Immunosensor Based on Zinc Oxide Nanocrystals Decorated with Copper for the Electrochemical Detection of Human Salivary Alpha-Amylase
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Apparatus for Characterization and Electrochemical Analysis
2.3. Electrochemical Selection and Use of the Nanocrystals
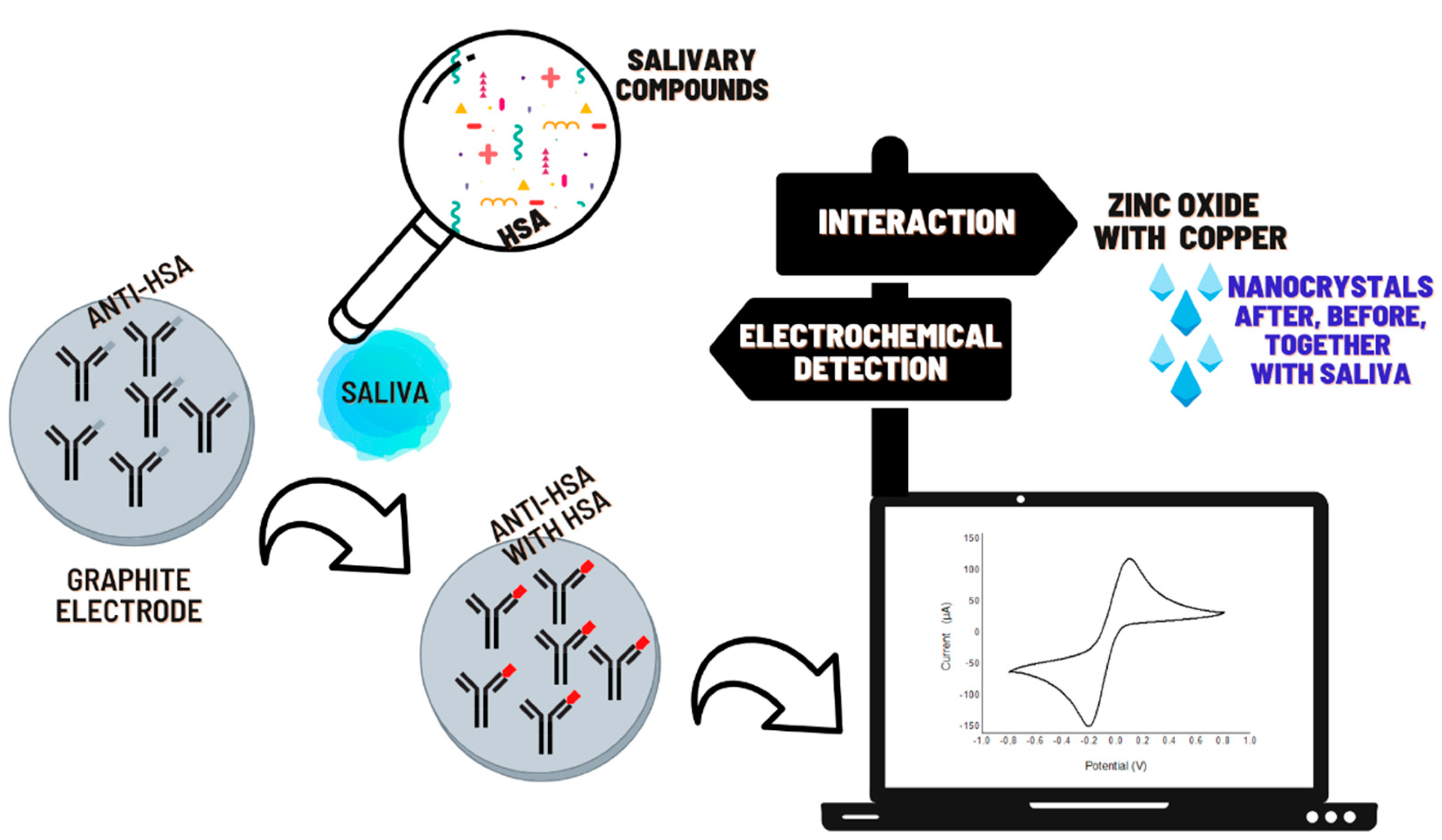
2.4. Immunosensor
3. Results
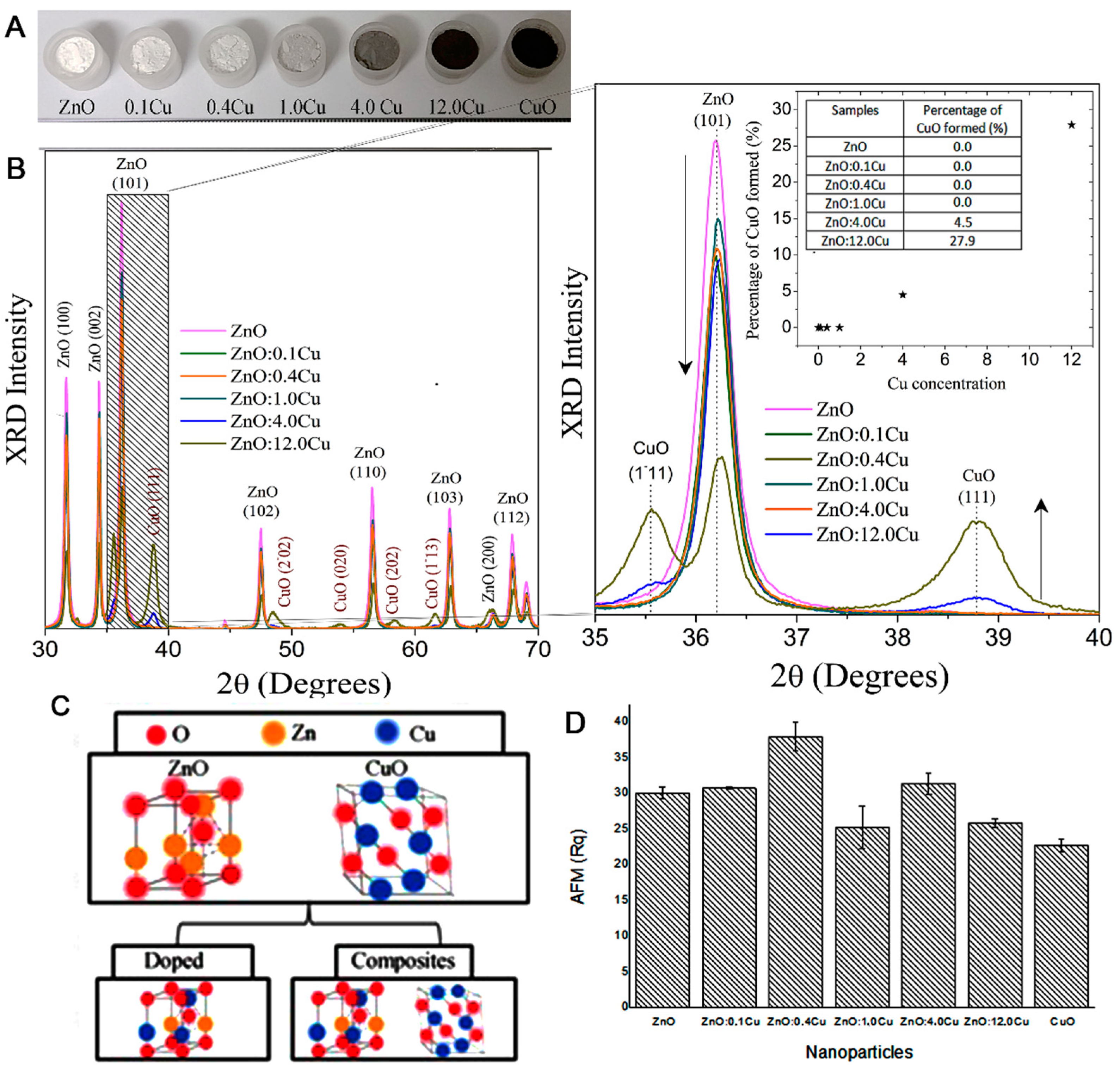
3.1. Characterization of ZnO:Cu Nanocrystals
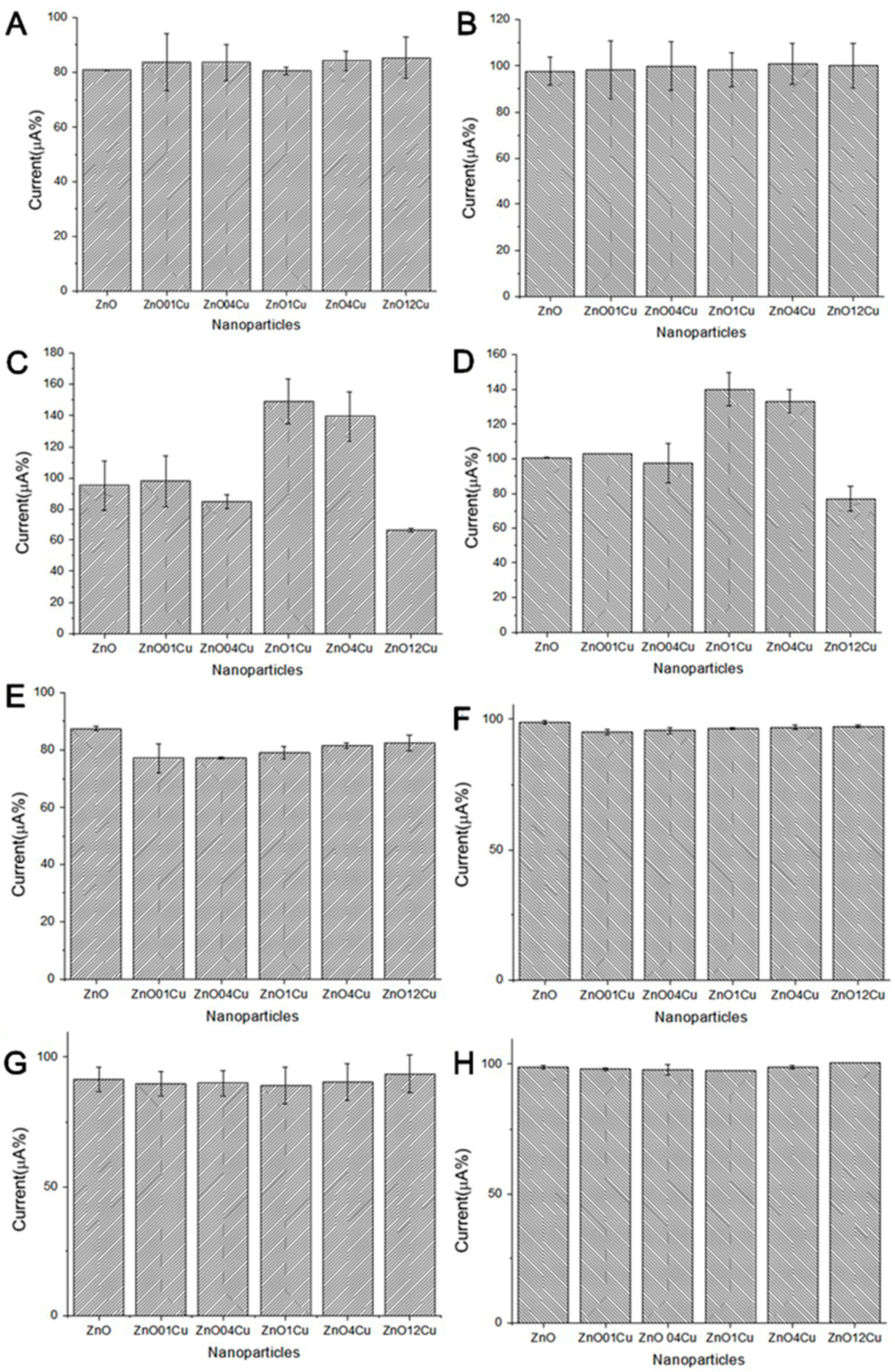
3.2. Electrochemical Analysis of ZnO:Cu Nanocrystals
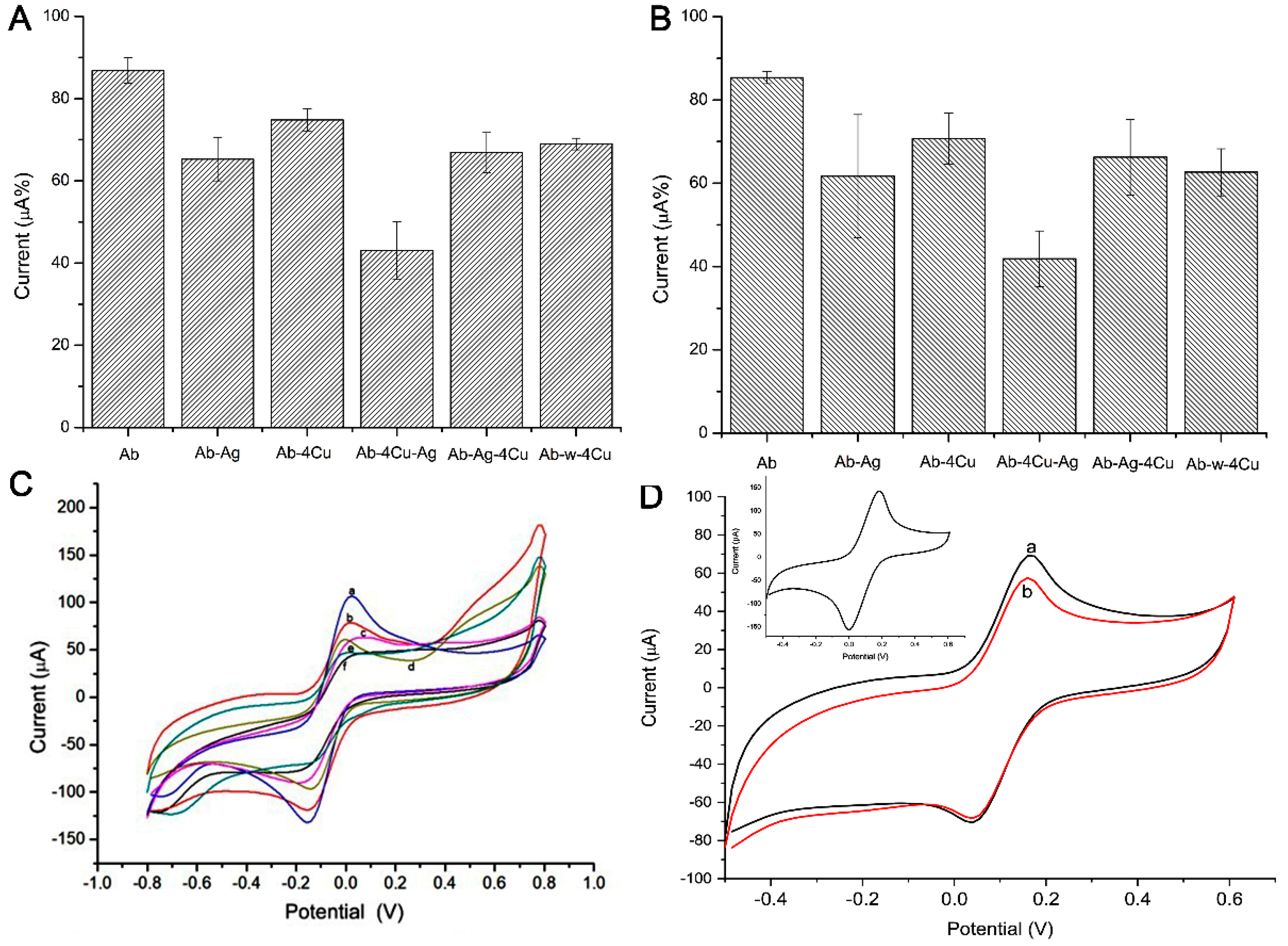
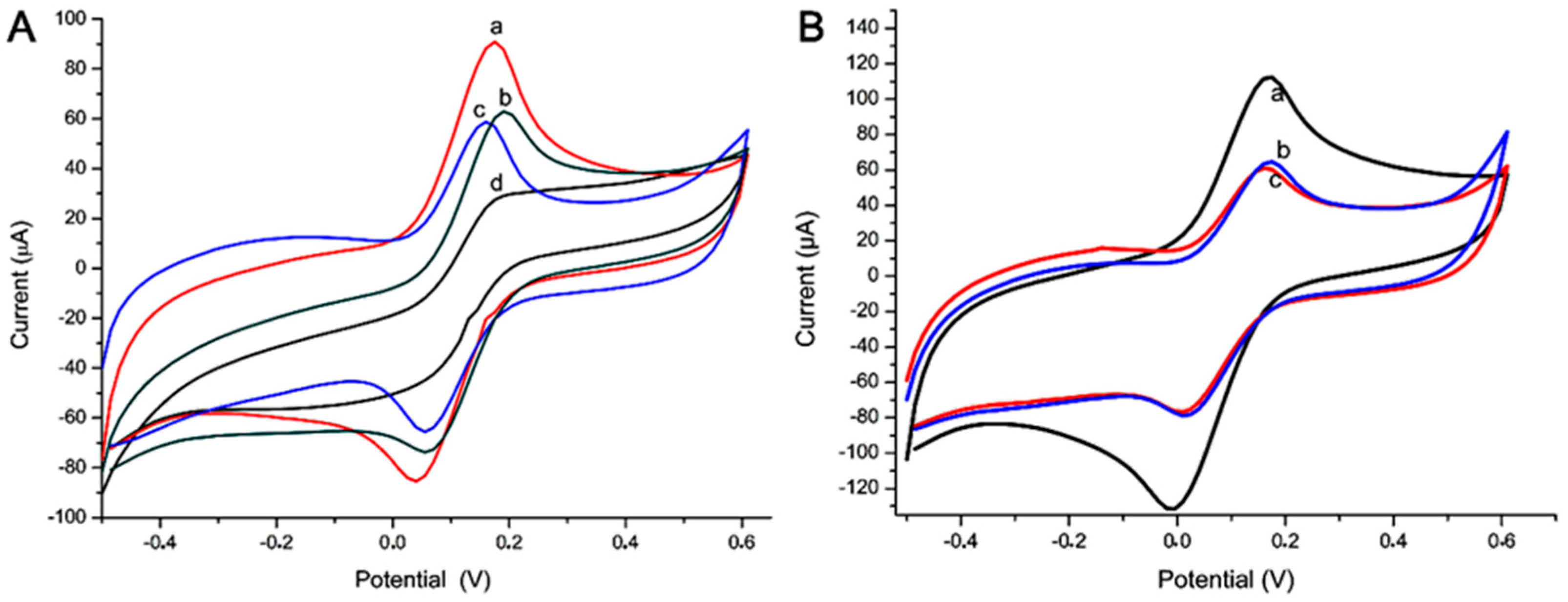
3.3. Immunosensor and Electrochemical Analysis
3.4. Sensor and Electrochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreuter, J. Nanoparticles-a historical perspective. Int. J. Pharm. 2006, 331, 1–10. [Google Scholar] [CrossRef]
- Colomban, P.; Gouadec, G. The ideal ceramic-fibre/oxide-matrix composite: How to reconcile antagonist physical and chemical requirements? Ann. Chim. Sci. Mater. 2005, 30, 673–688. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore. Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. J. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 1, 1–24. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D.; Van Vugt, L.K. ZnO nanowire lasers. Nanoscale 2011, 3, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal Synthesis of ZnO Nanoparticles and Anti-Infection Application in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef]
- Haq, A.N.U.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017, 2017, 8510342 . [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud. Univ. 2017, 31, 257–269. [Google Scholar] [CrossRef]
- Silva, R.S.; Gualdi, A.J.; Zabotto, F.L.; Cano, N.F.; Silva, A.C.A.; Dantas, N.O. Weak ferromagnetism in Mn2+ doped Bi2Te3 nanocrystals grown in glass matrix. J. Alloys Compd. 2017, 708, 619–622. [Google Scholar] [CrossRef]
- Rego-Filho, F.G.; Dantas, N.O.; Silva, A.C.A.; Vermelho, M.V.D.; Jacinto, C.; Gouveira-Neto, A.S. IR-to-visible frequency upconversion in Yb3+/Tm3+ co-doped phosphate glass. Opt. Mater. 2017, 73, 1–6. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schilda, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microb. 2015, 305, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Liu, X.; Liu, J.; Li, J.; Wu, T.; Li, H.; Lei, W.; Xu, Y.; Pan, L. Synergetic effect of Ag2O as co-catalyst for enhanced photocatalytic degradation of phenol on N-TiO. Sci. Eng. B Solid State Mater. Adv. Technol. 2016, 2211, 128–134. [Google Scholar] [CrossRef]
- Phoohinkong, W.; Foophow, T.; Pecharapa, W. Synthesis and characterization of copper zinc oxide nanoparticles obtained via metathesis process. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035003. [Google Scholar] [CrossRef]
- Morais, P.V.; Gomes-Junior, V.F.; Silva, A.C.A.; Dantas, N.O.; Schöning, M.J.; Siqueira-Junior, J.R. Nanofilm of ZnO nanocrystals/carbon nanotubes as biocompatible layer for enzymatic biosensors in capacitive field-effect devices. J. Mater. Sci. 2017, 52, 12314–12325. [Google Scholar] [CrossRef]
- Reis, É.M.; Rezende, A.A.A.; Oliveira, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.A.; Dantas, N.O.; Spanó, M.A. Evaluation of titanium dioxide nanocrystal-induced genotoxicity by the cytokinesis-block micronucleus assay and the Drosophila wing spot test. Food Chem. Toxicol. 2016, 84, 55–63. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.M.; Bocanegra, O.L.; Teixeira, R.R.; Soares, S.S.; Espindola, F.S. Response of salivary markers of autonomic activity to elite competition. Int. J. Sports Med. 2012, 33, 763–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teixeira, R.R.; Díaz, M.M.; Santos, T.V.; Bernardes, J.T.; Peixoto, L.G.; Bocanegra, O.L.; Neto, M.B.; Espindola, F.S. Chronic stress induces a hyporeactivity of the autonomic nervous system in response to acute mental stressor and impairs cognitive performance in business executives. PLoS ONE 2015, 10, e0119025. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Deguchi, M.; Wakasugi, J.; Ono, S.; Takai, N.; Higashi, T.; Mizuno, Y. Hand-held monitor of sympathetic nervous system using salivary amylase activity and its validation by driver fatigue assessment. Biosens. Bioelectron. 2006, 21, 1007–1014. [Google Scholar] [CrossRef]
- Santos, T.V.S.; Teixeira, R.R.; Franco, D.L.; Madurro, J.M.; Brito-Madurro, A.G.; Espindola, F.S. Bioelectrode for detection of human salivary amylase. Mater. Sci. Eng. C 2012, 32, 530–535. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Sadir, S.; Balakrishnan, M.; Corcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. BioMed Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Wang, K.; Zhang, W.; Li, Y.; Sun, Y.; Sun, X.; Li, C.; Dong, B.; Wang, L.; et al. Smart biosensors and intelligent devices for salivary biomarker detection. TrAC Trends Anal. Chem. 2021, 140, 116281. [Google Scholar] [CrossRef]
- Agarwal, R.P.; Henkin, R.I. Metal binding characteristics of human salivary and porcine pancreatic amylase. J. Biol. Chem. 1987, 262, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Duncan, S.E.; Dietrich, A.M.; O’Keefe, S.F.; Eigel, W.N.; Mallikarjunan, K. Interaction of copper and human salivary proteins. J. Agric. Food Chem. 2009, 57, 15–6967. [Google Scholar] [CrossRef] [PubMed]
- Linden, A.; Mayans, O.; Meyer-Klaucke, W.; Antranikian, G.; Wilmanns, M. Differential regulation of a hyperthermophilic α-amylase with a novel (Ca, Zn) two-metal center by zinc. J. Biol. Chem. 2003, 278, 9875–9884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Yu, W.; Sun, D.; Sun, X. Susceptibility to corrosion of laser welding composite arch wire in artificial saliva of salivary amylase and pancreatic amylase. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 267–271. [Google Scholar] [CrossRef]
