graphene based on microwave assisted solvothermal technique. Synth. Met. 2015, 200, 54–57. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y. Controllable Synthesis of Graphene and Its Applications. Adv. Mater. 2010, 22, 3225–3241. [Google Scholar] [CrossRef]
- Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Räder, H.J.; Müllen, K. Two-Dimensional Graphene Nanoribbons. J. Am. Chem. Soc. 2008, 130, 4216–4217. [Google Scholar] [CrossRef]
- Raidongia, K.; Tan, A.T.L.; Huang, J. Chapter 14—Graphene Oxide: Some New Insights into an Old Material. In Carbon Nanotubes and Graphene, 2nd ed.; Tanaka, K., Iijima, S., Eds.; Elsevier: Oxford, UK, 2014; pp. 341–374. [Google Scholar]
- Ahammad, A.J.S.; Islam, T.; Hasan, M.M. Chapter 12—Graphene-Based Electrochemical Sensors for Biomedical Applications. In Biomedical Applications of Graphene and 2D Nanomaterials; Nurunnabi, M., McCarthy, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–282. [Google Scholar]
- Ajayan, P. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Moses, J.C.; Gangrade, A.; Mandal, B.B. Chapter 5—Carbon Nanotubes and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amaterdam, The Netherlands, 2019; pp. 145–175. [Google Scholar]
- Szabó, A.; Perri, C.; Csató, A.; Giordano, G.; Vuono, D.; Nagy, J.B. Synthesis Methods of Carbon Nanotubes and Related Materials. Materials 2010, 3, 3092–3140. [Google Scholar] [CrossRef]
- Liu, B.; Lu, L.; Hua, E.; Jiang, S.; Xie, G. Detection of the human prostate-specific antigen using an aptasensor with gold nanoparticles encapsulated by graphitized mesoporous carbon. Microchim. Acta 2012, 178, 163–170. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Perera, V.S.; Park, B.-W.; Gao, M.; McGimpsey, G.W.; Huang, S.D.; Jaroniec, M. Graphitic Mesoporous Carbons with Embedded Prussian Blue-Derived Iron Oxide Nanoparticles Synthesized by Soft Templating and Low-Temperature Graphitization. Chem. Mater. 2013, 25, 2803–2811. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon Nanofibers and Their Composites: A Review of Synthesizing, Properties and Applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Hassler, M. 3—Other commonly used biomedical coatings: Pyrolytic carbon coatings. In Coatings for Biomedical Applications; Driver, M., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 75–105. [Google Scholar]
