A Microfluidic Platform for Cavitation-Enhanced Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Fabrication
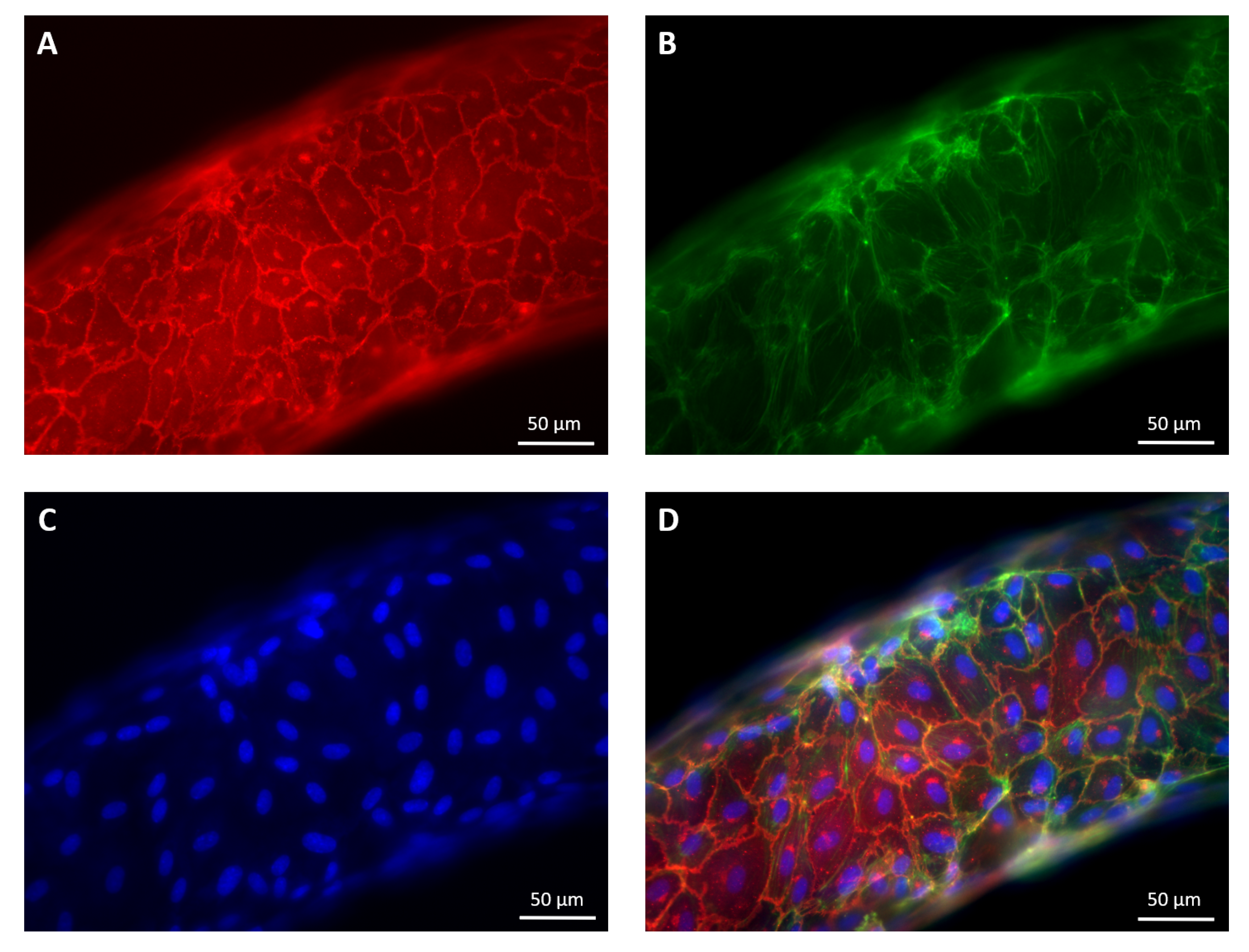
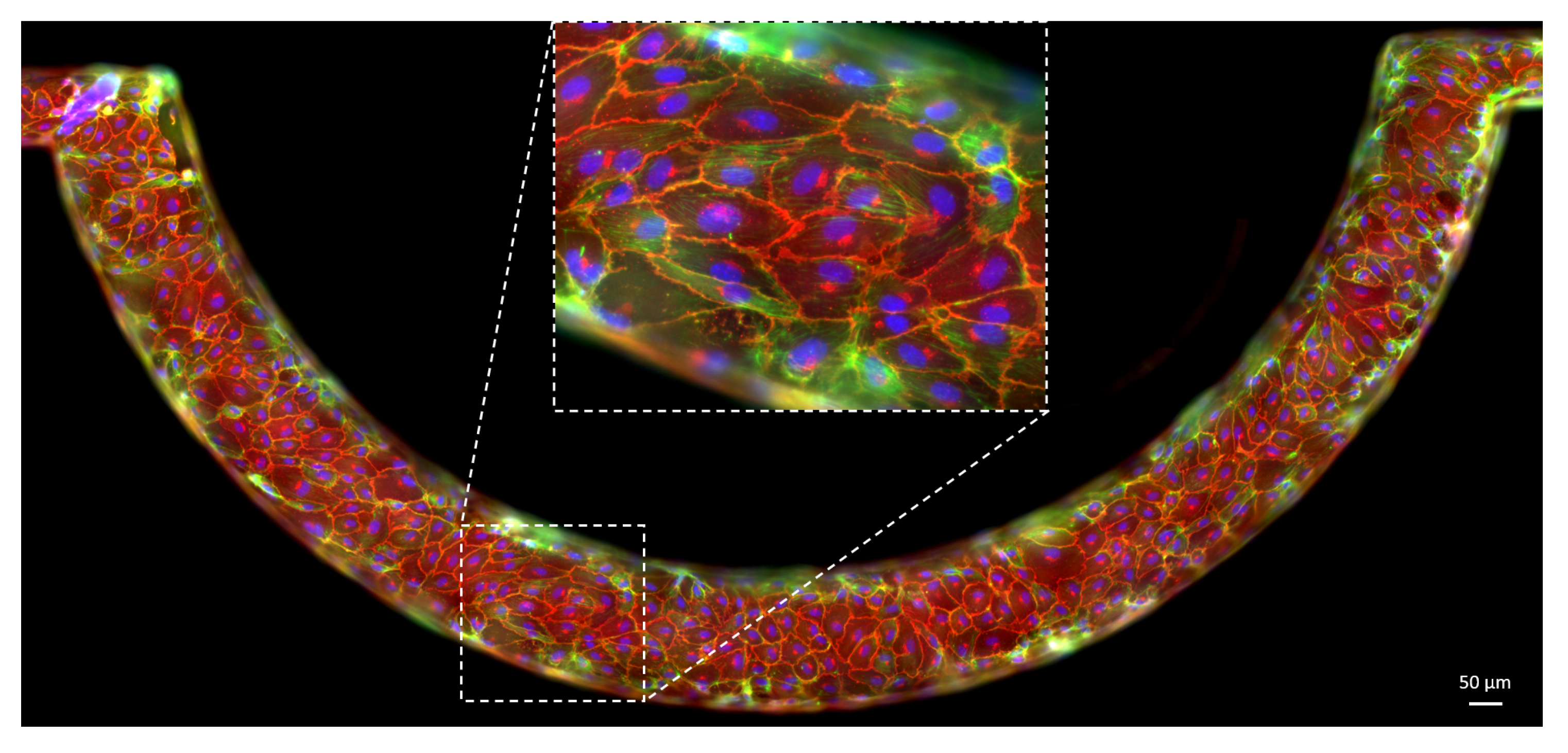
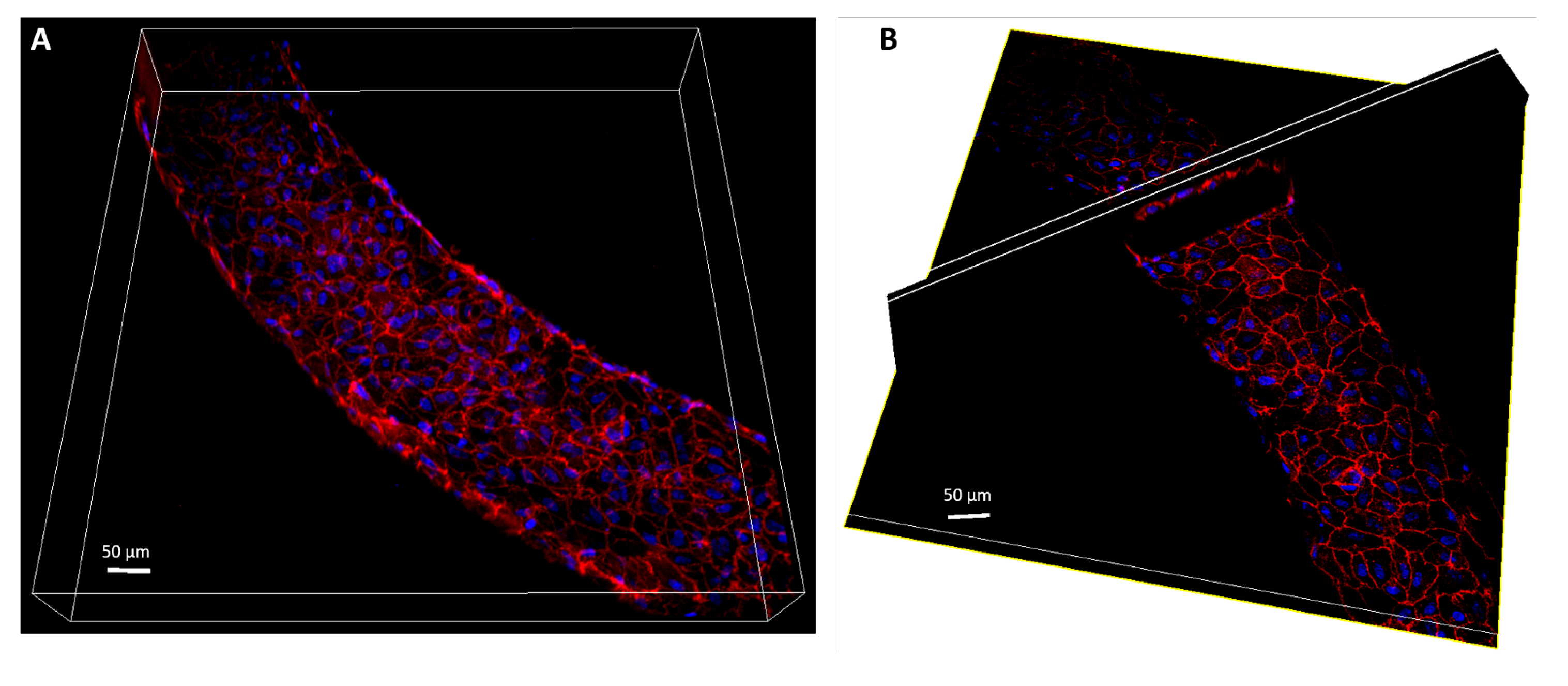
2.2. Cell Culture and of the Vasculature on Chip
2.3. Immunofluorescence Staining
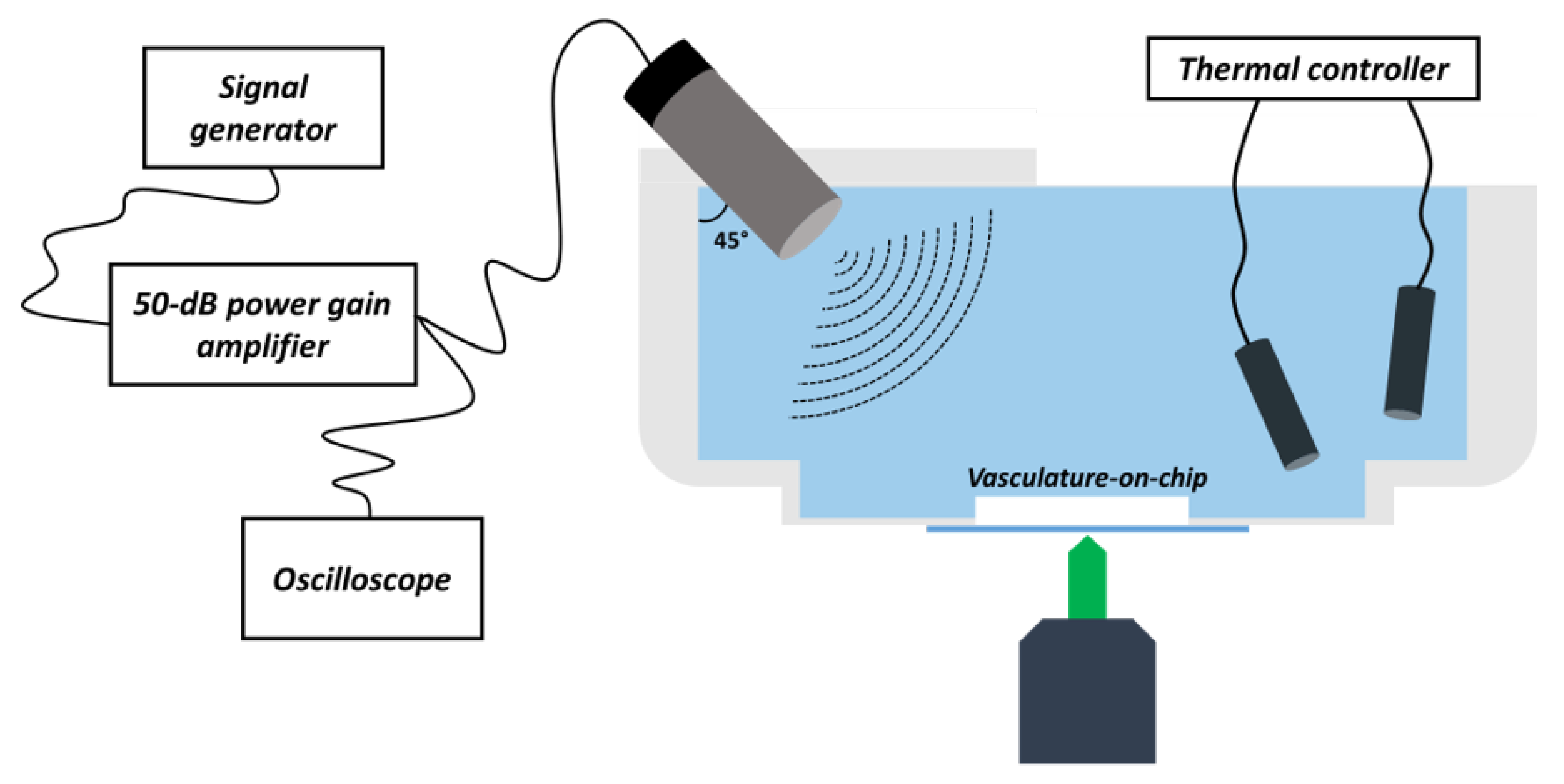
2.4. Insonation Chamber Design
2.5. Acoustic Set-Up
2.6. Microbubbles
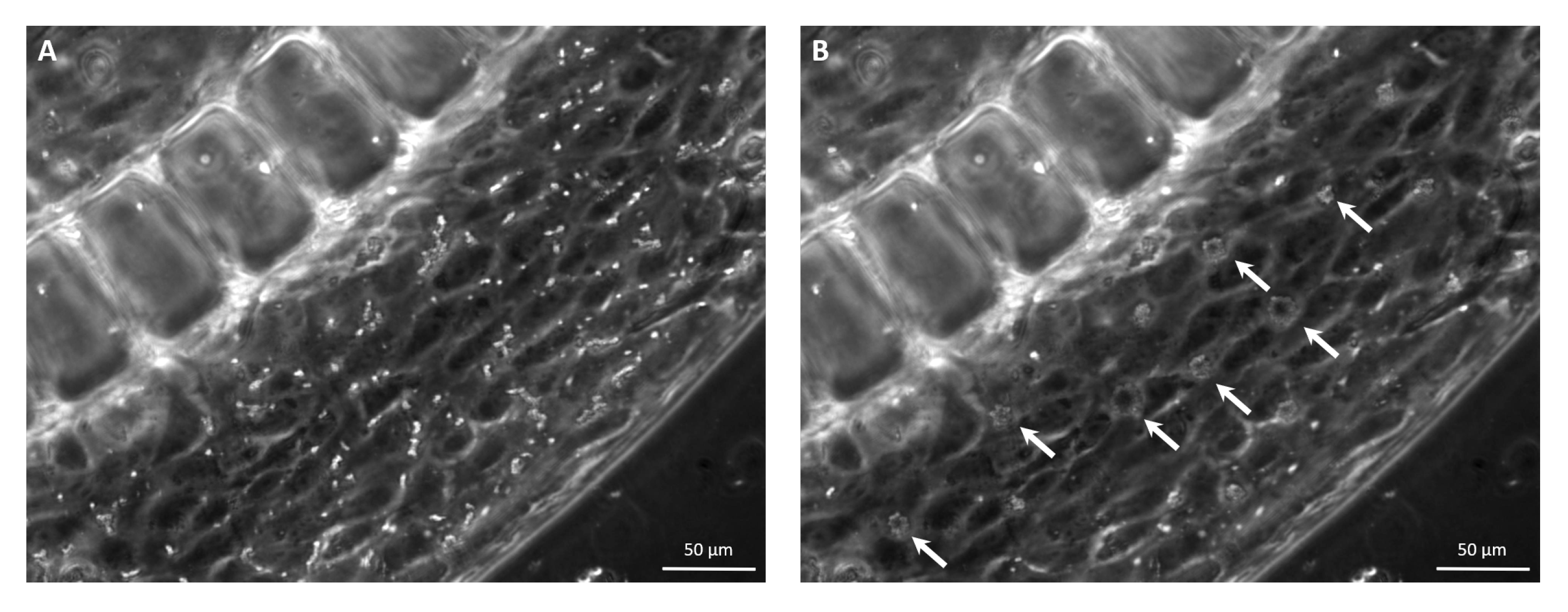
2.7. USMB-Mediated Cavitation Experiments
2.8. Optical Set-Up and IF Image Acquisition
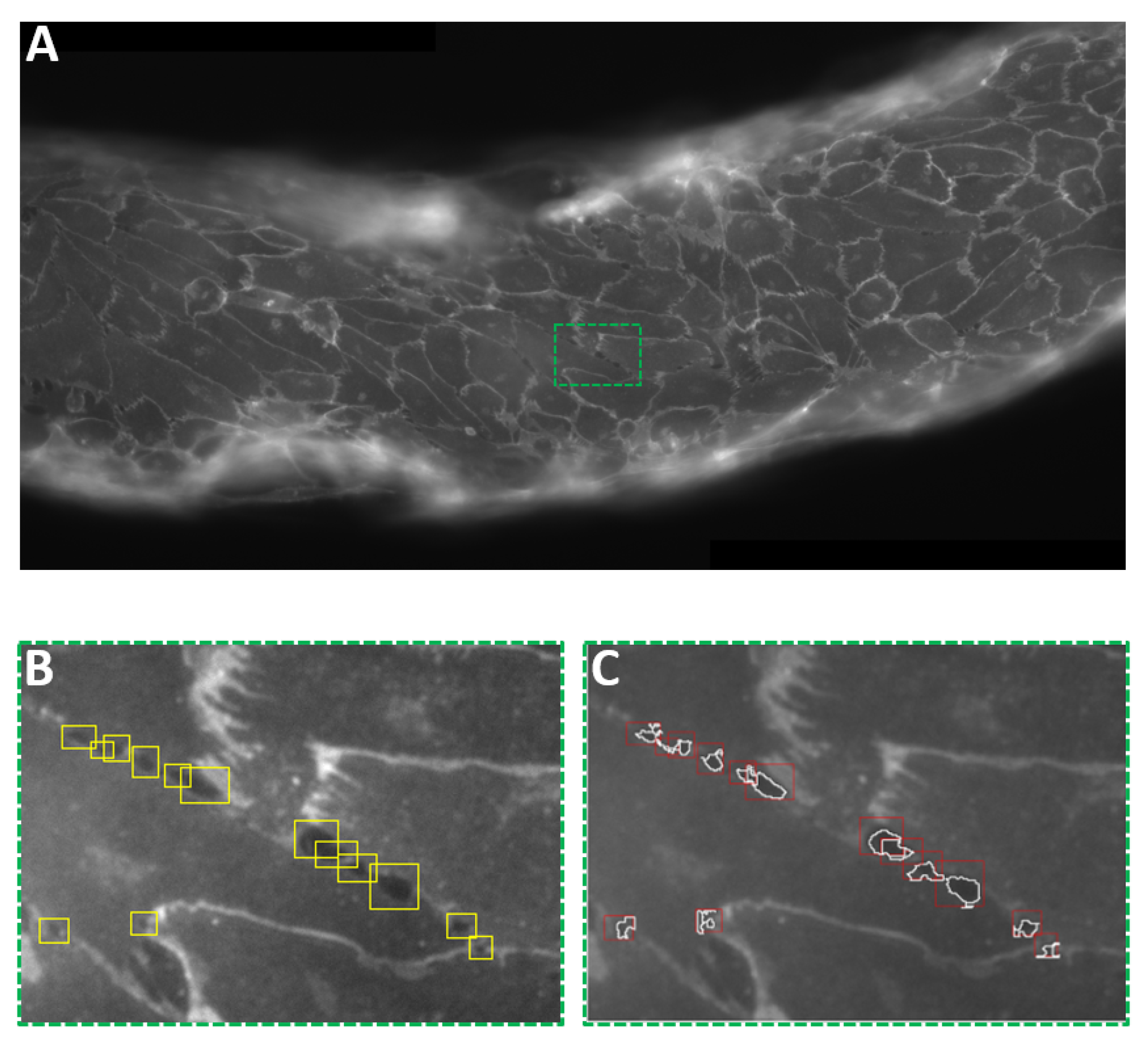
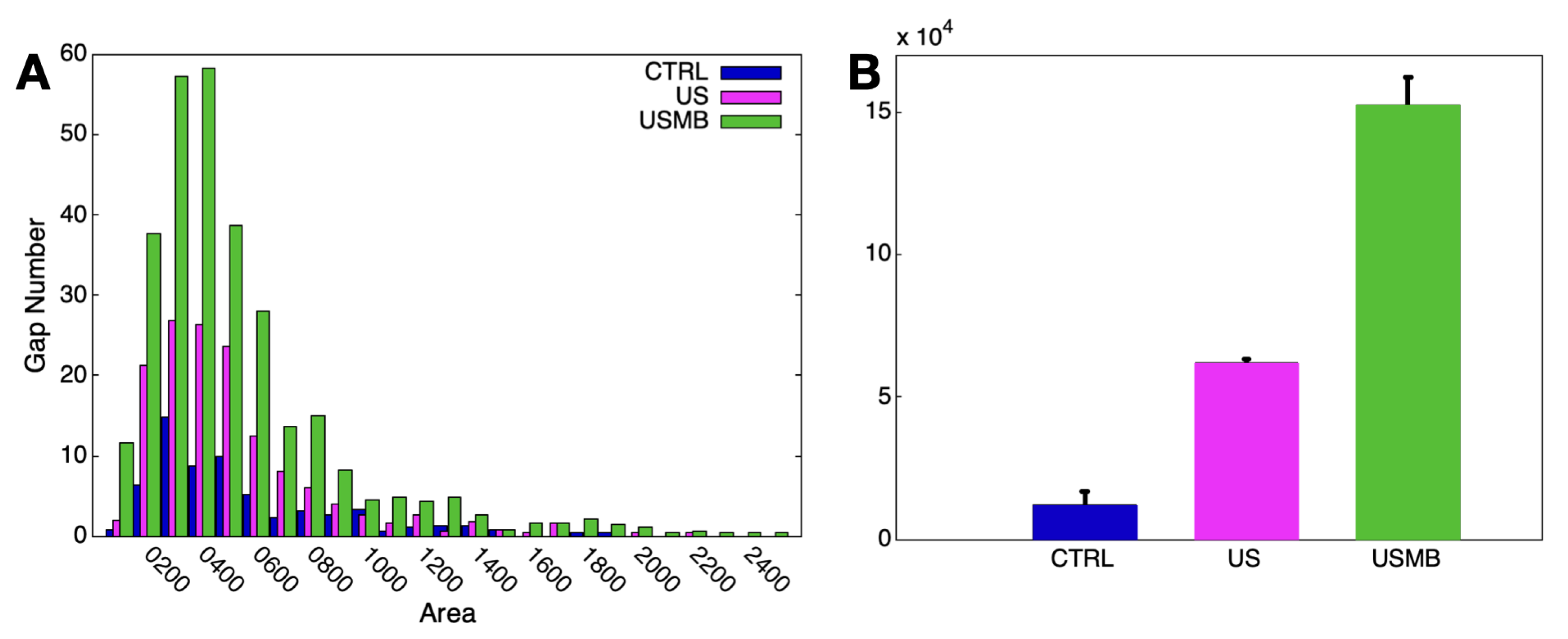
2.9. Interendothelial Gap Analysis
3. Results and Discussion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Drug targeting. Eur. J. Pharm. Sci. 2000, 11, S81–S91. [Google Scholar] [CrossRef]
- Needham, D. Materials engineering of lipid bilayers for drug carrier performance. Mrs Bull. 1999, 24, 32–40. [Google Scholar] [CrossRef]
- Krizbai, I.A.; Fazakas, C.; Haskó, J.; Molnár, J.; Nyúl-Tóth, Á.; Farkas, A.E.; Wilhelm, I. Molecular structure and function of biological barriers. Acta Biol. Szeged. 2015, 59, 39–50. [Google Scholar]
- Saladin, K.S. Anatomy & Physiology: The Unity of Form and Function; Mc Graw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Komarova, Y.; Malik, A.B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 2010, 72, 463–493. [Google Scholar] [CrossRef]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial cell–cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef]
- Radeva, M.; Waschke, J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol. 2018, 222, e12860. [Google Scholar] [CrossRef]
- Lampugnani, M.G. Endothelial adherens junctions and the actin cytoskeleton: An ‘infinity net’? J. Biol. 2010, 9, 16. [Google Scholar] [CrossRef]
