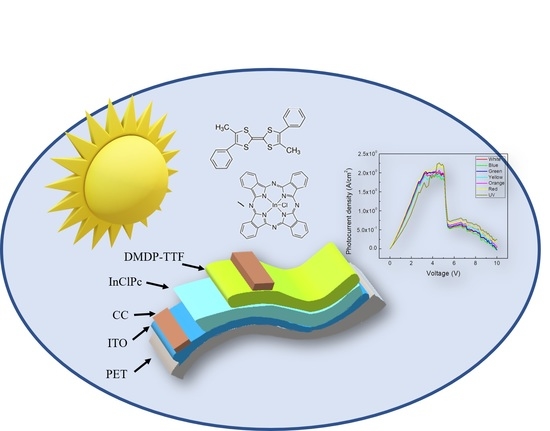
Innovative Implementation of an Alternative Tetrathiafulvene Derivative for Flexible Indium Phthalocyanine Chloride-Based Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Deposit and Characterization of Semiconductor Films
3.2. Manufacture and Characterization of the Device
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sevgili, O.; Canlı, S.; Akman, F.; Orak, I.; Karabulut, A.; Yıldırım, N. Characterization of Aluminum 8-Hydroxyquinoline Microbelts and Microdots, and Photodiode Applications. J. Phys. Chem. Solids 2020, 136, 109128. [Google Scholar] [CrossRef]
- Kayunkid, N.; Tammarugwattana, N.; Mano, K.; Rangkasikorn, A.; Nukeaw, J. Growth and Characterizations of Tin-Doped Nickel-Phthalocyanine Thin Film Prepared by Thermal Co-Evaporation as a Novel Nanomaterial. Surf. Coat. Technol. 2016, 306, 101–105. [Google Scholar] [CrossRef]
- Bandari, V.K.; Gu, Y.; Shi, S.; Nan, Y.; He, K.; Li, Y.; Bandari, N.; Moradi, S.; Tian, H.; Zhu, F.; et al. Fully Integrated Microscale Quasi-2D Crystalline Molecular Field-Effect Transistors. Adv. Funct. Mater. 2019, 29, 1903738. [Google Scholar] [CrossRef]
- Hubler, A.; Trnovec, B.; Zillger, T.; Ali, M.; Wetzold, N.; Mingebach, M.; Wagenpfahl, A.; Deibel, C.; Dyakonov, V. Printed Paper Photovoltaic Cells. Adv. Energy Mater. 2011, 1, 1018–1022. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bonasera, A.; Giuliano, G.; Arrabito, G.; Pignataro, B. Tackling Performance Challenges in Organic Photovoltaics: An Overview about Compatibilizers. Molecules 2020, 25, 2200. [Google Scholar] [CrossRef]
- Cheng, P.; Xiaowei, Z. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Du, X.; Heumueller, T.; Gruber, W.; Classen, A.; Unruh, T.; Li, N.; Brabec, C.J. Efficient Polymer Solar Cells Based on Non-fullerene Acceptors with Potential Device Lifetime Approaching 10 Years. Joule 2019, 3, 215. [Google Scholar] [CrossRef]
- Burlingame, Q.; Huang, X.; Liu, X.; Jeong, C.; Coburn, C.; Forrest, S.R. Intrinsically stable organic solar cells under high-intensity illumination. Nature 2019, 573, 394–397. [Google Scholar] [CrossRef]
- Heiber, M.C.; Baumbach, C.; Dyakonov, V.; Deibel, C. Encounter-Limited Charge-Carrier Recombination in Phase-Separated Organic Semiconductor Blends. Phys. Rev. Lett. 2015, 114, 136602. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Tang, C.W. Hole-transport limited S-shaped I-V curves in planar heterojunction organic photovoltaic cells. Appl. Phys. Lett. 2011, 99, 213506. [Google Scholar] [CrossRef]
- Menke, S.M.; Holmes, R.J. Exciton diffusion in organic photovoltaic cells. Energy Environ. Sci. 2014, 7, 499–512. [Google Scholar] [CrossRef]
- Sworakowski, J.; Ulański, J. Electrical properties of organic materials. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 87–125. [Google Scholar] [CrossRef]
- Wang, L.; Nan, G.; Yang, X.; Peng, Q.; Li, Q.; Shuai, Z. Computational methods for design of organic materials with high charge mobility. Chem. Soc. Rev. 2010, 39, 423–434. [Google Scholar] [CrossRef]