- Zittel, H.E.; Miller, F.J. A Glassy-Carbon Electrode for Voltammetry. Anal. Chem. 1965, 37, 200–203. [Google Scholar] [CrossRef]
- Valdevit, L.; Bauer, J. Chapter 13.1—Fabrication of 3D micro-/nanoarchitected materials. In Three-Dimensional Microfabrication Using Two-Photon Polymerization, 2nd ed.; Baldacchini, T., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2020; pp. 541–576. [Google Scholar]
- Krajewska, A.; Radecki, J.; Radecka, H. A Voltammetric Biosensor Based on Glassy Carbon Electrodes Modified with Single-Walled Carbon Nanotubes/Hemoglobin for Detection of Acrylamide in Water Extracts from Potato Crisps. Sensors 2008, 8, 5832–5844. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Grøndahl, L.; Jack, K.S.; Goonasekera, C.S. 5—Composite materials for bone repair. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 83–110. [Google Scholar]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Song, Y.; Xu, T.; Xu, L.-P.; Zhang, X. Nanodendritic gold/graphene-based biosensor for tri-mode miRNA sensing. Chem. Commun. 2019, 55, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Bahar, N.; Ekinci, D. Hollow porous gold nanoparticle/reduced graphene oxide composite films for electrochemical supercapacitor applications. Electrochim. Acta 2020, 337, 135844. [Google Scholar] [CrossRef]
- Siavash Moakhar, R.; AbdelFatah, T.; Sanati, A.; Jalali, M.; Flynn, S.E.; Mahshid, S.S.; Mahshid, S. A Nanostructured Gold/Graphene Microfluidic Device for Direct and Plasmonic-Assisted Impedimetric Detection of Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 23298–23310. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Deng, D.-H.; Shi, Q.; Liu, S.-R. Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim. Acta 2012, 177, 325–331. [Google Scholar] [CrossRef]
- Minati, L.; Antonini, V.; Dalla Serra, M.; Speranza, G. Multifunctional Branched Gold–Carbon Nanotube Hybrid for Cell Imaging and Drug Delivery. Langmuir 2012, 28, 15900–15906. [Google Scholar] [CrossRef]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. Plasmonic gold decorated MWCNT nanocomposite for localized plasmon resonance sensing. Sci. Rep. 2015, 5, 13181. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Huang, J.; Wang, J.; Wang, C.; Li, M.; Liu, J. Enhanced electrochemical detection of DNA hybridization based on Au/MWCNTs nanocomposites. Anal. Lett. 2007, 40, 3159–3169. [Google Scholar] [CrossRef]