- Seebach, J.; Taha, A.A.; Lenk, J.; Lindemann, N.; Jiang, X.; Brinkmann, K.; Bogdan, S.; Schnittler, H.J. The CellBorderTracker, a novel tool to quantitatively analyze spatiotemporal endothelial junction dynamics at the subcellular level. Histochem. Cell Biol. 2015, 144, 517–532. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Huh, D.; Torisawa, Y.S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol. 2020, 8, 1426. [Google Scholar] [CrossRef] [PubMed]
- Caprini, D.; Sinibaldi, G.; Marino, L.; Casciola, C.M. A T-junction device allowing for two simultaneous orthogonal views: Application to bubble formation and break-up. Microfluid. Nanofluidics 2018, 22, 85. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H. Organs-on-a-chip module: A review from the development and applications perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef]
- Weigl, B.; Domingo, G.; LaBarre, P.; Gerlach, J. Towards non-and minimally instrumented, microfluidics-based diagnostic devices. Lab Chip 2008, 8, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, S. Application of microfluidic chip technology in pharmaceutical analysis: A review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef]
- Ortseifen, V.; Viefhues, M.; Wobbe, L.; Grünberger, A. Microfluidics for Biotechnology: Bridging Gaps to Foster Microfluidic Applications. Front. Bioeng. Biotechnol. 2020, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- Marx, U.; Walles, H.; Hoffmann, S.; Lindner, G.; Horland, R.; Sonntag, F.; Klotzbach, U.; Sakharov, D.; Tonevitsky, A.; Lauster, R. ‘Human-on-a-chip’ developments: A translational cutting-edge alternative to systemic safety assessment and efficiency evaluation of substances in laboratory animals and man? Altern. Lab. Anim. 2012, 40, 235–257. