Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior
Abstract
1. Introduction
2. Experimental Sections
2.1. Synthesis of CdTe QDs
2.2. Detecting of Mercury in Aqueous Solution
2.3. Characterization
3. Results and Discussion
3.1. Morphology and Structure of CdTe QDs
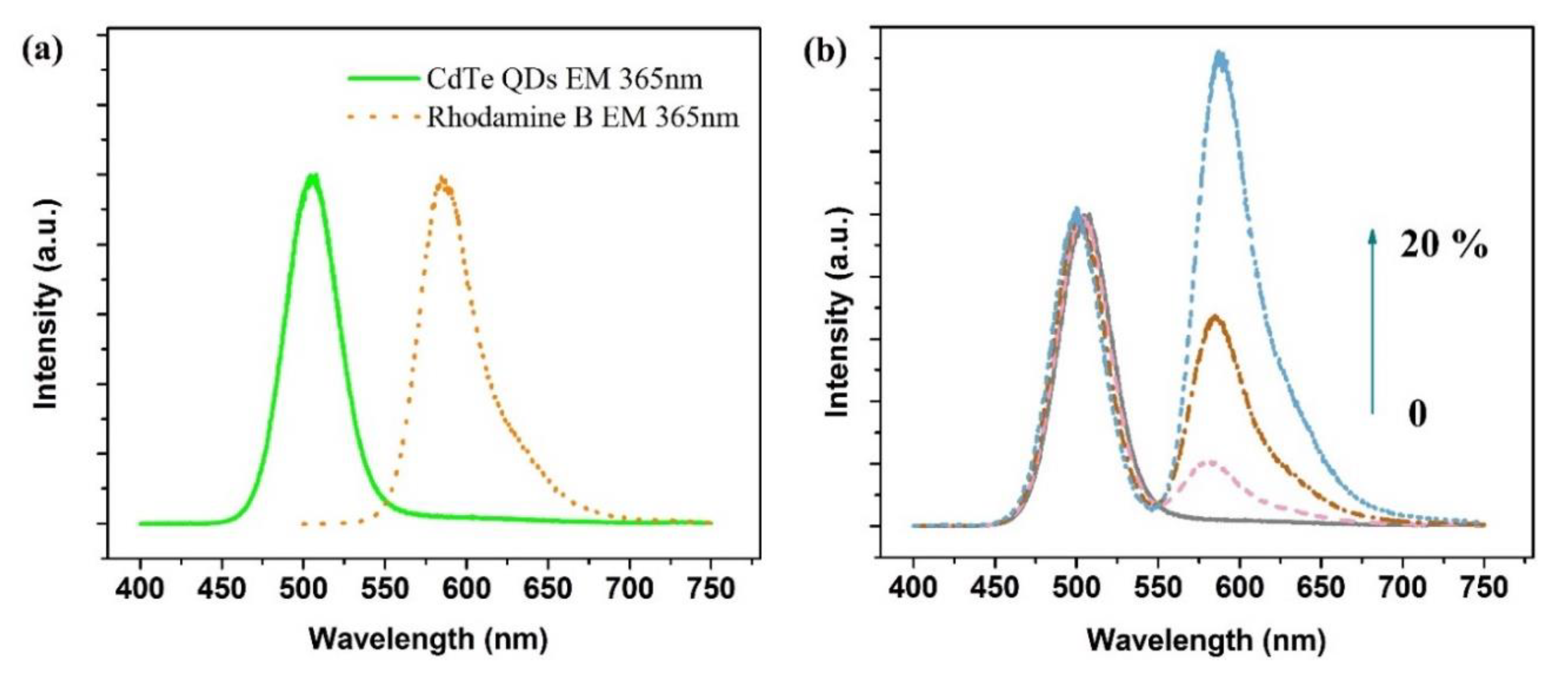
3.2. Luminescence Characterization of the Dual-Emission Probe
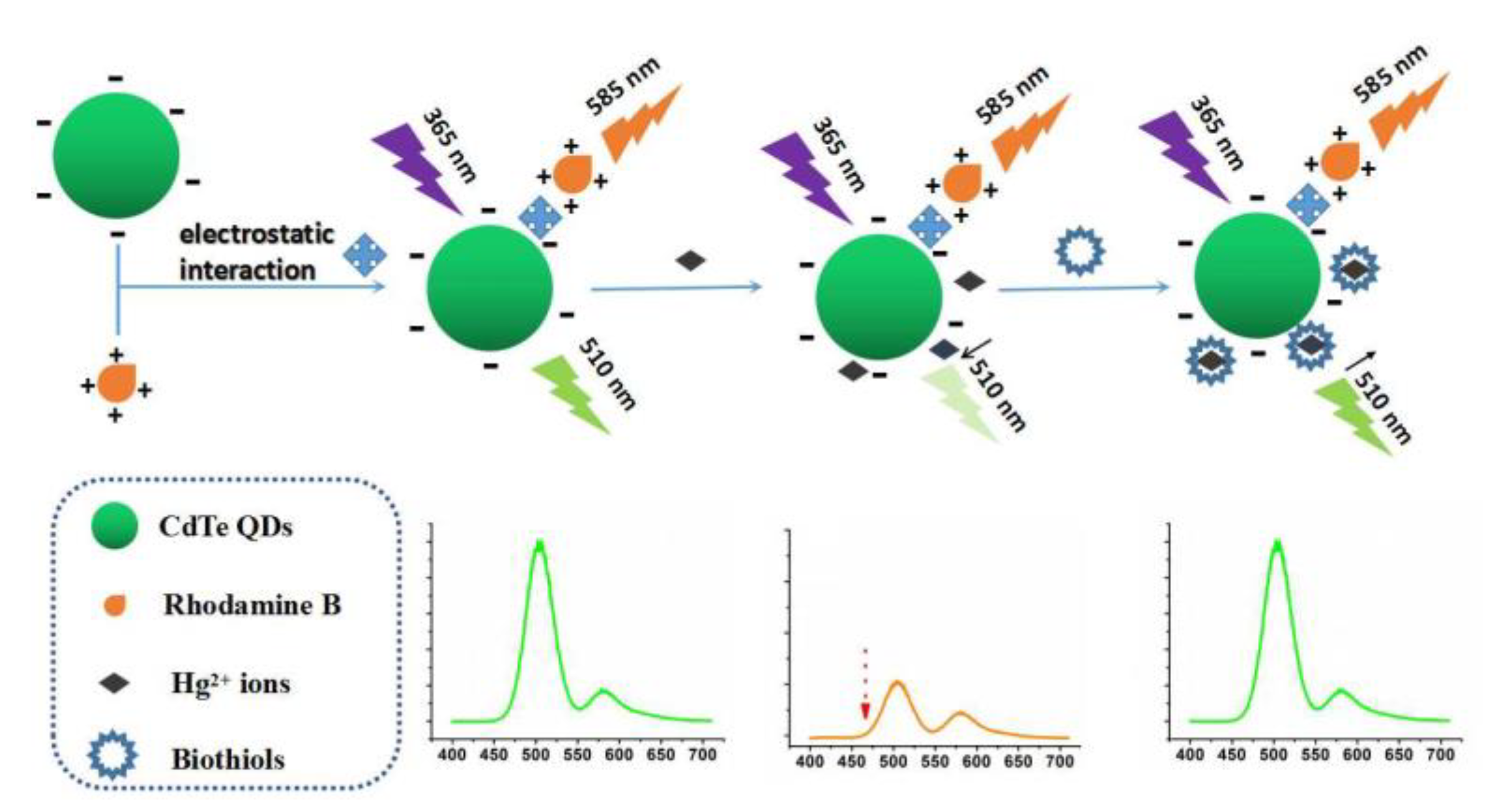
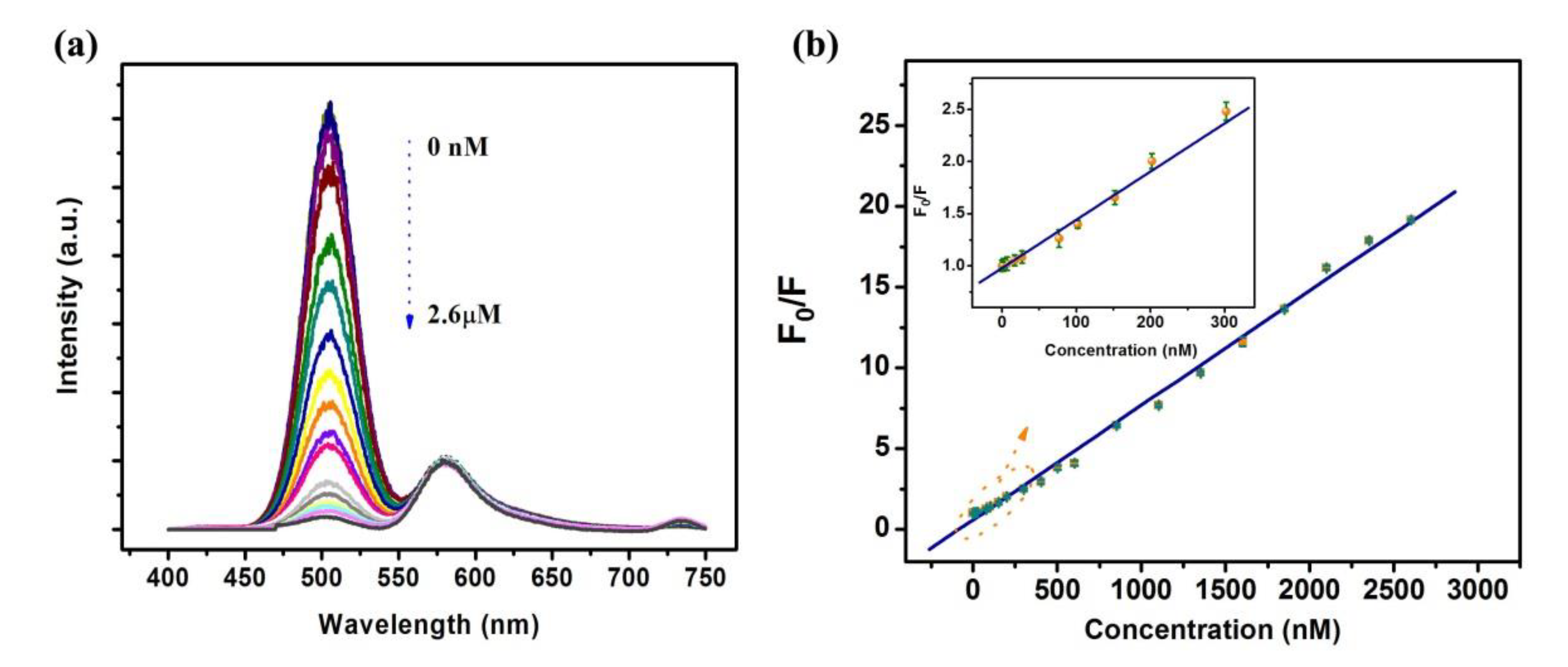
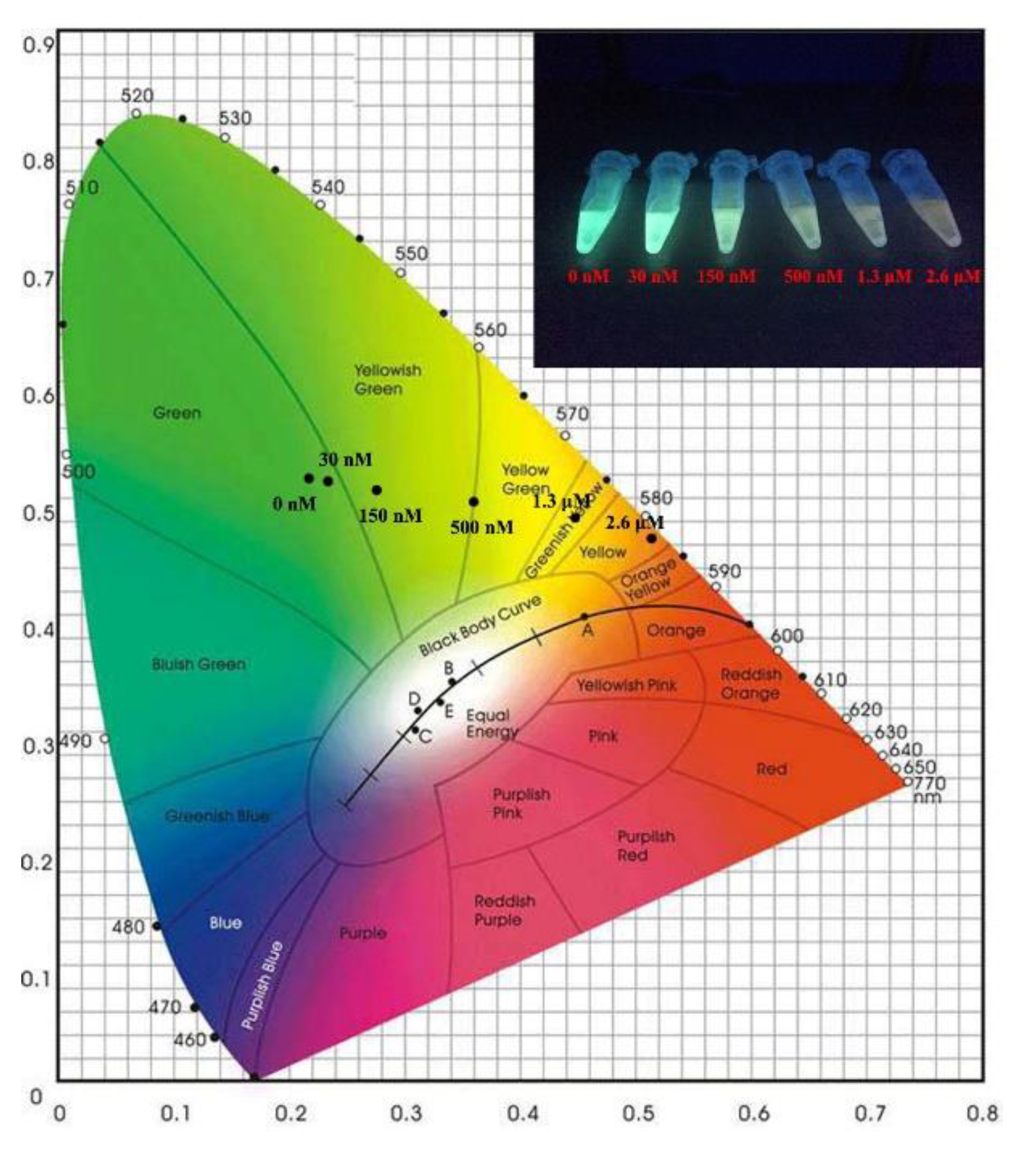
3.3. Fluorescence Response Characteristics of Dual-Emission Probe for Detecting Hg2+
3.4. Fluorescence Response Characteristics of QD-Based Probe for Hg2+ Detection
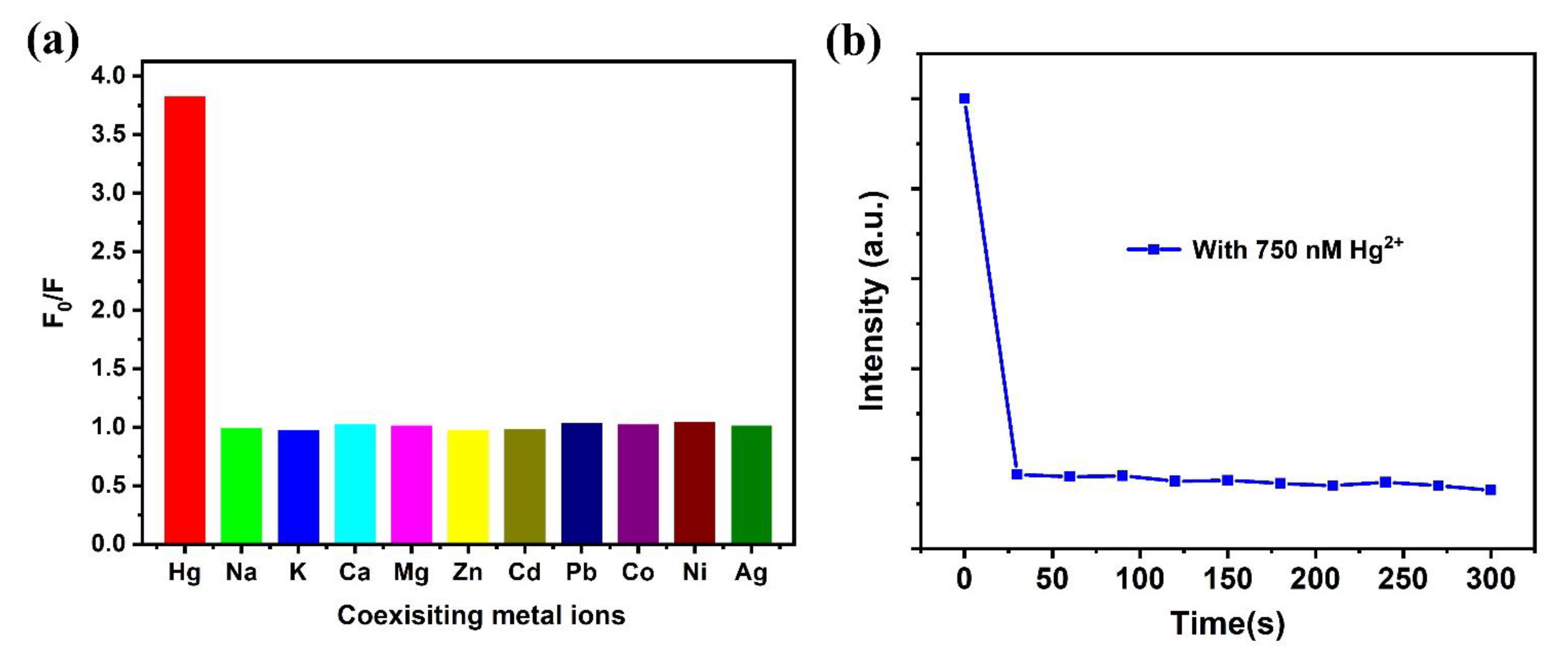
3.5. Selectivity to Metal Ions and Time Effect to Hg2+ of Dual-Emission Probe
3.6. Detection in Real Samples
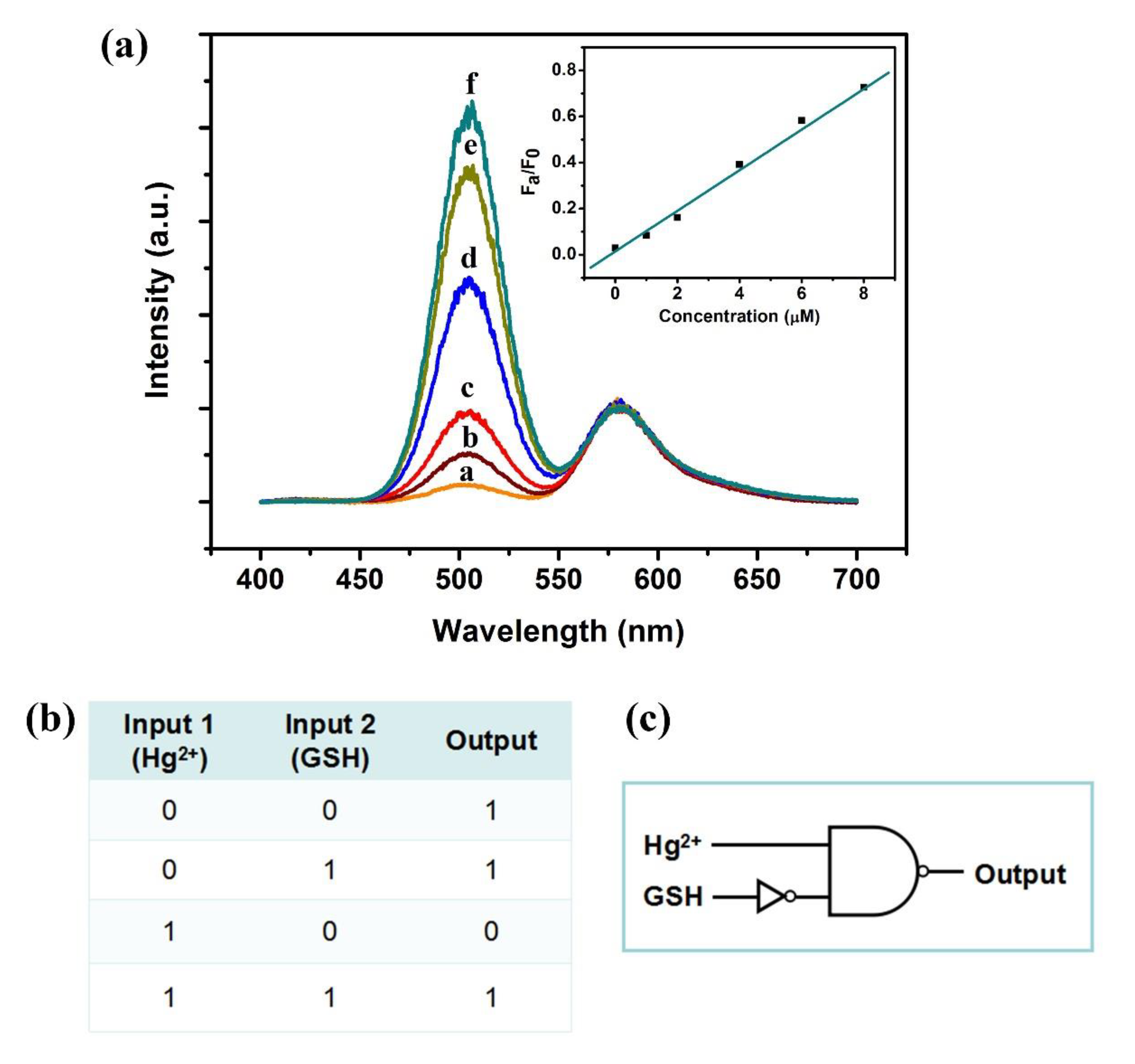
3.7. IMPLICATION Logic Gate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Janani, B.; Syed, A.; Thomas, A.M.; Bahkali, A.H.; Elgorban, A.M.; Raju, L.L.; Khan, S.S. UV-vis spectroscopic method for the sensitive and selective detection of mercury by silver nanoparticles in presence of alanine. Optik 2020, 204, 164160. [Google Scholar] [CrossRef]
- Chang, D.; Li, L.; Shi, L.H.; Yang, Y.X. Hg2+ detection, pH sensing and cell imaging based on bright blue-fluorescent n-doped carbon dots. Analyst 2020, 145, 8030–8037. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Lee, S.; Park, Y.; Min, T.; Bai, S.C. Evaluation of dietary selenium, vitamin c and e as the multi-antioxidants on the methylmercury intoxicated mice based on mercury bioaccumulation, antioxidant enzyme activity, lipid peroxidation and mitochondrial oxidative stress. Chemosphere 2021, 273, 129673. [Google Scholar] [CrossRef] [PubMed]