- Mohammadnezhad, M.; Selopal, G.S.; Cavuslar, O.; Barba, D.; Durmusoglu, E.G.; Acar, H.Y.; Wang, Z.M.; Lopinski, G.P.; Stansfield, B.; Zhao, H.; et al. Gold nanoparticle decorated carbon nanotube nanocomposite for dye-sensitized solar cell performance and stability enhancement. Chem. Eng. J. 2020, 127756. [Google Scholar] [CrossRef]
- Metz, K.M.; Colavita, P.E.; Tse, K.-Y.; Hamers, R.J. Nanotextured gold coatings on carbon nanofiber scaffolds as ultrahigh surface-area electrodes. J. Power Sources 2012, 198, 393–401. [Google Scholar] [CrossRef]
- Mondal, K.; Maitra, T.; Srivastava, A.K.; Pawar, G.; McMurtrey, M.D.; Sharma, A. 110th Anniversary: Particle Size Effect on Enhanced Graphitization and Electrical Conductivity of Suspended Gold/Carbon Composite Nanofibers. Ind. Eng. Chem. Res. 2020, 59, 1944–1952. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–Gold Nanoparticles Hybrid—Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.-C.; Arnaud, L.; Avilov, I.; Delgado, M.; Demoisson, F.; Espinosa, E.; Ewels, C.P.; Felten, A.; Guillot, J.; Ionescu, R. Carbon nanotubes randomly decorated with gold clusters: From nano2hybrid atomic structures to gas sensing prototypes. Nanotechnology 2009, 20, 375501. [Google Scholar] [CrossRef]
- Furue, R.; Koveke, E.P.; Sugimoto, S.; Shudo, Y.; Hayami, S.; Ohira, S.-I.; Toda, K. Arsine gas sensor based on gold-modified reduced graphene oxide. Sens. Actuators B Chem. 2017, 240, 657–663. [Google Scholar] [CrossRef]
- Choi, Y.; Bae, H.S.; Seo, E.; Jang, S.; Park, K.H.; Kim, B.-S. Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J. Mater. Chem. 2011, 21, 15431–15436. [Google Scholar] [CrossRef]
- Chang, H.; Wu, X.; Wu, C.; Chen, Y.; Jiang, H.; Wang, X. Catalytic oxidation and determination of β-NADH using self-assembly hybrid of gold nanoparticles and graphene. Analyst 2011, 136, 2735–2740. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Cai, J.; Qin, Y.; Zhang, A. One-step synthesis of multiwalled carbon nanotubes-gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sens. Actuators B Chem. 2010, 143, 524–529. [Google Scholar] [CrossRef]