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-chip: A fast track for engineered human tissues in drug development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Rosano, J.M.; Tousi, N.; Scott, R.C.; Krynska, B.; Rizzo, V.; Prabhakarpandian, B.; Pant, K.; Sundaram, S.; Kiani, M.F. A physiologically realistic in vitro model of microvascular networks. Biomed. Microdevices 2009, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A novel dynamic neonatal blood-brain barrier on a chip. PLoS ONE 2015, 10, e0142725. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Soroush, F.; Sun, S.; Liverani, E.; Langston, J.C.; Yang, Q.; Kilpatrick, L.E.; Kiani, M.F. Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. J. Neuroinflamm. 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Qiu, Y.; Ahn, B.; Sakurai, Y.; Hansen, C.E.; Tran, R.; Mimche, P.N.; Mannino, R.G.; Ciciliano, J.C.; Lamb, T.J.; Joiner, C.H.; et al. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat. Biomed. Eng. 2018, 2, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Parlato, S.; Grisanti, G.; Sinibaldi, G.; Peruzzi, G.; Casciola, C.M.; Gabriele, L. Tumor-on-a-chip platforms to study cancer–immune system crosstalk in the era of immunotherapy. Lab Chip 2021, 21, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef] [PubMed]
- Fritschen, A.; Blaeser, A. Biosynthetic, biomimetic, and self-assembled vascularized Organ-on-a-Chip systems. Biomaterials 2020, 268, 120556. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2020, 42, 119–133. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517. [Google Scholar] [CrossRef] [PubMed]
- Seebach, J.; Donnert, G.; Kronstein, R.; Werth, S.; Wojciak-Stothard, B.; Falzarano, D.; Mrowietz, C.; Hell, S.W.; Schnittler, H.J. Regulation of endothelial barrier function during flow-induced conversion to an arterial phenotype. Cardiovasc. Res. 2007, 75, 598–607. [Google Scholar] [CrossRef]