- Murugappan, G.; Sreeram, K.J. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids Surf. B Biointerfaces 2021, 197, 111386. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 81–99. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Wang, D.; Liu, C.; Zhang, C. An electrochemiluminescence cloth-based biosensor with smartphone-based imaging for detection of lactate in saliva. Analyst 2017, 142, 3715–3724. [Google Scholar] [CrossRef]
- Khanna, P.; Walt, D.R. Salivary diagnostics using a portable point-of-service platform: A review. Clin. Therapeut. 2015, 37, 498–504. [Google Scholar] [CrossRef]
- Vinitha, T.U.; Ghosh, S.; Milleman, A.; Nguyen, T.; Ahn, C.H. A new polymer lab-on-a-chip (LOC) based on a microfluidic capillary flow assay (MCFA) for detecting unbound cortisol in saliva. Lab Chip 2020, 20, 1961–1974. [Google Scholar] [CrossRef]
- Arunkumar, S.; Arunkumar, J.S.; Krishna, N.B.; Shakuntala, G.K. Developments in Diagnostic Applications of Saliva in Oral and Systemic Diseases-A Comprehensive Review. J. Sci. Innov. Res. 2014, 3, 372–387. Available online: http://www.jsirjournal.com/Vol3_Issue3_16.pdf (accessed on 5 January 2021).