- Akkemik, E.; Taser, P.; Bayindir, A.; Budak, H.; Ciftci, M. Purification and characterization of glutathione S-transferase from turkey liver and inhibition effects of some metal ions on enzyme activity. Environ. Toxicol. Pharmacol. 2012, 34, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Ma, Q.F.; Cheng, H.Y.; Liu, J.H.; Wang, Y.C. Simultaneous enrichment of inorganic and organic species of lead and mercury in pg L-1 levels by solid phase extraction online combined with high performance liquid chromatography and inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2021, 1157, 338388. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Butler, O.T.; Cairns, W.R.L.; Cook, J.M.; Davidson, C.M.; Cavoura, O.; Mertz-Kraus, R. Atomic spectrometry update—A review of advances in environmental analysis. J. Anal. At. Spectrom. 2020, 35, 9–53. [Google Scholar] [CrossRef]
- Nguyen, T.T.K.; Luu, H.T.; Vu, L.D.; Ta, T.T.; Le, G.T.H. Determination of total mercury in solid samples by anodic stripping voltammetry. J. Chem. 2021, 2021, 8888879. [Google Scholar] [CrossRef]
- Alharthi, S.S.; Fallatah, A.M.; Al-Saidi, H.M. Design and characterization of electrochemical sensor for the determination of mercury(II) ion in real samples based upon a new schiff base derivative as an ionophore. Sensors 2021, 21, 3020. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.-Y.; Woo, M.