- DeStefano, J.G.; Williams, A.; Wnorowski, A.; Yimam, N.; Searson, P.C.; Wong, A.D. Real-time quantification of endothelial response to shear stress and vascular modulators. Integr. Biol. 2017, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Silvani, G.; Scognamiglio, C.; Caprini, D.; Marino, L.; Chinappi, M.; Sinibaldi, G.; Peruzzi, G.; Kiani, M.F.; Casciola, C.M. Reversible Cavitation-Induced Junctional Opening in an Artificial Endothelial Layer. Small 2019, 15, 1905375. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Boarescu, P.M.; Melincovici, C.S.; Mihu, C.M. Microfluidic endothelium-on-a-chip development, from in vivo to in vitro experimental models. Rom. J. Morphol. Embryol. 2020, 61, 15. [Google Scholar] [CrossRef]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Searson, P.C. Live-cell imaging of invasion and intravasation in an artificial microvessel platform. Cancer Res. 2014, 74, 4937–4945. [Google Scholar] [CrossRef] [PubMed]
- Price, G.M.; Wong, K.H.; Truslow, J.G.; Leung, A.D.; Acharya, C.; Tien, J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31, 6182–6189. [Google Scholar] [CrossRef]
- Price, G.M.; Tien, J. Methods for forming human microvascular tubes in vitro and measuring their macromolecular permeability. In Biological Microarrays; Springer: Berlin/Heidelberg, Germany, 2011; pp. 281–293. [Google Scholar]
- Wolff, A.; Antfolk, M.; Brodin, B.; Tenje, M. In vitro blood–brain barrier models—An overview of established models and new microfluidic approaches. J. Pharm. Sci. 2015, 104, 2727–2746. [Google Scholar] [CrossRef]
- Lee, C.S.; Leong, K.W. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr. Opin. Biotechnol. 2020, 66, 78–87. [Google Scholar] [CrossRef]
- Helfield, B. A review of phospholipid encapsulated ultrasound contrast agent microbubble physics. Ultrasound Med. Biol. 2019, 45, 282–300. [Google Scholar] [CrossRef]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-responsive cavitation nuclei for therapy and drug delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef]