- Sun, S.S.; Dalton, L.R. Introduction to Organic Electronic and Optoelectronic Materials and Devices; CRC Press: London, UK, 2005; ISBN 9781315374185. [Google Scholar]
- Ganesamoorthy, R.; Sathiyan, G.; Sakthivel, P. Review: Fullerene based acceptors for efficient bulk heterojunction organic solar cell applications. Sol. Energy Mater. Sol. Cells 2017, 161, 102–148. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Simon, J.; Andre, J.-J. Molecular Semiconductors: Photoelectrical Properties and Solar Cells; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-70012-5. [Google Scholar]
- Lee, H.; Lee, J.; Yi, Y.; Cho, S.W.; Kim, J.W. Anomalous Hole Injection Deterioration of Organic Light-Emitting Diodes with a Manganese Phthalocyanine Layer. J. Appl. Phys. 2015, 117, 035503. [Google Scholar] [CrossRef]
- Kwong, C.Y.; Djurišiĉ, A.B.; Chui, P.C.; Lam, L.S.M.; Chan, W.K. Improvement of the Efficiency of Phthalocyanine Organic Schottky Solar Cells with ITO Electrode Treatment. Appl. Phys. A 2003, 77, 555–560. [Google Scholar] [CrossRef]
- El-Menyawy, E.M. Optical Properties of Amorphous and Crystalline Tris(8-Hydroxyquinoline) Indium Films. J. Alloys Compd. 2016, 683, 393–398. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic Photoresponse Materials and Devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vergara, M.E.; Hamui, L.; González Habib, S. New Approaches in Flexible Organic Field-Effect Transistors (FETs) Using InClPc. Materials 2019, 12, 1712. [Google Scholar] [CrossRef]
- Aziz, F.; Sulaiman, K.; Karimov, K.S.; Muhammad, M.R.; Sayyad, M.H.; Majlis, B.Y. Investigation of Optical and Humidity-Sensing Properties of Vanadyl Phthalocyanine-Derivative Thin Films. Mol. Cryst. Liq. Cryst. 2012, 566, 22–32. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Motomochi-Lozano, J.D.; Cosme, I.; Hamui, L.; Olivares, A.J.; Galván-Hidalgo, J.M.; Gómez, E. Growth of Films with Seven-Coordinated Diorganotin(IV) Complexes and PEDOT:PSS Structurally Modified for Electronic Applications. Semicond. Sci. Technol. 2020, 35, 105016. [Google Scholar] [CrossRef]
- Abdullah, S.M.; Ahmad, Z.; Aziz, F.; Sulaiman, K. Investigation of VOPcPhO as an Acceptor Material for Bulk Heterojunction Solar Cells. Org. Electron. 2012, 13, 2532–2537. [Google Scholar] [CrossRef]
- Aziz, F.; Ahmad, Z.; Abdullah, S.M.; Sulaiman, K.; Sayyad, M.H. Photovoltaic Effect in Single-Junction Organic Solar Cell Fabricated Using Vanadyl Phthalocyanine Soluble Derivative. Pigment Resin Technol. 2015, 44, 26–32. [Google Scholar] [CrossRef]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 104, 1324–1338. [Google Scholar] [CrossRef]
- Peumans, P.; Bulović, V.; Forrest, S.R. Efficient Photon Harvesting at High Optical Intensities in Ultrathin Organic Double-Heterostructure Photovoltaic Diodes. Appl. Phys. Lett. 2000, 76, 2650–2652. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Beyer, A.; Plönnigs, B.; Nollau, A.; Fritz, T.; Leo, K.; Schlettwein, D.; Hiller, S.; Wöhrle, D. Controlled P-Doping of Pigment Layers by Cosublimation: Basic Mechanisms and Implications for Their Use in Organic Photovoltaic Cells. Sol. Energy Mater. Sol. Cells 2000, 63, 83–99. [Google Scholar] [CrossRef]