- Jha, N.; Ramaprabhu, S. Development of Au nanoparticles dispersed carbon nanotube-based biosensor for the detection of paraoxon. Nanoscale 2010, 2, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.V.; Charaya, H.; Lahiri, I. Noble Metal Decorated Graphene-Based Gas Sensors and Their Fabrication: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 499–526. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Molavi, R.; Sheikhi, M.H. Facile wet chemical synthesis of Al doped CuO nanoleaves for carbon monoxide gas sensor applications. Mater. Sci. Semicond. Process. 2020, 106, 104767. [Google Scholar] [CrossRef]
- Wan, H.; Gan, Y.; Sun, J.; Liang, T.; Zhou, S.; Wang, P. High sensitive reduced graphene oxide-based room temperature ionic liquid electrochemical gas sensor with carbon-gold nanocomposites amplification. Sens. Actuators B Chem. 2019, 299, 126952. [Google Scholar] [CrossRef]
- Zou, C.; Hu, J.; Su, Y.; Zhou, Z.; Cai, B.; Tao, Z.; Huo, T.; Hu, N.; Zhang, Y. Highly repeatable and sensitive three-dimensional γ-Fe2O3@reduced graphene oxide gas sensors by magnetic-field assisted assembly process. Sens. Actuators B Chem. 2020, 306, 127546. [Google Scholar] [CrossRef]
- Wang, J.; Fan, S.; Xia, Y.; Yang, C.; Komarneni, S. Room-temperature gas sensors based on ZnO nanorod/Au hybrids: Visible-light-modulated dual selectivity to NO2 and NH3. J. Hazard. Mater. 2020, 381, 120919. [Google Scholar] [CrossRef] [PubMed]
- Van Toan, N.; Viet Chien, N.; Van Duy, N.; Si Hong, H.; Nguyen, H.; Duc Hoa, N.; Van Hieu, N. Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Lee, K.; Scardaci, V.; Kim, H.-Y.; Hallam, T.; Nolan, H.; Bolf, B.E.; Maltbie, G.S.; Abbott, J.E.; Duesberg, G.S. Highly sensitive, transparent, and flexible gas sensors based on gold nanoparticle decorated carbon nanotubes. Sens. Actuators B Chem. 2013, 188, 571–575. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Ma, X.; Yang, D. Homogeneous coating of Au and SnO2 nanocrystals on carbon nanotubes via layer-by-layer assembly: A new ternary hybrid for a room-temperature CO gas sensor. Chem. Commun. 2008, 6182–6184. [Google Scholar] [CrossRef]