- Nolsøe, C.P.; Lorentzen, T. International guidelines for contrast-enhanced ultrasonography: Ultrasound imaging in the new millennium. Ultrasonography 2016, 35, 89. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surf. B Biointerfaces 2018, 168, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control. Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Leighton, T. The principles of cavitation. In Ultrasound in Food Process; Powey, M.J.W., Mason, T.J., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; p. 151. [Google Scholar]
- Apfel, R.E.; Holland, C.K. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 1991, 17, 179–185. [Google Scholar] [CrossRef]
- De Cock, I.; Zagato, E.; Braeckmans, K.; Luan, Y.; de Jong, N.; De Smedt, S.C.; Lentacker, I. Ultrasound and microbubble mediated drug delivery: Acoustic pressure as determinant for uptake via membrane pores or endocytosis. J. Control. Release 2015, 197, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E. Physical basis and principles of action of microbubble-based contrast agents. In Contrast Media in Ultrasonography; Springer: Berlin/Heidelberg, Germany, 2005; pp. 15–30. [Google Scholar]
- Blake, F., Jr. The Onset of Cavitation in Liquids; Acoustics Research Laboratory, Harvard University: Cambridge, MA, USA, 1949; Volume 12. [Google Scholar]
- Appel, R. Possibility of microcavitation from diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1986, 33, 139–142. [Google Scholar] [CrossRef]
- Magaletti, F.; Marino, L.; Casciola, C.M. Shock wave formation in the collapse of a vapor nanobubble. Phys. Rev. Lett. 2015, 114, 064501. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef]