- Yoon, S.M.; Lou, S.J.; Loser, S.; Smith, J.; Chen, L.X.; Facchetti, A.; Marks, T. Fluorinated Copper Phthalocyanine Nanowires for Enhancing Interfacial Electron Transport in Organic Solar Cells. Nano Lett. 2012, 12, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Rajaputra, S.; Vallurupalli, S.; Singh, V.P. Copper Phthalocyanine Based Schottky Diode Solar Cells. J. Mater. Sci. Mater. Electron. 2007, 18, 1147–1150. [Google Scholar] [CrossRef]
- Khalil, S.; Tazarki, H.; Souli, M.; Guasch, C.; Jamoussi, B.; Kamoun, N. Synthesis and Characterization of Novel 4-Tetra-4-Tolylsulfonyl ZnPc Thin Films for Optoelectronic Applications. Appl. Surf. Sci. 2017, 421, 205–212. [Google Scholar] [CrossRef]
- Cid, J.-J.; García-Iglesias, M.; Yum, J.-H.; Forneli, A.; Albero, J.; Martínez-Ferrero, E.; Vázquez, P.; Grätzel, M.; Nazeeruddin, M.K.; Palomares, E.; et al. Structure–Function Relationships in Unsymmetrical Zinc Phthalocyanines for Dye-Sensitized Solar Cells. Chem. Eur. J. 2009, 15, 5130–5137. [Google Scholar] [CrossRef] [PubMed]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef]
- Del Caño, T.; Parra, V.; Rodríguez-Méndez, M.L.; Aroca, R.F.; De Saja, J.A. Characterization of evaporated trivalent and tetravalent phthalocyanines thin films: Different degree of organization. Appl. Surf. Sci. 2005, 246, 327–333. [Google Scholar] [CrossRef]
- Farren, C.; Christensen, C.A.; FitzGerald, S.; Bryce, M.R.; Beeby, A. Synthesis of Novel Phthalocyanine−Tetrathiafulvalene Hybrids; Intramolecular Fluorescence Quenching Related to Molecular Geometry. J. Org. Chem. 2002, 67, 9130–9139. [Google Scholar] [CrossRef]
- de Caro, D.; Souque, M.; Faulmann, C.; Coppel, Y.; Valade, L.; Fraxedas, J.; Vendier, O.; Courtade, F. Colloidal Solutions of Organic Conductive Nanoparticles. Langmuir 2013, 29, 8983–8988. [Google Scholar] [CrossRef]
- Otsubo, T.; Takimiya, K. Recent Synthetic Advances of Tetrathiafulvalene-Based Organic Conductors. Bull. Chem. Soc. Jpn. 2004, 77, 43–58. [Google Scholar] [CrossRef]
- Park, G.E.; Shin, J.; Lee, D.H.; Lee, T.W.; Shim, H.; Cho, M.J.; Pyo, S.; Choi, D.H. Acene-Containing Donor–Acceptor Conjugated Polymers: Correlation between the Structure of Donor Moiety, Charge Carrier Mobility, and Charge Transport Dynamics in Electronic Devices. Macromolecules 2014, 47, 3747–3754. [Google Scholar] [CrossRef]
- Back, J.Y.; Kim, Y.; An, T.K.; Kang, M.S.; Kwon, S.-K.; Park, C.E.; Kim, Y.-H. Synthesis and Electrical Properties of Novel Oligomer Semiconductors for Organic Field-Effect Transistors (OFETs): Asymmetrically End-Capped Acene-Heteroacene Conjugated Oligomers. Dyes Pigm. 2015, 112, 220–226. [Google Scholar] [CrossRef]
- Motoyama, T.; Sugii, S.; Ikeda, S.; Yamaguchi, Y.; Yamada, H.; Nakayama, K. Bulk-Heterojunction Organic Photovoltaic Devices Fabricated Using Asymmetric Soluble Anthracene Core Photoprecursors. Jpn. J. Appl. Phys. 2013, 53, 01AB02. [Google Scholar] [CrossRef]