-A. Trends in sensor development toward next-generation point-of-care testing for mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Wang, F.X.; Hua, Y.; Liu, H.; Yin, M.Y.; Zhang, C.X.; Zhang, Y.; Wang, H. A fluorimetric testing strip for the visual evaluation of mercury in blood using copper nanoclusters with DMSO-enhanced fluorescence and stability. Nanoscale 2020, 12, 24079–24084. [Google Scholar] [CrossRef]
- Yao, J.; He, Y.; Li, L.; Li, P.F.; Yang, M. Magnified fluorescent aptasensors based on a gold nanoparticle-DNA hybrid and dnase i for the cycling detection of mercury(II) ions in aqueous solution. Ind. Eng. Chem. Res. 2019, 58, 21201–21207. [Google Scholar] [CrossRef]
- Tripathy, M.; Subuddhi, U.; Patel, S. A styrylpyridinium dye as chromogenic and fluorogenic dual mode chemosensor for selective detection of mercuric ion: Application in bacterial cell imaging and molecular logic gate. Dye Pigment 2020, 174, 108054. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, W.; Wang, Z.J.; Lv, Q.; Bai, H.; Zhang, Q. Analyte-enhanced photocatalytic activity of cdse/zns quantum dots for paper-based colorimetric sensing of Hg2+ under visible light. Microchem J. 2021, 164, 106037. [Google Scholar] [CrossRef]
- Tong, Y.J.; Qi, J.X.; Song, A.M.; Zhong, X.L.; Jiang, W.; Zhang, L.; Liang, R.P.; Qiu, J.D. Electronic synergy between ligands of luminol and isophthalic acid for fluorescence ratiometric detection of Hg2+. Anal. Chim. Acta 2020, 1128, 11–18. [Google Scholar] [CrossRef]
