A Novel Preparation of Ag Agglomerates Paste with Unique Sintering Behavior at Low Temperature
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods and Characterizations
3. Results and Discussion
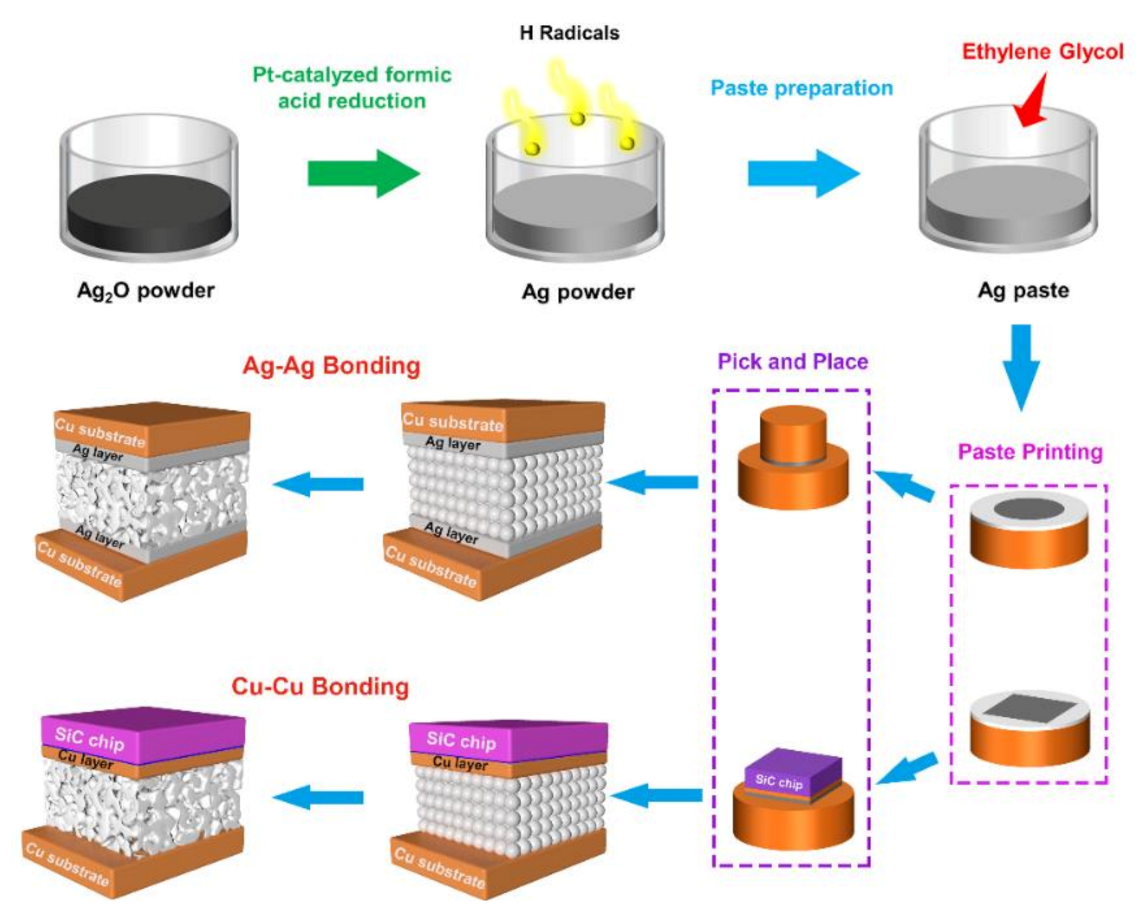
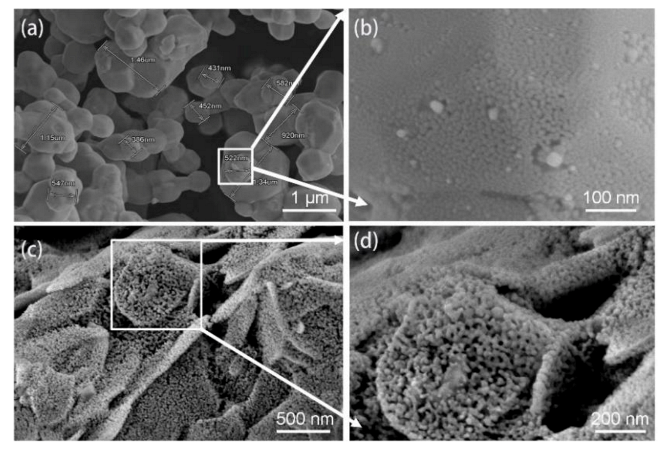
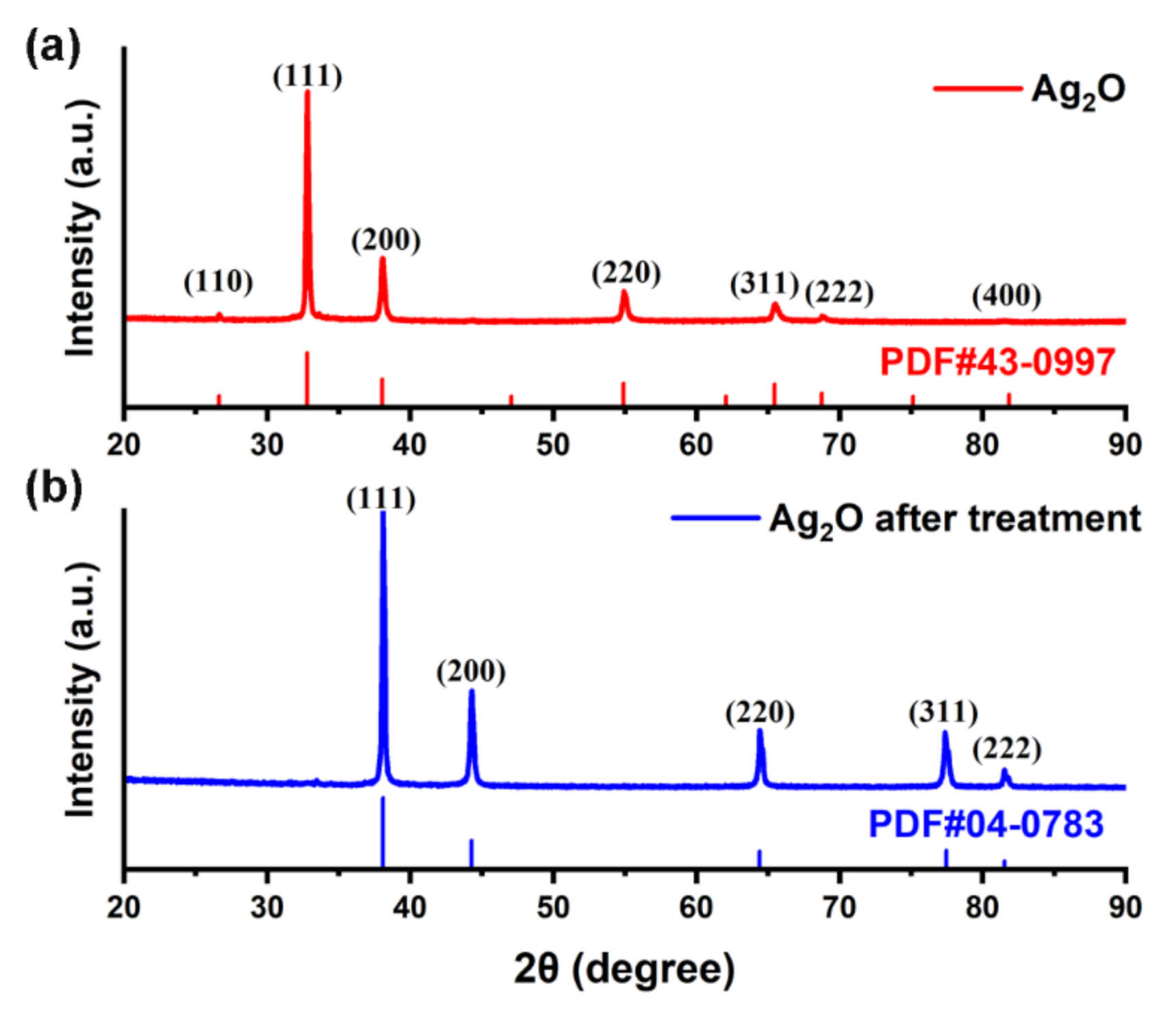
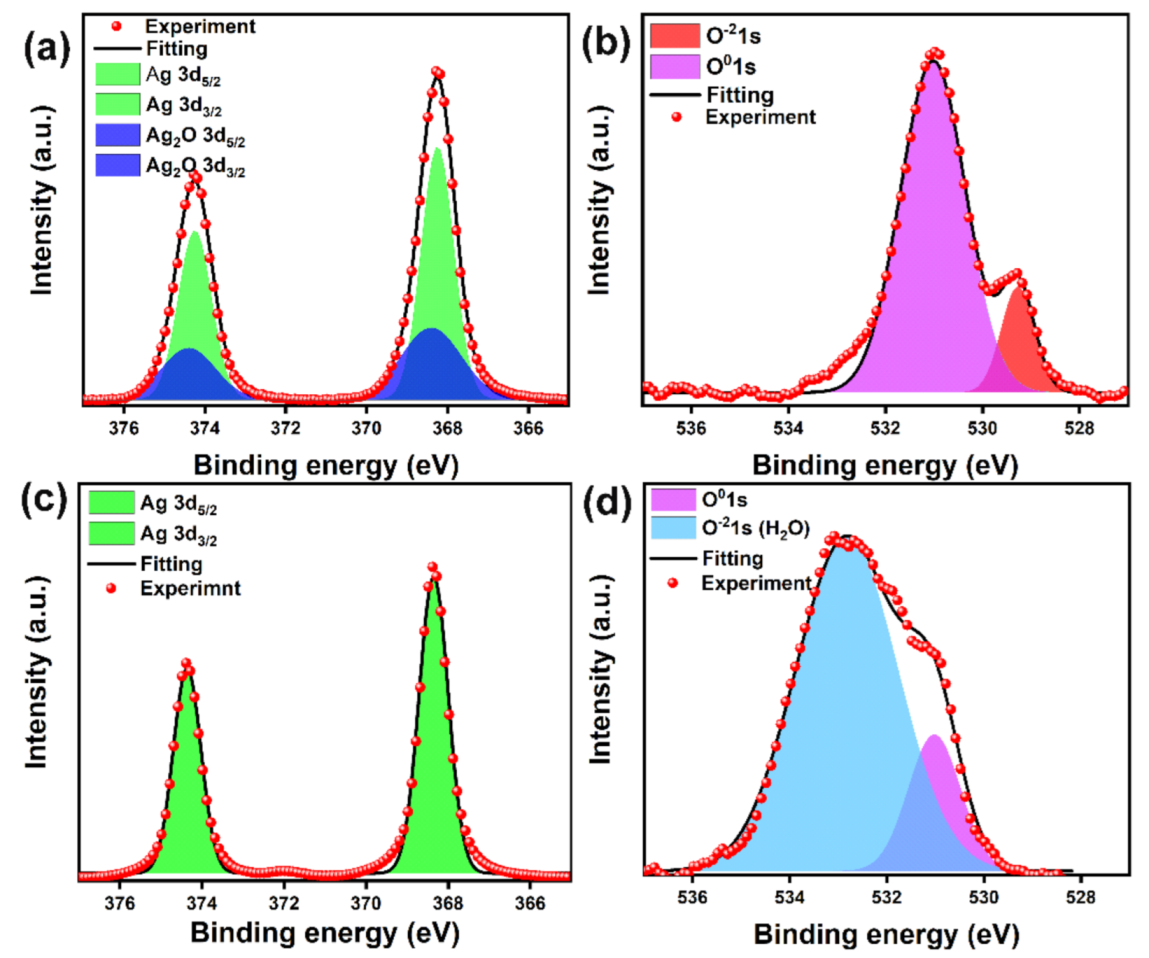
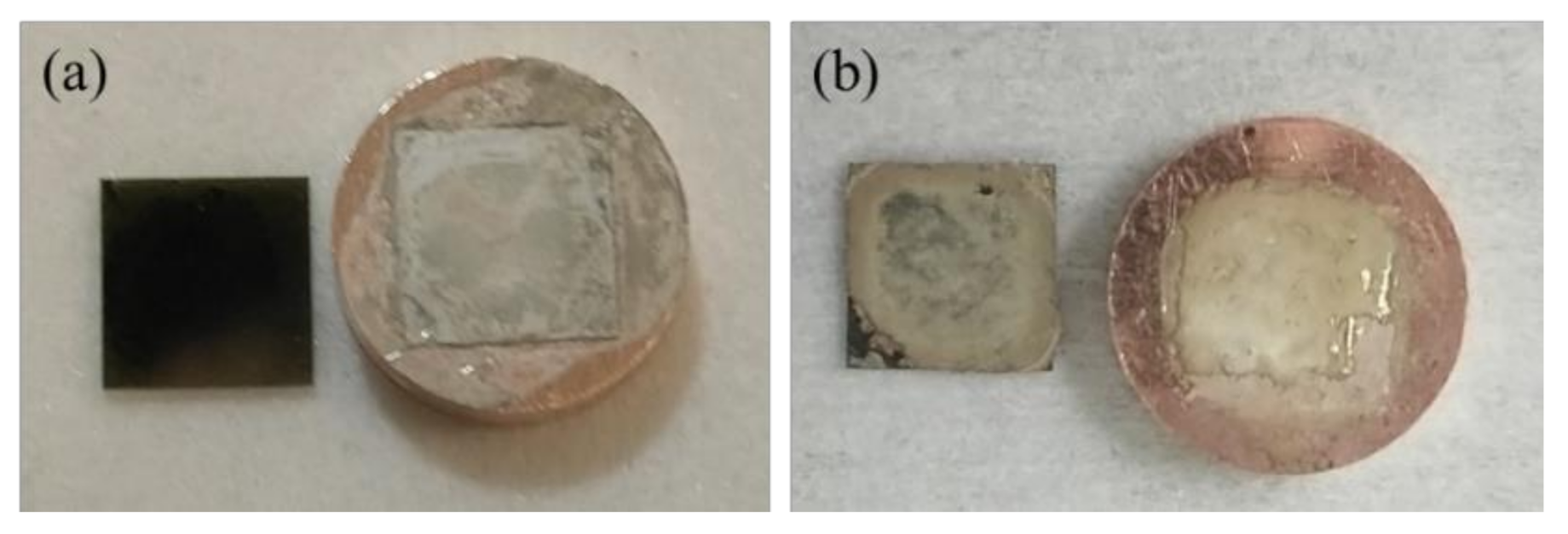
3.1. Characteristics of Ag Paste
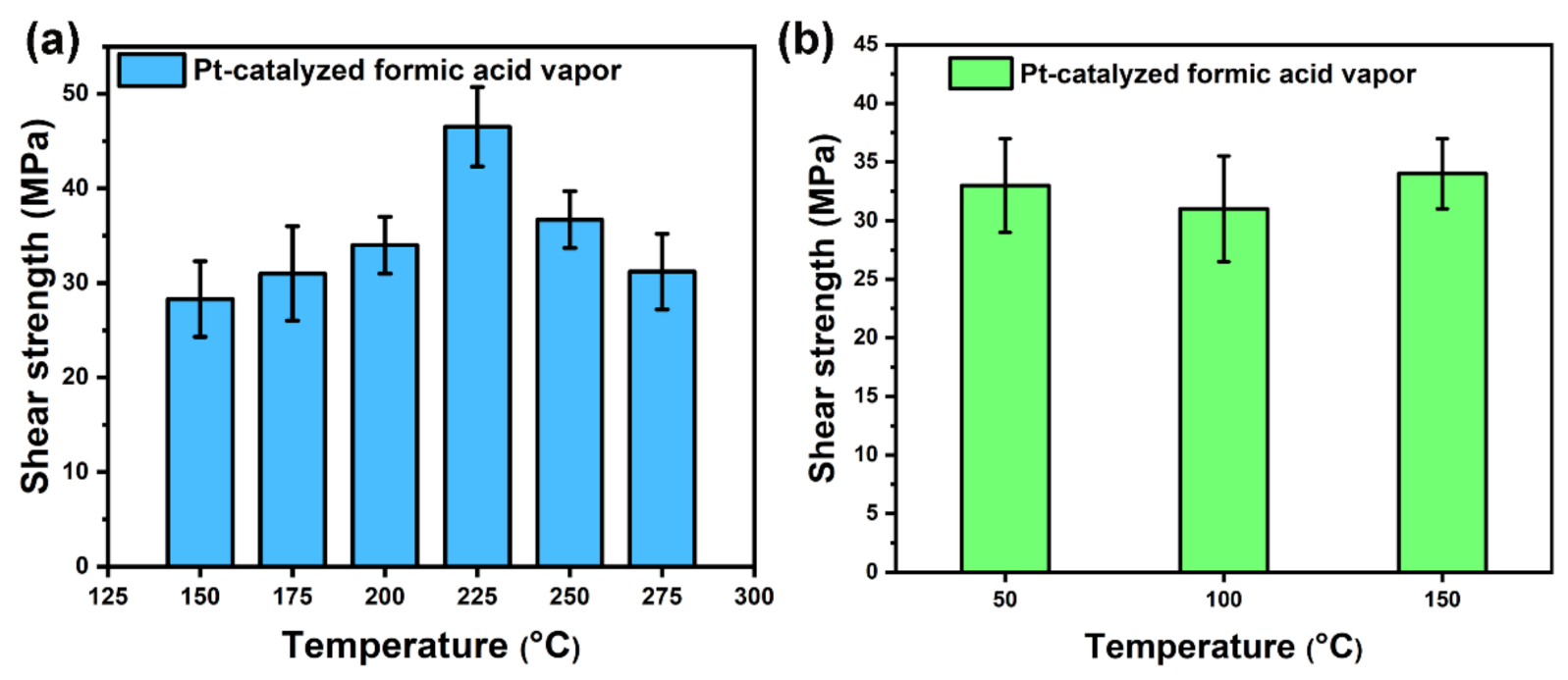
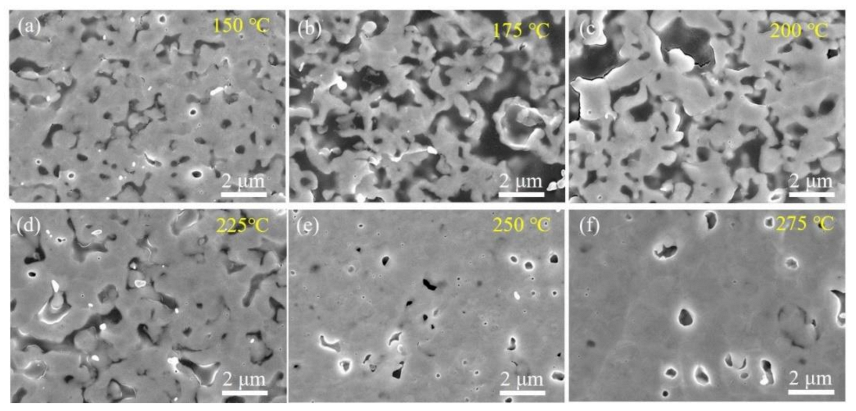
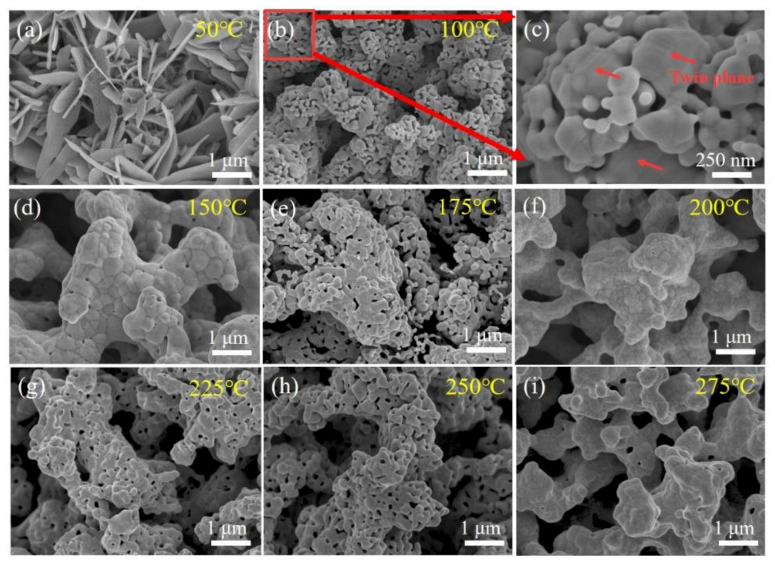
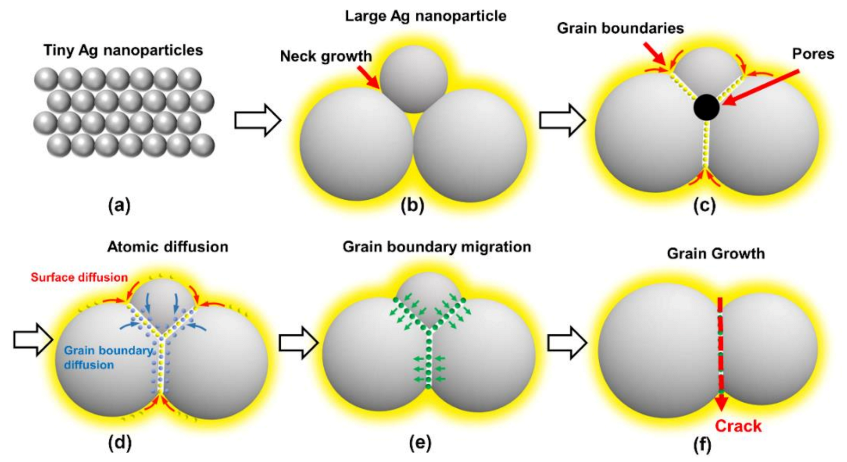
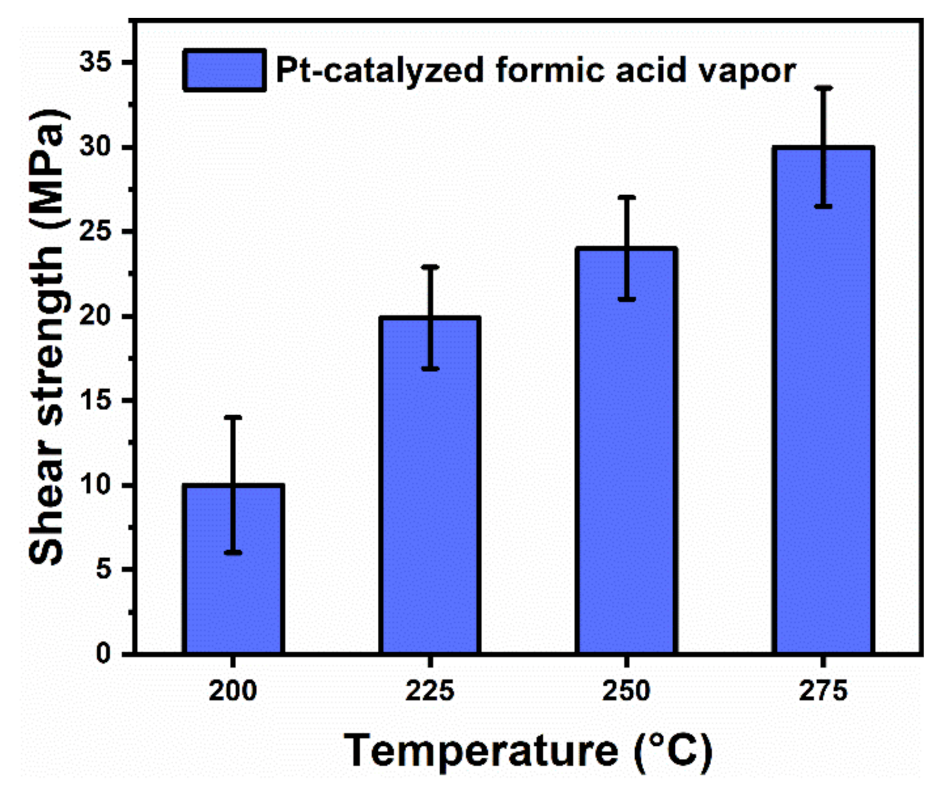
3.2. Sintering Processes and Mechanism for Ag–Ag Bonding
4. Sintering Processes and Mechanism for Cu–Cu Bonding
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chuang, R.W.; Lee, C.C. Silver-indium joints produced at low temperature for high temperature devices. Trans. Compon. Packag. Technol. 2002, 25, 453–458. [Google Scholar] [CrossRef]