- Kilicoglu, T.; Tombak, A.; Ocak, Y.S.; Aydemir, M. Electrical and Photoelectrical Characterization of a TTF/p-InP Organic–Inorganic Heterojunction. Microelectron. Eng. 2014, 129, 91–95. [Google Scholar] [CrossRef]
- Bouit, P.-A.; Villegas, C.; Delgado, J.L.; Viruela, P.M.; Pou-Amérigo, R.; Ortí, E.; Martín, N. ExTTF-Based Dyes Absorbing over the Whole Visible Spectrum. Org. Lett. 2011, 13, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Akiyama, Y.; Kojima, H.; Kawamoto, T.; Mori, T. Organic Semiconductors and Conductors with Tert-Butyl Substituents. Crystals 2012, 2, 1222–1238. [Google Scholar] [CrossRef]
- Kanno, M.; Bando, Y.; Shirahata, T.; Inoue, J.-i.; Wada, H.; Mori, T. Stabilization of Organic Field-Effect Transistors in Hexamethylenetetrathiafulvalene Derivatives Substituted by Bulky Alkyl Groups. J. Mater. Chem. 2009, 36, 6548–6555. [Google Scholar] [CrossRef]
- Inoue, J.-I.; Kanno, M.; Ashizawa, M.; Seo, C.; Tanioka, A.; Mori, T. Organic transistors based on octamethylenetetrathiafulvalenes. Chem. Lett. 2010, 39, 538–540. [Google Scholar] [CrossRef]
- Nagakubo, J.; Ashizawa, M.; Kawamoto, T.; Tanioka, A.; Mori, T. Stabilization of organic field-effect transistors by tert-butyl groups in dibenzotetrathiafulvalene derivatives. Phys. Chem. Chem. Phys. 2011, 13, 14370–14377. [Google Scholar] [CrossRef] [PubMed]
- Molas, S.; Caro, J.; Santiso, J.; Figueras, A.; Fraxedas, J.; Méziére, C.; Fourmigué, M.; Batail, P. Thin molecular films of neutral tetrathiafulvalene-derivatives. J. Cryst. Growth 2000, 218, 399–409. [Google Scholar] [CrossRef]
- Mahar, F.K.; Mehdi, M.; Qureshi, U.A.; Brohi, K.M.; Zahid, B.; Ahmed, F.; khatri, Z. Dyeability of recycled electrospun polyethylene terephthalate nanofibers Kinetics and thermodynamic study. J. Mol. Liq. 2017, 248, 911–919. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Ahn, M.H.; Cho, E.S.; Kwon, S.J. Characteristics of ITO-resistive touch film deposited on a PET substrate by in-line DC magnetron sputtering. Vacuum 2014, 101, 221–227. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.J. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Gaffo, L.; Cordeiro, M.R.; Freitas, A.R.; Moreira, W.C.; Girotto, E.M.; Zucolotto, V. The effects of temperature on the molecular orientation of zinc phthalocyanine films. J. Mater. Sci. 2010, 45, 1366–1370. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Solimana, H.S.; Khalifab, B.A.; Soliman, I.M. Structural and optical properties of nanocrystalline aluminum phthalocyanine chloride thin films. Mater. Sci. Semicon. Proc. 2015, 38, 177–183. [Google Scholar] [CrossRef]
- Melby, L.R.; Hartzler, H.D.; Sheppard, W.A. Improved Synthesis of tetrathiafulvalene. J. Org. Chem. 1974, 39, 2456–2458. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Al-Ghamdi, A.A.; Asiri, A.M. Optical properties of thermally evaporated tin-phthalocyanine dichloride thin films SnPcC12. Phys. B Condens. Matter 2004, 334, 398–406. [Google Scholar] [CrossRef]