- Hasan, M.T.; Lee, B.H.; Lin, C.W.; McDonald-Boyer, A.; Gonzalez-Rodriguez, R.; Vasireddy, S.; Tsedev, U.; Coffer, J.; Belcher, A.M.; Naumov, A.V. Near-infrared emitting graphene quantum dots synthesized from reduced graphene oxide for in vitro/in vivo/ex vivo bioimaging applications. 2D Mater. 2021, 8, 035013. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Li, H.; Zhou, J.; Zhang, H.; Fu, L. A sensitive immunosensor based on fret between gold nanoparticles and inp/zns quantum dots for arginine kinase detection. Food Chem. 2021, 354, 129536. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, H.; Liu, Y.; Yang, L.; Xiao, Q.; Huang, S. Investigation on conformational variation and activity of trypsin affected by black phosphorus quantum dots via multi-spectroscopy and molecular modeling. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 256, 119746. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kumar, S.; Ortega, G.A.; Srinivasan, S.; Rajabzadeh, A.R. Target specific aptamer-induced self-assembly of fluorescent graphene quantum dots on palladium nanoparticles for sensitive detection of tetracycline in raw milk. Food Chem. 2021, 346, 128893. [Google Scholar] [CrossRef]
- Gan, Z.Y.; Hu, X.T.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y.; Zhang, W.; Li, Y.X.; Xu, Y.W. A dual-emission fluorescence sensor for ultrasensitive sensing mercury in milk based on carbon quantum dots modified with europium (III) complexes. Sens. Actuators B Chem. 2021, 328, 128997. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, C.; Chen, X.C.; Yang, B.; Yang, L.; Jiang, C.L.; Zhang, Z.P. Ratiometric fluorescent paper sensor utilizing hybrid carbon dots-quantum dots for the visual determination of copper ions. Nanoscale 2016, 8, 5977–5984. [Google Scholar] [CrossRef]