- Balázsi, C.; Sedlácková, K.; Llobet, E.; Ionescu, R. Novel hexagonal WO3 nanopowder with metal decorated carbon nanotubes as NO2 gas sensor. Sens. Actuators B Chem. 2008, 133, 151–155. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Serra, E. Functional characterization of carbon nanotube networked films functionalized with tuned loading of Au nanoclusters for gas sensing applications. Sens. Actuators B Chem. 2009, 140, 176–184. [Google Scholar] [CrossRef]
- Young, S.-J.; Lin, Z.-D.; Hsiao, C.-H.; Huang, C.-S. Ethanol gas sensors composed of carbon nanotubes with adsorbed gold nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 11634–11640. [Google Scholar] [CrossRef]
- Kozlova, O.; Zwinderman, M.; Christofi, N. A new short-term toxicity assay using Aspergillus awamori with recombinant aequorin gene. BMC Microbiol. 2005, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkin, S.; Gu, M.B. Whole Cell Sensing System II: Applications; Springer Science & Business Media: Berlin, Germany, 2010; Volume 118. [Google Scholar]
- Zhang, Y.; Zeng, G.M.; Tang, L.; Chen, J.; Zhu, Y.; He, X.X.; He, Y. Electrochemical sensor based on electrodeposited graphene-Au modified electrode and nanoAu carrier amplified signal strategy for attomolar mercury detection. Anal. Chem. 2015, 87, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.-P.N.; Brockgreitens, J.; Ahmed, S.; Abbas, A. Dual detection of nitrate and mercury in water using disposable electrochemical sensors. Biosens. Bioelectron. 2016, 85, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive electrochemical detection of arsenic (III) using gold nanoparticle modified carbon nanotubes via anodic stripping voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef]
- Wang, Y.C.; Cokeliler, D.; Gunasekaran, S. Reduced graphene oxide/carbon nanotube/gold nanoparticles nanocomposite functionalized screen-printed electrode for sensitive electrochemical detection of endocrine disruptor bisphenol A. Electroanalysis 2015, 27, 2527–2536. [Google Scholar] [CrossRef]
- Fan, L.; Li, X.; Kan, X. Disposable graphite paper based sensor for sensitive simultaneous determination of hydroquinone and catechol. Electrochim. Acta 2016, 213, 504–511. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, D.; Hu, X.; Han, H.; Lin, M.; Wang, C. An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters. Sens. Actuators B Chem. 2017, 243, 1–7. [Google Scholar] [CrossRef]