- She, X.; Huang, A.Q.; Lucia, O.; Ozpineci, B. Review of Silicon Carbide Power Devices and Their Applications. IEEE Trans. Ind. Electron. 2017, 64, 8193–8205. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.S.; Kim, S.S. Suganuma, K. Interfacial Reaction and Die Attach Properties of Zn-Sn High-Temperature Solders. J. Electron. Mater. 2009, 38, 266–272. [Google Scholar] [CrossRef]
- Yoon, J.W.; Noh, B.I.; Jung, S.B. Interfacial reaction between Au-Sn solder and Au/Ni-metallized Kovar. J. Mater. Sci. 2011, 22, 84–90. [Google Scholar] [CrossRef]
- Lau, F.L.; Made, R.I.; Putra, W.N.; Lim, J.Z.; Nachiappan, V.C.; Aw, J.L.; Gan, C.L. Electrical behavior of Au-Ge eutectic solder under aging for solder bump application in high temperature Electronics. Microelectron. Reliab. 2013, 53, 1581–1586. [Google Scholar] [CrossRef]
- Wakuda, D.; Hatamura, M.; Suganuma, K. Novel method for room temperature sintering of Ag nanoparticle paste in air. Chem. Phys. Lett. 2007, 441, 305–308. [Google Scholar] [CrossRef]