- Su, D.D.; Wang, M.K.; Liu, Q.; Chen, J.Y.; Su, X.G. Dual-emission ratio fluorescence detection of bleomycin based on nitrogen doped graphene quantum dots@gold nanoclusters assembly. Sens. Actuators B Chem. 2019, 290, 163–169. [Google Scholar] [CrossRef]
- Gui, R.J.; An, X.Q.; Huang, W.X. An improved method for ratiometric fluorescence detection of pH and Cd2+ using fluorescein isothiocyanate-quantum dots conjugates. Anal. Chim. Acta 2013, 767, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Guo, S.; Yuan, Z.Q.; Lu, C. Carbon quantum dot-gold nanocluster nanosatellite for ratiometric fluorescence probe and imaging for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 241, 821–827. [Google Scholar] [CrossRef]
- Liu, Y.F.; Tang, X.S.; Deng, M.; Zhu, T.; Edman, L.; Wang, J. Hydrophilic aginzns quantum dots as a fluorescent turn-on probe for cd2+ detection. J. Alloy Compd. 2021, 864, 158109. [Google Scholar] [CrossRef]
- Han, B.Y.; Yuan, J.P.; Wang, E.K. Sensitive and selective sensor for biothiols in the cell based on the recovered fluorescence of the CdTe Quantum Dots-Hg(II) System. Anal. Chem. 2009, 81, 5569–5573. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.B.; Xu, S.; Song, H.W.; Xu, W.; Chen, X.; Zhou, D.L.; Yin, Z.; Han, W. Highly sensitive and selective detection of mercury ions based on up-conversion FRET from NaYF4:Yb3+/Er3+ nanophosphors to CdTe quantum dots. RSC Adv. 2015, 5, 99099–99106. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.H.; Guo, W.Z.; Peng, X.G. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mat. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Ali, E.M.; Zheng, Y.G.; Yu, H.H.; Ying, J.Y. Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal. Chem. 2007, 79, 9452–9458. [Google Scholar]