- Souza, A.V.; Giolo, J.S.; Teixeira, R.R.; Vilela, D.D.; Peixoto, L.G.; Justino, A.B.; Caixeta, D.C.; Puga, G.M.; Espindola, F.S. Salivary and Plasmatic Antioxidant Profile following Continuous, Resistance, and High-Intensity Interval Exercise: Preliminary Study. Oxid. Med. Cell Longev. 2019, 5425021. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano-García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W. Significance of nanomaterials in wearables: A review on wearable actuators and sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef]
- Mishra, S.; Saadat, D.; Lee, O.Y.; Choi, W.S.; Kim, J.H.; Yeo, W.H. Recent advances in salivary cancer diagnostics enabled by biosensors and bioelectronics. Biosens. Bioelectron. 2016, 81, 181–197. [Google Scholar] [CrossRef]
- Ilea, A.; Andrei, V.; Feurdean, C.N.; Băbțan, A.-M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Ciui, B.; Tertiș, M.; Săndulescu, R. Saliva, a magic biofluid available for multilevel assessment and a mirror of general health—A systematic review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef]
- Ehtesabi, H. Carbon nanomaterials for salivary-based biosensors: A review. Mater. Today Chem. 2020, 17, 100342. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors targeting salivary biomarkers: A comprehensive review. TrAC. Trends Anal. Chem. 2020, 116164. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K. Characterization of Nanomaterials by Physical Methods. Annu. Rev. Anal. Chem. 2009, 2, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Gómez, A.; Baró, M.D.; Suriñach, S.; Pellicer, E.; Sort, J. Self-templating faceted and spongy single-crystal ZnO nanorods: Resistive switching and enhanced piezoresponse. Mater. Des. 2017, 133, 54–61. [Google Scholar] [CrossRef]
- Li, F.; Gou, Q.; Xing, J.; Tan, Z.; Jiang, L.; Xie, L.; Wu, J.; Zhang, W.; Xiao, D.; Wu, J. The piezoelectric and dielectric properties of sodium–potassium niobate ceramics with new multiphase boundary. J. Mater. Sci. Mater. Electron. 2017, 28, 18090–18098. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bharti, A.; Singh, J.P.; Chae, K.H.; Goyal, N. Influence of Cu doping on the local electronic and magnetic properties of ZnO nanostructures. Nanoscale Adv. 2020, 2, 4450–4463. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; de la Guardia, M. Nanomaterial-based electrochemical immunosensors as advanced diagnostic tools. Anal. Methods. 2014, 6, 3891–3900. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Rathod, A.P.; Sonawane, S.H.; Shimpi, N.G.; Parate, V.R. Copper-Doped Zinc Oxide Nanoparticles: Influence on Thermal, Thermo Mechanical, and Tribological Properties of Polycarbonate. Polym. Compos. 2017, 39, E1398–E1406. [Google Scholar] [CrossRef]
- Thiwawong, T.; Onlaor, K.; Chaithanatkun, N.; Tunhoo, T. Preparation of Copper Doped Zinc Oxide Nanoparticles by Precipitation Process for Humidity Sensing Device. AIP Conf. Proc. 2018, 2010, 020022. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. An overview of the synthesis of CuO-ZnO nanocomposite for environmental and other applications. Nanotechnol. Rev. 2018, 7, 267–282. [Google Scholar] [CrossRef]