- Van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; Folkert, J.; Versluis, M.; De Jong, N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J. Control. Release 2006, 112, 149–155. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Juffermans, L.J.; van Dijk, A.; Jongenelen, C.A.; Drukarch, B.; Reijerkerk, A.; de Vries, H.E.; Kamp, O.; Musters, R.J. Ultrasound and microbubble-induced intra-and intercellular bioeffects in primary endothelial cells. Ultrasound Med. Biol. 2009, 35, 1917–1927. [Google Scholar] [CrossRef]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Miller, D.L.; Brayman, A.A. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med. Biol. 1996, 22, 1131–1154. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- Fatar, M.; Griebe, M.; Stroick, M.; Kern, R.; Hennerici, M.; Meairs, S. Neuroprotective effect of combined ultrasound and microbubbles in a rat model of middle cerebral artery infarction. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA; AIP Publishing LLC: Melville, NY, USA, 2005; Volume 754, pp. 62–64. [Google Scholar]
- Izadifar, Z.; Babyn, P.; Chapman, D. Mechanical and biological effects of ultrasound: A review of present knowledge. Ultrasound Med. Biol. 2017, 43, 1085–1104. [Google Scholar] [CrossRef]
- Kost, J. Ultrasound for controlled delivery of therapeutics. Clin. Mater. 1993, 13, 155–161. [Google Scholar] [CrossRef]
- Ng, K.y.; Liu, Y. Therapeutic ultrasound: Its application in drug delivery. Med. Res. Rev. 2002, 22, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Unger, E.C.; Hersh, E.; Vannan, M.; McCreery, T. Gene delivery using ultrasound contrast agents. Echocardiography 2001, 18, 355–361. [Google Scholar] [CrossRef]
- Unger, E.; Porter, T.; Lindner, J.; Grayburn, P. Cardiovascular drug delivery with ultrasound and microbubbles. Adv. Drug Deliv. Rev. 2014, 72, 110–126. [Google Scholar] [CrossRef]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef]