- Wang, Y.H.; Bao, L.; Liu, Z.H.; Pang, D.W. Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal. Chem. 2011, 83, 8130–8137. [Google Scholar] [CrossRef]
- He, J.L.; Zhang, H.R.; Zou, J.L.; Liu, Y.L.; Zhuang, J.L.; Xiao, Y.; Lei, B.F. Carbon dots-based fluorescent probe for “off-on” sensing of Hg(II) and I. Biosens. Bioelectron. 2016, 79, 531–535. [Google Scholar] [CrossRef]
- Long, C.; Li, X.; Jiang, Z.; Zhang, P.; Qing, Z.; Qing, T.; Feng, B. Adsorption-improved mose2 nanosheet by heteroatom doping and its application for simultaneous detection and removal of mercury (II). J. Hazard. Mater. 2021, 413, 125470. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.; Xie, H.L.; Wei, L.N.; Xu, L.; Zhang, L.; Lan, W.; Zhou, C.S.; She, Y.B.; Fu, H.Y. A novel thioctic acid-carbon dots fluorescence sensor for the detection of Hg2+ and thiophanate methyl via S-Hg affinity. Food Chem. 2021, 346, 128923. [Google Scholar] [CrossRef]
- Huang, L.; Li, P.; Lin, C.; Wu, Y.; Chen, Z.; Fu, F. DNA-templated fluorescent silver nanoclusters on-off switch for specific and sensitive determination of organic mercury in seafood. Biosens. Bioelectron. 2021, 183, 113217. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Z.; Yang, G.H.; Wan, X.J. Ultra-high quantum yield nitrogen-doped carbon quantum dots and their versatile application in fluorescence sensing, bioimaging and anti-counterfeiting. Spectroc. Acta A Mol. Biomol. Spectr. 