- Robinet, S.; Clarisse, C.; Gauneau, M.; Salvi, M.; Delamar, M.; Leclerc, M.; Lacharme, J.P. Spectroscopic and Structural Studies of Scandium Diphthalocyanine Films. Thin Solid Films 1989, 182, 307–318. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Farag, A.M.; Abd-El-Rahman, K.F.; Darwish, A.A.A. Dispersion studies and electronic transitions in nickel phthalocyanine thin films. Opt. Laser Technol. 2005, 37, 513–523. [Google Scholar] [CrossRef]
- Laidani, N.; Bartali, R.; Gottardi, G.; Anderle, M.; Cheyssac, P. Optical absorption parameters of amorphous carbon films from Forouhi-Bloomer and Tauc-Lorentz models a comparative study. J. Phys. Condens. Matter 2007, 20, 015226. [Google Scholar] [CrossRef]
- Wang, C.; Bryce, M.R.; Batsanov, A.S.; Stanley, C.F.; Beeby, A.; Howard, J.A.K. Synthesis, spectroscopy and electrochemistry of phthalocyanine derivatives functionalized with four and eight peripheral tetrathiafulvalene units. J. Chem. Soc. Perkin Trans. 2 1997, 9, 1671–1678. [Google Scholar] [CrossRef]
- Blower, M.A.; Bryce, M.R.; Devonport, W. Synthesis and aggregation of a Phthalocyanine symmetrically-functionalized with eight tetrathiafulvalene units. Adv. Mater. 1996, 8, 63–65. [Google Scholar] [CrossRef]
- Rajesh, K.R.; Menon, C.S. D.C. Electrical and optical properties of vacuum-deposited organic semiconductor FePcCl thin films. Can. J. Phys. 2005, 83, 1151–1159. [Google Scholar] [CrossRef]
- Neghabi, M.; Zadsar, M.; Ghorashi, S.M.B. Investigation of structural and optoelectronic properties of annealed nickel phthalocyanine thin films. Mater. Sci. Semicond. Process. 2014, 17, 13–20. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Costas, A.; Breazu, C.; Stanculescu, A.; Rasoga, O.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Hybrid organic-inorganic thin films based on zinc phthalocyanine and zinc oxide deposited by MAPLE. Appl. Surf. Sci. 2020, 503, 144317. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Lei, Y.; Zhu, F.; Wu, B.; Zhang, T.; Niu, G.; Xiong, Z.; Song, Q. Photocurrent generation through electron–exciton interaction at the organic semiconductor donor/acceptor interface. Phys. Chem. Chem. Phys. 2013, 15, 16891–16897. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vergara, M.E.; Carrera-Téllez, R.; Smith-Ruiz, P.; Rios, C.; Salcedo, R. The Effect of the Indium (III) Phthalocyanine Chloride Films on the Behavior of Flexible Devices of Flat and Disperse Heterojunction. Coatings 2019, 9, 673. [Google Scholar] [CrossRef]
- Islam, Z.U.; Thahir, M.; Syed, W.A.; Aziz, F.; Wahab, F.; Said, S.M.; Sarker, M.R.; Ali, S.H.M.; Sabri, M.F.M. Fabrication and Photovoltaic Properties of Organic Solar Cell Based on Zinc Phthalocyanine. Energies 2020, 13, 962. [Google Scholar] [CrossRef]
- Varghese, A.C.; Menon, C.S. Influence of iodine on the electrical properties of magnesium phthalocyanines thin film devices. Centr. Eur. J. Phys. 2006, 4, 20–29. [Google Scholar] [CrossRef]
- Abdel-Malik, T.G.; Abdel-Latif, R.M. Transport properties in oxygen-doped cobalt phthalocyanine thin film devices. Thin Solid Films 1996, 286, 277–281. [Google Scholar] [CrossRef]