- Aragay, G.; Pino, F.; Merkoci, A. Nanomaterials for sensing and destroying pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Du, D.; Li, J.; Tu, H.; Liu, D.; Zhang, A. Methyl parathion hydrolase based nanocomposite biosensors for highly sensitive and selective determination of methyl parathion. Biosens. Bioelectron. 2011, 26, 4320–4325. [Google Scholar] [CrossRef]
- Liu, T.; Su, H.; Qu, X.; Ju, P.; Cui, L.; Ai, S. Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide–gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides. Sens. Actuators B Chem. 2011, 160, 1255–1261. [Google Scholar] [CrossRef]
- Xie, C.; Li, H.; Li, S.; Wu, J.; Zhang, Z. Surface molecular self-assembly for organophosphate pesticide imprinting in electropolymerized poly (p-aminothiophenol) membranes on a gold nanoparticle modified glassy carbon electrode. Anal. Chem. 2009, 82, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Du, D.; Shao, Y.; Li, Z.; Wang, J.; Engelhard, M.H.; Li, J.; Lin, Y. Self assembly of acetylcholinesterase on a gold nanoparticles–graphene nanosheet hybrid for organophosphate pesticide detection using polyelectrolyte as a linker. J. Mater. Chem. 2011, 21, 5319–5325. [Google Scholar] [CrossRef]


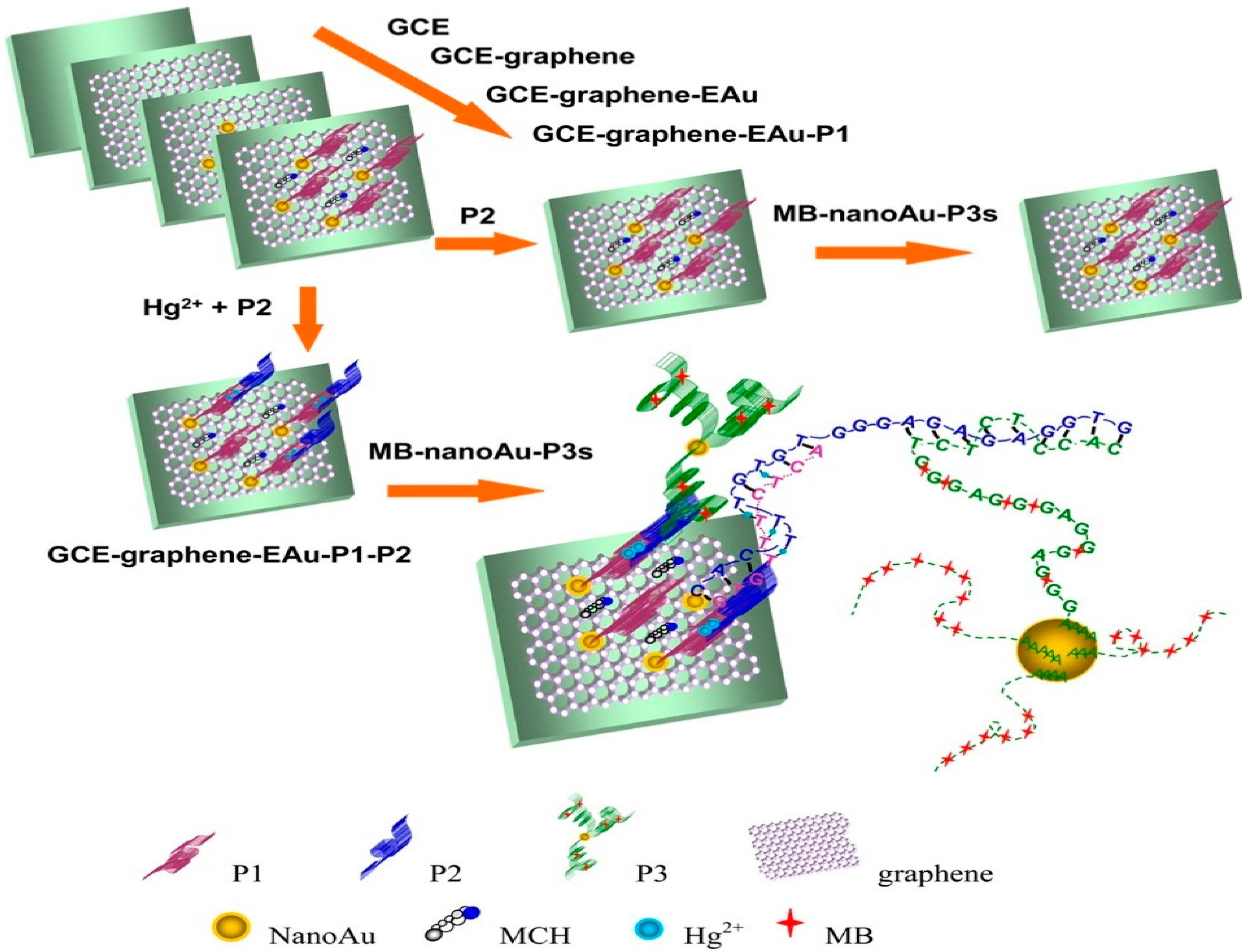
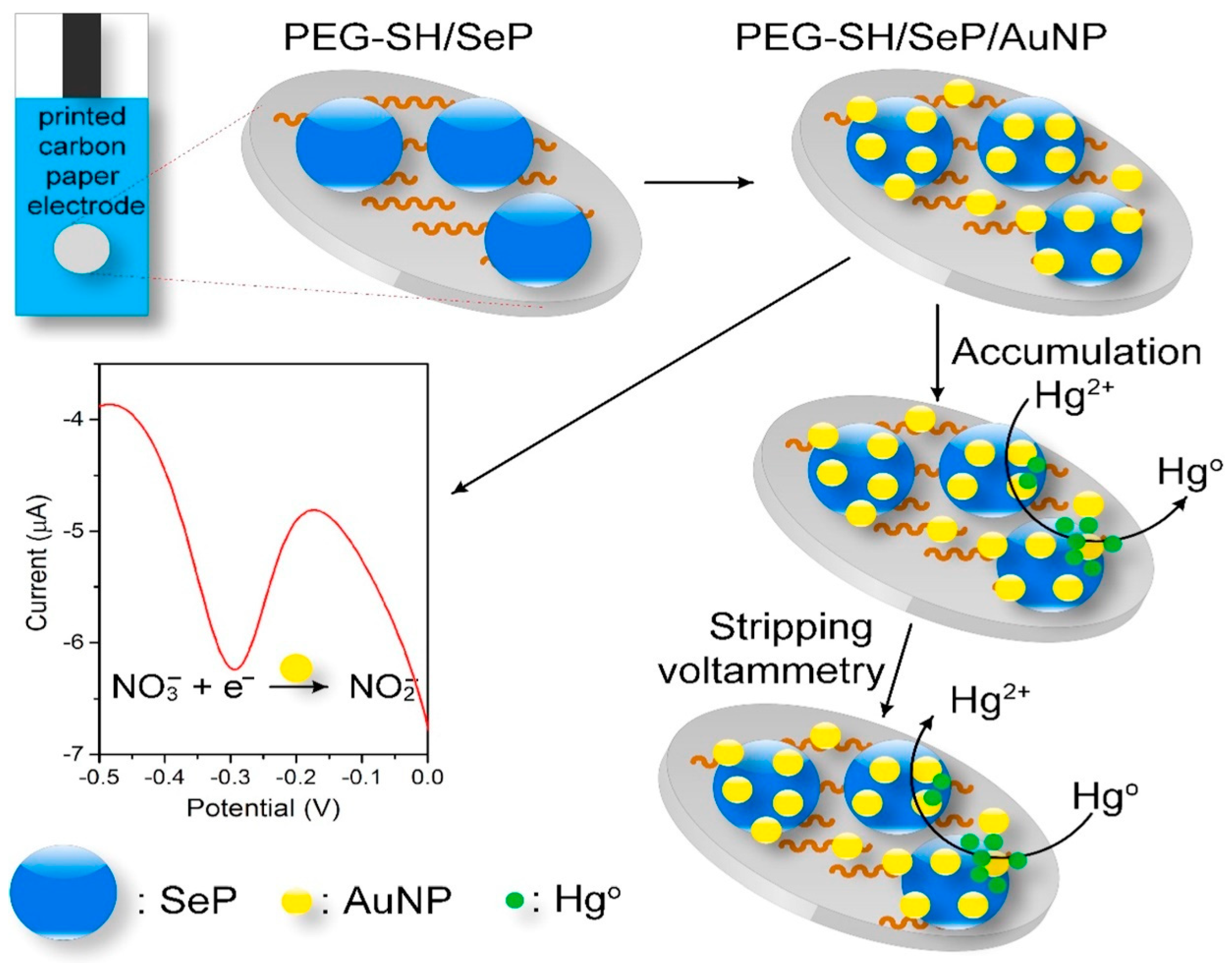
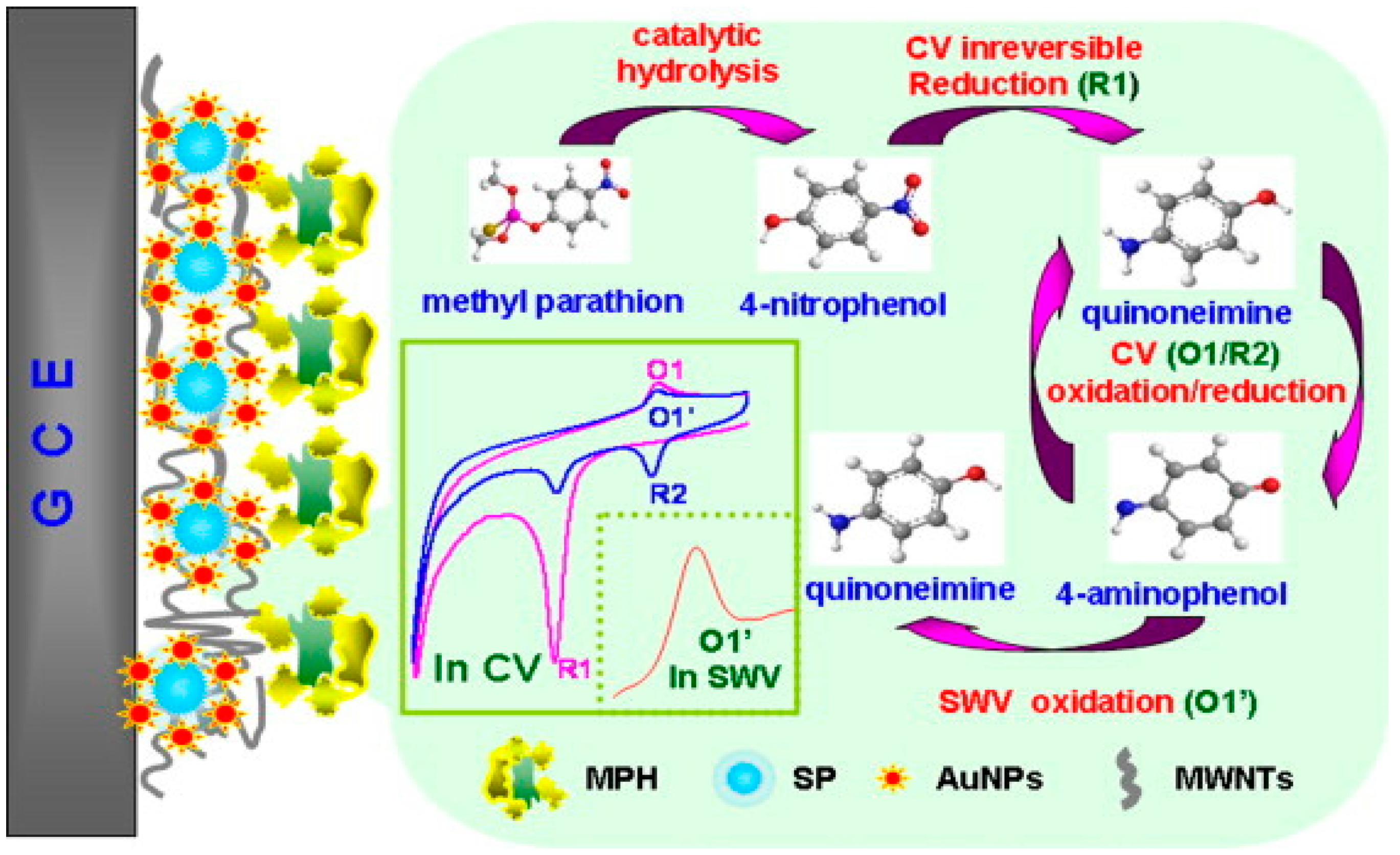
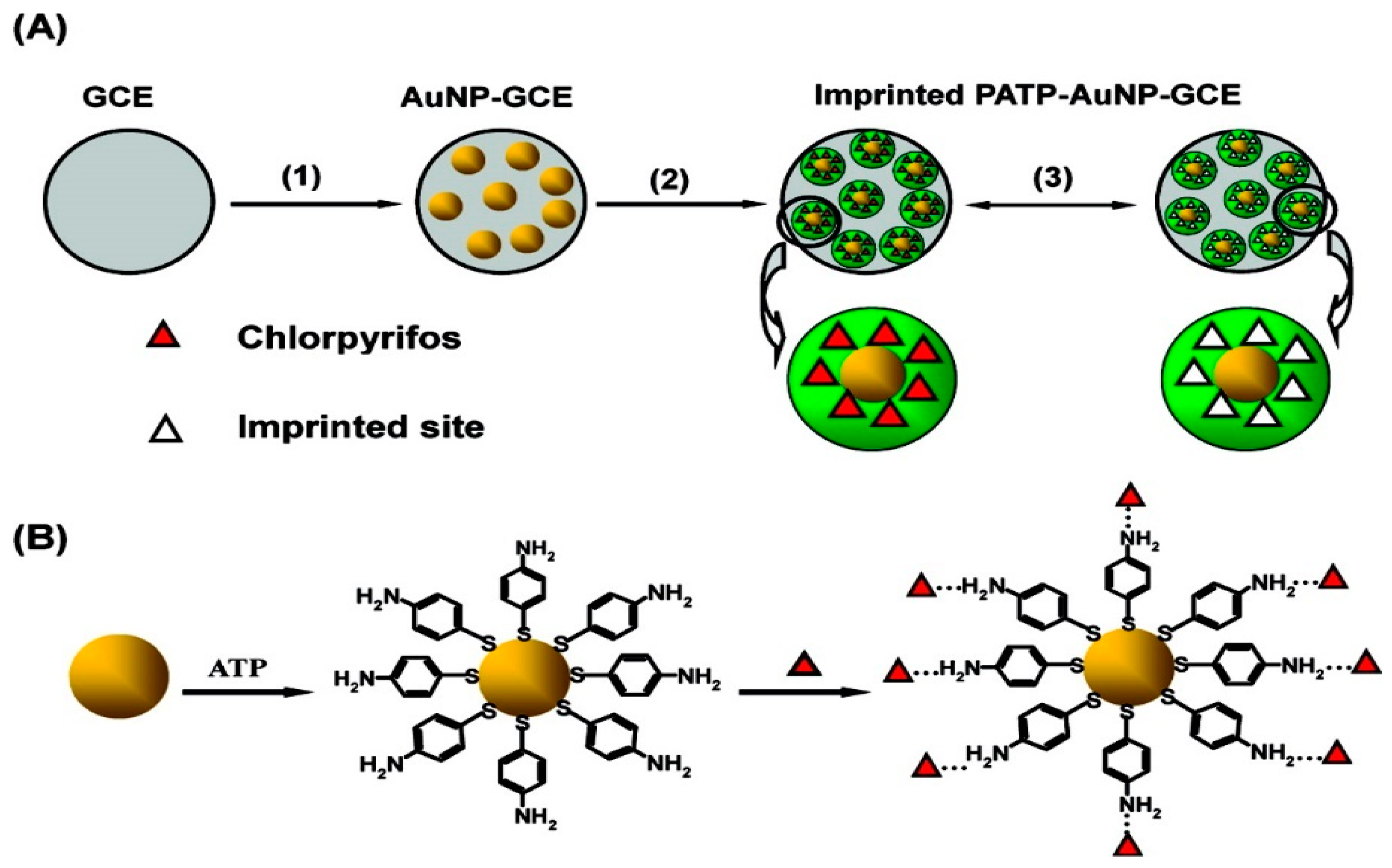
| Sensors | Nanocomposites | Modification | Detected Items | Sensitivity/LOD | Ref |
|---|---|---|---|---|---|
| Gas sensor | rGO–CGN | Carbon–gold nanocomposites (CGN) on an rGO-based electrochemical gas sensor. | O2 | Sensitivity of 0.289–0.168 μA/% O2 for low and high concentration range, respectively. | [171] |
| SWCNT–AuNPs | SWCNT films spray deposited on transparent and flexible plastic substrates and then decorated with AuNPs. | NH3 | 255 ppb | [177] | |
| CNT/Au/SnO2 nanotubes | CNT/Au/SnO2 nanotubes synthesised via homogeneous coating of Au and SnO2 nanocrystals on CNTs. | CO | Sensitivity of about 70 Ig/Ia for 2500 ppm concentration of CO. | [178] | |
| Au-MWCNTs/hex–WO3 | Metal decorated MWCNTs embedded into the hex–WO3 nanocomposites. | NO2 | 100 ppb | [179] | |