- Yasuda, Y.; Ide, E.; Morita, T. Low-Temperature Bonding Using Silver Nanoparticles Stabilized by Short-Chain Alkylamines. Jpn. J. Appl. Phys. 2009, 48, 125004. [Google Scholar] [CrossRef]
- Morisada, Y.; Nagaoka, T.; Fukusumi, M.; Kashiwagi, Y.; Yamamoto, M.; Nakamoto, M. A Low-Temperature Bonding Process Using Mixed Cu-Ag Nanoparticles. J. Electron. Mater. 2010, 39, 1283–1288. [Google Scholar] [CrossRef]
- Nishikawa, H.; Hirano, T.; Takemoto, T.; Terada, N. Effects of Joining Conditions on Joint Strength of Cu/Cu Joint Using Cu Nanoparticle Paste. Open Surf. Sci. J. 2010, 3, 60–64. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, H. Densification and grain growth during sintering of nanosized particles. J. Metall. Rev. 2008, 53, 326–352. [Google Scholar] [CrossRef]
- Yu, F.; Cui, J.; Zhou, Z.; Fang, K.; Johnson, R.; Hamilton, M. Reliability of Ag Sintering for Power Semiconductor Die Attach in High Temperature Applications. IEEE Trans. Power Electron. 2016, 32, 7083–7095. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, H.; Jia, Q.; Guo, W.; Zou, G.; Liu, L. Stabilizing the sintered nanopore bondline by residual organics for high temperature electronics. Microelectron. Reliab. 2020, 111, 113727. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zou, G.; Wang, W.; Lei, L.; Zhang, G.; Zhou, Y. Failure analysis and reliability evaluation of silver-sintered die attachment for high-temperature applications. Microelectron. Reliab. 2019, 94, 46–55. [Google Scholar] [CrossRef]
- Yan, J.; Zou, G.; Wu, A.; Ren, J.; Yan, J.; Hu, A.; Lei, L.; Zhou, Y.N. Effect of PVP on the low temperature bonding process using polyol prepared Ag nanoparticle paste for electronic packaging application. J. Phys. Conf. Ser. 2012, 379, 012024. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, L.; Mei, Y.; Lu, G. A novel multiscale silver paste for die bonding on bare copper by low-temperature pressure-free sintering in air. Mater. Des. 2017, 140, 64–72. [Google Scholar] [CrossRef]