- Egelhaaf, H.J.; Oelkrug, D. Luminescence and nonradiative deactivation of excited states involving oxygen defect centers in polycrystalline ZnO. J. Cryst. Growth 1996, 161, 190–194. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, H.; Li, J.; Chen, L. Highly sensitive and selective voltammetric detection of mercury (II) using an ITO electrode modified with 5-methyl-2-thiouracil, graphene oxide and gold nanoparticles. Microchim. Acta 2013, 180, 493–499. [Google Scholar] [CrossRef]
- Martins, B.R.; Barbosa, Y.O.; Andrade, C.M.R.; Pereira, L.Q.; Simão, G.F.; de Oliveira, C.J.; Correia, D.; Oliveira, R.T.S., Jr.; da Silva, M.V.; Silva, A.C.A.; et al. Development of an Electrochemical Immunosensor for Specific Detection of Visceral Leishmaniasis Using Gold-Modified Screen-Printed Carbon Electrodes. Biosensors 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Shatzman, A.R.; Henkin, R.I. Metal-binding characteristics of the parotid salivary protein gustin. Biochim. Biophys. Acta. 1980, 623, 107–108. [Google Scholar] [CrossRef]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A. Metal and anion binding sites in lactoferrin and related proteins. Pure Appl. Chem. 1990, 62, 1067–1070. [Google Scholar] [CrossRef]
- Sadik, O.A.; Aluoch, A.O.; Zhou, A. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009, 24, 2749–2765. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.R.; Sampaio, T.M.; de Farias, A.K.S.R.; de Paula Martins, R.; Teixeira, R.R.; Oliveira, R.T.S., Jr.; Oliveira, C.J.F.; da Silva, M.V.; Rodrigues, V., Jr.; Dantas, N.O.; et al. Immunosensor Based on Zinc Oxide Nanocrystals Decorated with Copper for the Electrochemical Detection of Human Salivary Alpha-Amylase. Micromachines 2021, 12, 657. https://doi.org/10.3390/mi12060657
Martins BR, Sampaio TM, de Farias AKSR, de Paula Martins R, Teixeira RR, Oliveira RTS Jr., Oliveira CJF, da Silva MV, Rodrigues V Jr., Dantas NO, et al. Immunosensor Based on Zinc Oxide Nanocrystals Decorated with Copper for the Electrochemical Detection of Human Salivary Alpha-Amylase. Micromachines. 2021; 12(6):657. https://doi.org/10.3390/mi12060657
Chicago/Turabian StyleMartins, Beatriz Rodrigues, Tainá Marques Sampaio, Ana Karoline Silva Rocha de Farias, Rheltheer de Paula Martins, Renata Roland Teixeira, Robson Tadeu Soares Oliveira, Jr., Carlo Jose Freire Oliveira, Marcos Vinícius da Silva, Virmondes Rodrigues, Jr., Noelio Oliveira Dantas, and et al. 2021. "Immunosensor Based on Zinc Oxide Nanocrystals Decorated with Copper for the Electrochemical Detection of Human Salivary Alpha-Amylase" Micromachines 12, no. 6: 657. https://doi.org/10.3390/mi12060657
APA StyleMartins, B. R., Sampaio, T. M., de Farias, A. K. S. R., de Paula Martins, R., Teixeira, R. R., Oliveira, R. T. S., Jr., Oliveira, C. J. F., da Silva, M. V., Rodrigues, V., Jr., Dantas, N. O., Espindola, F. S., Silva, A. C. A., & Alves-Balvedi, R. P. (2021). Immunosensor Based on Zinc Oxide Nanocrystals Decorated with Copper for the Electrochemical Detection of Human Salivary Alpha-Amylase. Micromachines, 12(6), 657. https://doi.org/10.3390/mi12060657