- De Luca, R.; Silvani, G.; Scognamiglio, C.; Sinibaldi, G.; Peruzzi, G.; Chinappi, M.; Kiani, M.; Casciola, C. Towards cavitation-enhanced permeability in blood vessel on a chip. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1873, p. 020010. [Google Scholar]
- Cheng, M.; Li, F.; Han, T.; Alfred, C.; Qin, P. Effects of ultrasound pulse parameters on cavitation properties of flowing microbubbles under physiologically relevant conditions. Ultrason. Sonochem. 2019, 52, 512–521. [Google Scholar] [CrossRef]
- Nam, K.H.; Eddington, D.T. Size-based separation of microparticles in a multilayered microfluidic device. J. Microelectromechanical Syst. 2010, 19, 375–383. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Accounts Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Lamberti, G.; Tang, Y.; Prabhakarpandian, B.; Wang, Y.; Pant, K.; Kiani, M.F.; Wang, B. Adhesive interaction of functionalized particles and endothelium in idealized microvascular networks. Microvasc. Res. 2013, 89, 107–114. [Google Scholar] [CrossRef]
- Marmottant, P.; Van Der Meer, S.; Emmer, M.; Versluis, M.; De Jong, N.; Hilgenfeldt, S.; Lohse, D. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. J. Acoust. Soc. Am. 2005, 118, 3499–3505. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Kooiman, K.; Emmer, M.; Foppen-Harteveld, M.; van Wamel, A.; de Jong, N. Increasing the endothelial layer permeability through ultrasound-activated microbubbles. IEEE Trans. Biomed. Eng. 2009, 57, 29–32. [Google Scholar] [CrossRef]
- Lelu, S.; Afadzi, M.; Berg, S.; Åslund, A.; Torp, S.H.; Sattler, W.; Davies, C.d.L. Primary porcine brain endothelial cells as in vitro model to study effects of ultrasound and microbubbles on blood–brain barrier function. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 64, 281–290. [Google Scholar] [CrossRef]