2021, 253, 119583. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Wang, J.; Wang, Q.S.; Tang, D.P.; Lin, Y. Distance-dependent visual fluorescence immunoassay on CdTe quantum dot-impregnated paper through silver ion-exchange reaction. Microchim. Acta 2020, 187, 536. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gou, X. An investigation of preparation, properties, characterization and the mechanism of zinc blende CdCe/CdS core/shell quantum dots for sensitive and selective detection of trace mercury. J. Mater. Chem. C 2016, 4, 9856–9863. [Google Scholar] [CrossRef]
- Chen, J.L.; Gao, Y.C.; Xu, Z.B.; Wu, G.H.; Chen, Y.C.; Zhu, C.Q. A novel fluorescent array for mercury (II) ion in aqueous solution with functionalized cadmium selenide nanoclusters. Anal. Chim. Acta 2006, 577, 77–84. [Google Scholar] [CrossRef]
- Li, H.Z.; Lu, W.W.; Zhao, G.L.; Song, B.; Zhou, J.; Dong, W.X.; Han, G.R. Silver ion-doped cdte quantum dots as fluorescent probe for Hg2+ detection. RSC Adv. 2020, 10, 38965–38973. [Google Scholar] [CrossRef]
- Liu, Y.F.; Tang, X.S.; Deng, M.; Cao, Y.L.; Li, Y.J.; Zheng, H.; Li, F.H.; Yan, F.B.; Lan, T.Y.; Shi, L.L.; et al. Nitrogen doped graphene quantum dots as a fluorescent probe for mercury(II) ions. Microchim. Acta 2019, 186, 140. [Google Scholar] [CrossRef]


| Probes | Linear Range (nM) | LOD (nM) | Ref. |
|---|---|---|---|
| C-dots | 0–25,000 | 23.4 | [30] |
| N, S-MoSe2 | 0–5000 | 10 | [31] |
| Thioctic acid-carbon dots | 50–580 | 33.3 | [32] |
| DNA-templated AgNCs | 0–2000 | 5 | [33] |
| Nitrogen-doped C-dots | 300–5000 | 24.1 | [34] |