| Au-modified CNTs networks | Au nanoclusters deposited onto CNTs networks by sputtering. | NH3, CO, N2O, H2S, SO2 | 200 ppb NO2 | [180] | |
| AuH–rGO | GO flakes deposited over a monolayer of AuNPs, chemically attached to a functionalised, fused silica substrate. | H2, CO, NO2 | Sensitivity of 0.1% (for 100 ppm) up to 0.5% (for 10,000 ppm) for H2 and a variation of 0.1% for 1 ppm NO2, while CO not detected. | [15] | |
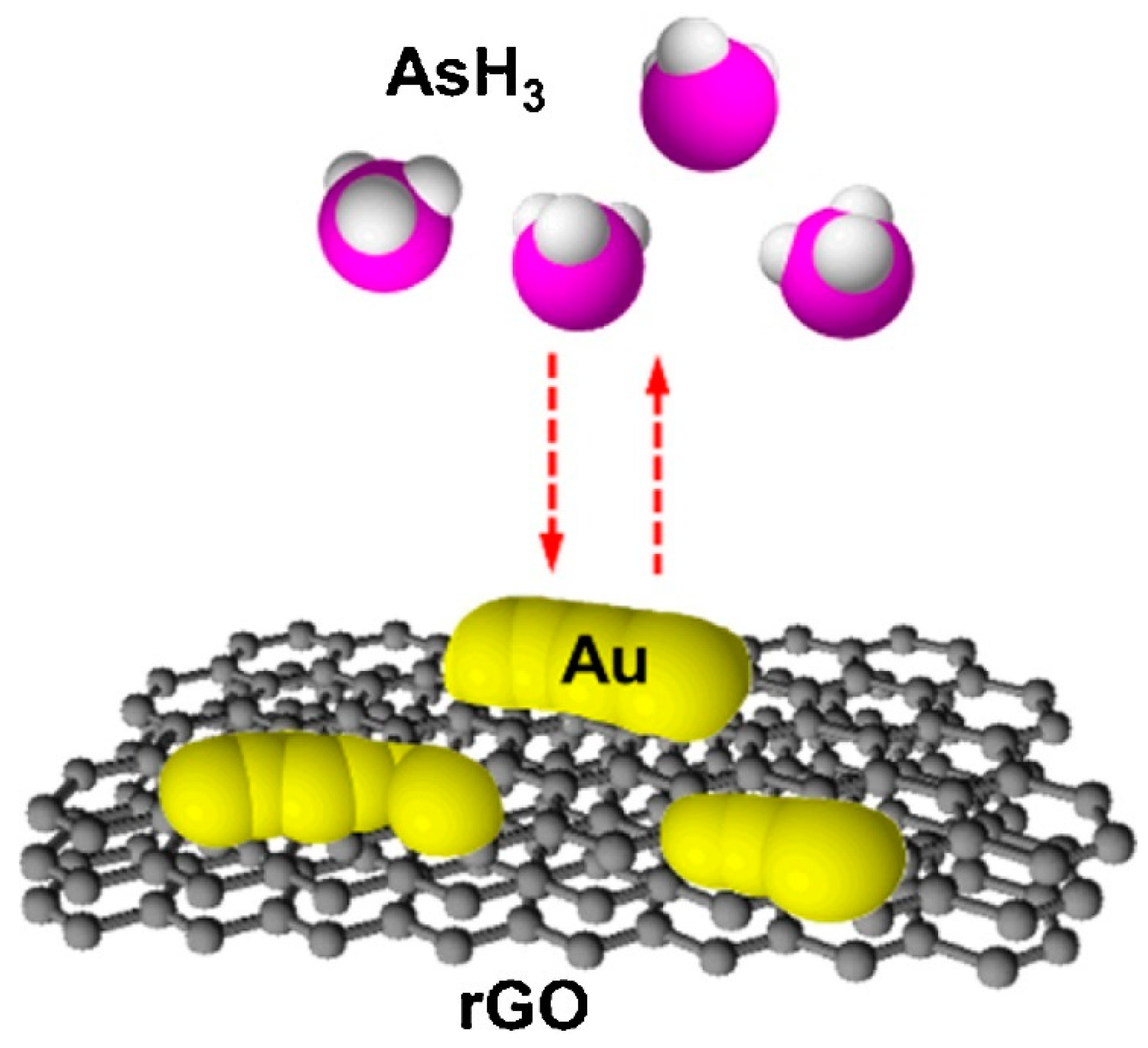
| Au/rGO | rGO-modified with a thin gold film on an interdigitated array electrode. | AsH3 | 0.01 ppmv | [163] | |
| Au–CNT | CNTs from a SiO2/Si substrate transferred to the flexible substrate and deposited with a controlled load of Au. | Ethanol Gas | Sensitivity of 5.39% for 800 ppm concentration of ethanol gas. | [181] | |
| Toxicant sensor | GCE–GR–EAu | GR and nanoAu electrodeposited on the surface of GCE, then functionalised with the 10-mer thymine-rich DNA probe. | Hg2+ | 0.001 aM | [184] |
| PEG–SH/SePs/AuNPs | Disposable carbon paper electrodes functionalised with SePs and AuNPs. | NO3–, Hg2+ | 8.6 µM and 1.0 ppb for NO3– and Hg2+. | [185] | |
| Au-MWCNTs | AuNPs deposited on MWCNTs via reduction of HAuCl4 by NaBH4 followed by fixing it onto the GCE surface via evaporation of a suspension in chloroform. | As(III) | Sensitivity of 1985 μA/μM with square wave voltammetry and a LOD of 0.1 μg/L. | [186] | |
| RGO/CNT/AuNPs | GO/CNT nanocomposite reduced to RGO/CNT on SPE, followed by electrochemical deposition of AuNPs on modified SPE. | BPA | 800 pM | [187] | |
| AuNPs/EGP | AuNPs electrodeposited on EGP to fabricate AuNPs/EGP sensor. | CC, HQ | 4.13 × 10−8 mol/L and 2.73 × 10−8 mol/L for CC and HQ. | [188] | |
| GCE/rGO/AuNPs | A modified GCE based on the rGO and AuNPs fabricated with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) as complexing agents. | Fe(III) | 3.5 nM | [189] | |
| Pesticide sensor | Au–MWNTs–GCE | AuNPs dispersed on the outer surface of MWNTs used to modify GCE. | Paraoxon | 0.025 ppb | [167] |
| AuNP–CHIT/GCE, MWCNT–Au–CHIT/GCE | Chitosan modified GCE (CHIT/GCE) coated with AuNPs and MWCNT–Au nanocomposites to fabricate AuNPs modified GCE (AuNP–CHIT/GCE) and MWCNT–Au nanocomposites modified GCE (MWCNT–Au–CHIT/GCE), respectively. | Malathion | 0.6 ng/mL | [166] | |