- Morita, T.; Yasuda, Y.; Ide, E.; Akada, Y.; Hirose, A. Bonding Technique Using Micro-Scaled Silver-Oxide Particles for In-Situ Formation of Silver Nanoparticles. Mater. Trans. 2008, 49, 2875–2880. [Google Scholar] [CrossRef]
- Hirose, A.; Takeda, N.; Konaka, Y.; Tatsumi, H.; Akada, Y.; Ogura, T.; Ide, E.; Morita, T. Low Temperature Sintering Bonding Process Using Ag Nanoparticles Derived from Ag2O for Packaging of High-Temperature Electronics. Mater. Sci. Forum 2012, 706, 2962–2967. [Google Scholar] [CrossRef]
- Morita, T.; Yasuda, Y.; Ide, E.; Hirose, A. Direct Bonding to Aluminum with Silver-Oxide Microparticles. Mater. Trans. 2009, 50, 226–228. [Google Scholar] [CrossRef]
- Hirose, A.; Tatsumi, H.; Takeda, N.; Akada, Y.; Morita, T. A novel metal-to-metal bonding process through in-situ formation of Ag nanoparticles using Ag2O microparticles. J. Phys. Conf. Ser. 2009, 165, 012074. [Google Scholar] [CrossRef]
- Takata, S.; Ogura, T.; Ide, E.; Morita, T.; Hirose, A. Effects of Solvents in the Polyethylene Glycol Series on the Bonding of Copper Joints Using Ag2O Paste. J. Electron. Mater. 2013, 42, 507–515. [Google Scholar] [CrossRef]
- Mu, F.; Zhao, Z.; Zou, G.; Bai, H.; Wu, A.; Liu, L.; Zhang, D.; Norman, Z. Mechanism of Low Temperature Sintering-Bonding through In-Situ Formation of Silver Nanoparticles Using Silver Oxide Microparticles. Mater. Trans. 2013, 54, 872–878. [Google Scholar] [CrossRef]
- Ogura, T.; Takata, S.; Takahashi, M.; Hirose, A. Effects of Reducing Solvent on Copper, Nickel, and Aluminum Joining Using Silver Nanoparticles Derived from a Silver Oxide Paste. J. Mater. Trans. 2015, 56, 1030–1036. [Google Scholar] [CrossRef]
- He, L.; Li, J.; Wu, X.; Mu, F.; Wang, Y.; Lu, Y.; Suga, T. Robust Ag-Cu Sintering Bonding at 160 °C via Combining Ag2O Microparticle Paste and Pt-Catalyzed Formic Acid Vapor. Metals 2020, 10, 315. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, Z.; Wang, C.; Ding, S.; Wen, J.; Liu, Z.Q.; Wang, C. Sintering mechanism of the Cu-Ag core-shell nanoparticle paste at low temperature in ambient air. RSC Adv. 2016, 6, 91783–91790. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Ji, H.; Wang, C. Rapid pressureless low-temperature sintering of Ag nanoparticles for high-power density electronic packaging. Scr. Mater. 2013, 69, 789–792. [Google Scholar] [CrossRef]
- Du, C.; Li, X.; Mei, Y.; Lu, G. Bonding performance of sintered nanosilver joints on bare copper substrates with different grain structures. J. Mater. Sci. Mater. Electron. 2019, 30, 12860–12868. [Google Scholar] [CrossRef]
- Wang, X.; Mei, Y.; Li, X.; Wang, M.; Cui, Z.; Lu, G. Pressureless sintering of nanosilver paste as die attachment on substrates with ENIG finish for semiconductor applications. J. Alloys Compd. 2018, 777, 578–585. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, Y.; Meng, Y.; Yin, Z.; Zhao, X.; Wang, Y.; Suga, T. A Novel Preparation of Ag Agglomerates Paste with Unique Sintering Behavior at Low Temperature. Micromachines 2021, 12, 521. https://doi.org/10.3390/mi12050521
Li J, Xu Y, Meng Y, Yin Z, Zhao X, Wang Y, Suga T. A Novel Preparation of Ag Agglomerates Paste with Unique Sintering Behavior at Low Temperature. Micromachines. 2021; 12(5):521. https://doi.org/10.3390/mi12050521
Chicago/Turabian StyleLi, Junlong, Yang Xu, Ying Meng, Zhen Yin, Xuelong Zhao, Yinghui Wang, and Tadatomo Suga. 2021. "A Novel Preparation of Ag Agglomerates Paste with Unique Sintering Behavior at Low Temperature" Micromachines 12, no. 5: 521. https://doi.org/10.3390/mi12050521
APA StyleLi, J., Xu, Y., Meng, Y., Yin, Z., Zhao, X., Wang, Y., & Suga, T. (2021). A Novel Preparation of Ag Agglomerates Paste with Unique Sintering Behavior at Low Temperature. Micromachines, 12(5), 521. https://doi.org/10.3390/mi12050521