- Ivanov, K.; Kalinina, M.; Levkovich, Y.I. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc. Res. 1981, 22, 143–155. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Jiménez, N.; Krouwer, V.J.; Post, J.A. A new, rapid and reproducible method to obtain high quality endothelium in vitro. Cytotechnology 2013, 65, 1–14. [Google Scholar] [CrossRef]
- Esch, M.B.; Post, D.J.; Shuler, M.L.; Stokol, T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A 2011, 17, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Boissenot, T.; Bordat, A.; Fattal, E.; Tsapis, N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release 2016, 241, 144–163. [Google Scholar] [CrossRef]
- Barnett, S.B.; Ter Haar, G.R.; Ziskin, M.C.; Rott, H.D.; Duck, F.A.; Maeda, K. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med. Biol. 2000, 26, 355–366. [Google Scholar] [CrossRef]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. USA 2012, 109, 16486–16491. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grisanti, G.; Caprini, D.; Sinibaldi, G.; Scognamiglio, C.; Silvani, G.; Peruzzi, G.; Casciola, C.M. A Microfluidic Platform for Cavitation-Enhanced Drug Delivery. Micromachines 2021, 12, 658. https://doi.org/10.3390/mi12060658
Grisanti G, Caprini D, Sinibaldi G, Scognamiglio C, Silvani G, Peruzzi G, Casciola CM. A Microfluidic Platform for Cavitation-Enhanced Drug Delivery. Micromachines. 2021; 12(6):658. https://doi.org/10.3390/mi12060658
Chicago/Turabian StyleGrisanti, Giulia, Davide Caprini, Giorgia Sinibaldi, Chiara Scognamiglio, Giulia Silvani, Giovanna Peruzzi, and Carlo Massimo Casciola. 2021. "A Microfluidic Platform for Cavitation-Enhanced Drug Delivery" Micromachines 12, no. 6: 658. https://doi.org/10.3390/mi12060658
APA StyleGrisanti, G., Caprini, D., Sinibaldi, G., Scognamiglio, C., Silvani, G., Peruzzi, G., & Casciola, C. M. (2021). A Microfluidic Platform for Cavitation-Enhanced Drug Delivery. Micromachines, 12(6), 658. https://doi.org/10.3390/mi12060658