| MPH/SP@AuNPs/MWCNTs/GCE | The sensing film prepared via the formation of AuNPs on SP (SP@AuNP), mixing with MWCNTs on the surface of a GCE followed by covalent immobilisation of MPH. | Methyl parathion | 0.3 ng/mL | [191] | |
| CPBA/AuNPs/RGO-CS/GCE | An amperometric biosensor based on immobilising acetylcholinesterase on the modified GCE with nanocomposites of CPBA/rGO–AuNPs. | Chlorpyrifos, malathion, carbofuran, isoprocarb | 0.1, 0.5, 0.05, and 0.5 ppb for chlorpyrifos, malathion, carbofuran, and isoprocarb, respectively. | [192] | |
| PATP–AuNP–GCE | Electropolmerisable PATP assembled on the AuNPs at the surface of GCE by the formation of Au-S bonds, then, the CPF template assembled onto the monolayer of ATP through the hydrogen-bonding interaction between amino group and CPF. | Chlorpyrifos | 0.33 μM | [193] | |
| AuNPs/cr-Gs | In the presence of PDDA, a nanohybrid of AuNPs and cr-Gs synthesised by the growth of AuNPs on the surface of graphene nanosheets. Then, an enzyme nanoassembly (AChE/AuNPs/cr-Gs) was prepared by self-assembling of AChE on AuNP/cr-Gs nanohybrid. | Paraoxon | 0.1 pM | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmati, S.; Doherty, W.; Amani Babadi, A.; Akmal Che Mansor, M.S.; Julkapli, N.M.; Hessel, V.; Ostrikov, K. Gold–Carbon Nanocomposites for Environmental Contaminant Sensing. Micromachines 2021, 12, 719. https://doi.org/10.3390/mi12060719
Rahmati S, Doherty W, Amani Babadi A, Akmal Che Mansor MS, Julkapli NM, Hessel V, Ostrikov K. Gold–Carbon Nanocomposites for Environmental Contaminant Sensing. Micromachines. 2021; 12(6):719. https://doi.org/10.3390/mi12060719
Chicago/Turabian StyleRahmati, Shahrooz, William Doherty, Arman Amani Babadi, Muhamad Syamim Akmal Che Mansor, Nurhidayatullaili Muhd Julkapli, Volker Hessel, and Kostya (Ken) Ostrikov. 2021. "Gold–Carbon Nanocomposites for Environmental Contaminant Sensing" Micromachines 12, no. 6: 719. https://doi.org/10.3390/mi12060719
APA StyleRahmati, S., Doherty, W., Amani Babadi, A., Akmal Che Mansor, M. S., Julkapli, N. M., Hessel, V., & Ostrikov, K. (2021). Gold–Carbon Nanocomposites for Environmental Contaminant Sensing. Micromachines, 12(6), 719. https://doi.org/10.3390/mi12060719







_Ostrikov.png)