- Rani, V.; Sharma, A.; Kumar, P.; Singh, B.; Ghosh, S. Charge transport mechanism in copper phthalocyanine thin film with and witout traps. RSC Adv. 2017, 7, 54911–54919. [Google Scholar] [CrossRef]
- Kayunkid, N.; Rangkasikorn, A.; Saributr, C.; Nukeaw, J. Growth and characterizations of tin-doped zinc-phthalocyanine prepared by thermal coevaporation in high vacuum as a novel nanomaterial. Jpn. J. Appl. Phys. 2016, 55, BB1–BB12. [Google Scholar] [CrossRef]
- Noda, B.; Wada, H.; Shibata, K.; Yoshino, T.; Katsuhara, M.; Aoyagi, I.; Mori, T.; Taguchi, T.; Kambayashi, T.; Ishikawa, K.; et al. Crystal structures and transistor properties of phenyl-substituted tetrathiafulvalene derivatives. Nanotechnology 2007, 18, 424009. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Shafai, T.S. Oxygen induced p-doping of α-nickel phthalocyanine vacuum sublimed films: Implication for its use in organic photovoltaics. Appl. Phys. Lett. 2003, 82, 1628. [Google Scholar] [CrossRef]
- Choi, S.A.; Kim, K.; Lee, S.J.; Lee, H.; Babajanyan, A.; Friedman, B.; Lee, K. Effects of thermal preparation on Copper Phthalocyanine organic light emitting diodes. J. Lumin. 2016, 171, 149–153. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd El-Rahman, K.F. Investigation of electrical conductivity in Schottky-barrier devices based on nickel phthalocyanine thin films. J. Alloys Compd. 2007, 430, 194–199. [Google Scholar] [CrossRef]
- Cheung, S.K.; Cheung, N.W. Extraction of Schottky diode parameters from forward current-voltage characteristics. Appl. Phys. Lett. 1986, 49, 85–87. [Google Scholar] [CrossRef]







| Parameter | Units | Darkness |
|---|---|---|
| SLOPE OHMIC | - | 1.09 |
| SLOPE T-CLC | - | 1.69 |
| SLOPE SCLC | - | 0.8 |
| Mobility | cm2/V s | 3.58 × 10−6 |
| Trapping Factor | - | 3.48 |
| Photocurrent density @ 0 V | A/cm2 | 1.17 × 10−5 |
| Trap concentration | cm−3 | 7.74 × 1016 |
| VTFL | V | 5.39 |
| Jsc | A/cm2 | 1.64 × 10−5 |
| Ideality Factor (n) | - | 0.97 |
| Barrier Height (φb) | eV | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamui, L.; Sánchez-Vergara, M.E. Innovative Implementation of an Alternative Tetrathiafulvene Derivative for Flexible Indium Phthalocyanine Chloride-Based Solar Cells. Micromachines 2021, 12, 633. https://doi.org/10.3390/mi12060633
Hamui L, Sánchez-Vergara ME. Innovative Implementation of an Alternative Tetrathiafulvene Derivative for Flexible Indium Phthalocyanine Chloride-Based Solar Cells. Micromachines. 2021; 12(6):633. https://doi.org/10.3390/mi12060633
Chicago/Turabian StyleHamui, Leon, and María Elena Sánchez-Vergara. 2021. "Innovative Implementation of an Alternative Tetrathiafulvene Derivative for Flexible Indium Phthalocyanine Chloride-Based Solar Cells" Micromachines 12, no. 6: 633. https://doi.org/10.3390/mi12060633
APA StyleHamui, L., & Sánchez-Vergara, M. E. (2021). Innovative Implementation of an Alternative Tetrathiafulvene Derivative for Flexible Indium Phthalocyanine Chloride-Based Solar Cells. Micromachines, 12(6), 633. https://doi.org/10.3390/mi12060633