| CdTe QDs | 0–2600 | 11.4 | This work |
| Samples | - | By Our Method | By ICP-MS | ||
|---|---|---|---|---|---|
| Added (nM) | Measured (nM) | Recovery (%) | Measured (nM) | Recovery (%) | |
| Drinking water | 100.0 | 97.5 | 97.5 | 98.9 | 98.9 |
| 500.0 | 512.8 | 102.6 | 510.7 | 102.1 | |
| 2000.0 | 2025.8 | 101.3 | 2026.5 | 101.3 | |
| Sea Water | 100.0 | 103.7 | 103.7 | 103.9 | 103.9 |
| 500.0 | 523.9 | 104.8 | 527.5 | 105.5 | |
| 2000.0 | 2043.8 | 102.2 | 2028.7 | 101.4 | |
| Wastewater | 100.0 | 110.6 | 110.6 | 108.4 | 108.4 |
| 500.0 | 530.5 | 106.1 | 527.8 | 105.6 | |
| 2000.0 | 2065.4 | 103.3 | 2041.8 | 102.1 | |
| Deionized Water | 100.0 | 97.5 | 97.5 | 98.6 | 98.6 |
| 500.0 | 517.6 | 103.5 | 505.3 | 101.1 | |
| 2000.0 | 2037.9 | 101.9 | 2020.5 | 101.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Xu, S.; Liu, Z.; Yu, K.; Pan, X. Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior. Micromachines 2021, 12, 713. https://doi.org/10.3390/mi12060713
Gao Y, Xu S, Liu Z, Yu K, Pan X. Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior. Micromachines. 2021; 12(6):713. https://doi.org/10.3390/mi12060713
Chicago/Turabian StyleGao, Yuefeng, Sai Xu, Zhijian Liu, Kezhen Yu, and Xinxiang Pan. 2021. "Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior" Micromachines 12, no. 6: 713. https://doi.org/10.3390/mi12060713
APA StyleGao, Y., Xu, S., Liu, Z., Yu, K., & Pan, X. (2021). Dual-Emission Fluorescence Probe Based on CdTe Quantum Dots and Rhodamine B for Visual Detection of Mercury and Its Logic Gate Behavior. Micromachines, 12(6), 713. https://doi.org/10.3390/mi12060713






