Microsphere-Based Microfluidic Device for Plasma Separation and Potential Biochemistry Analysis Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
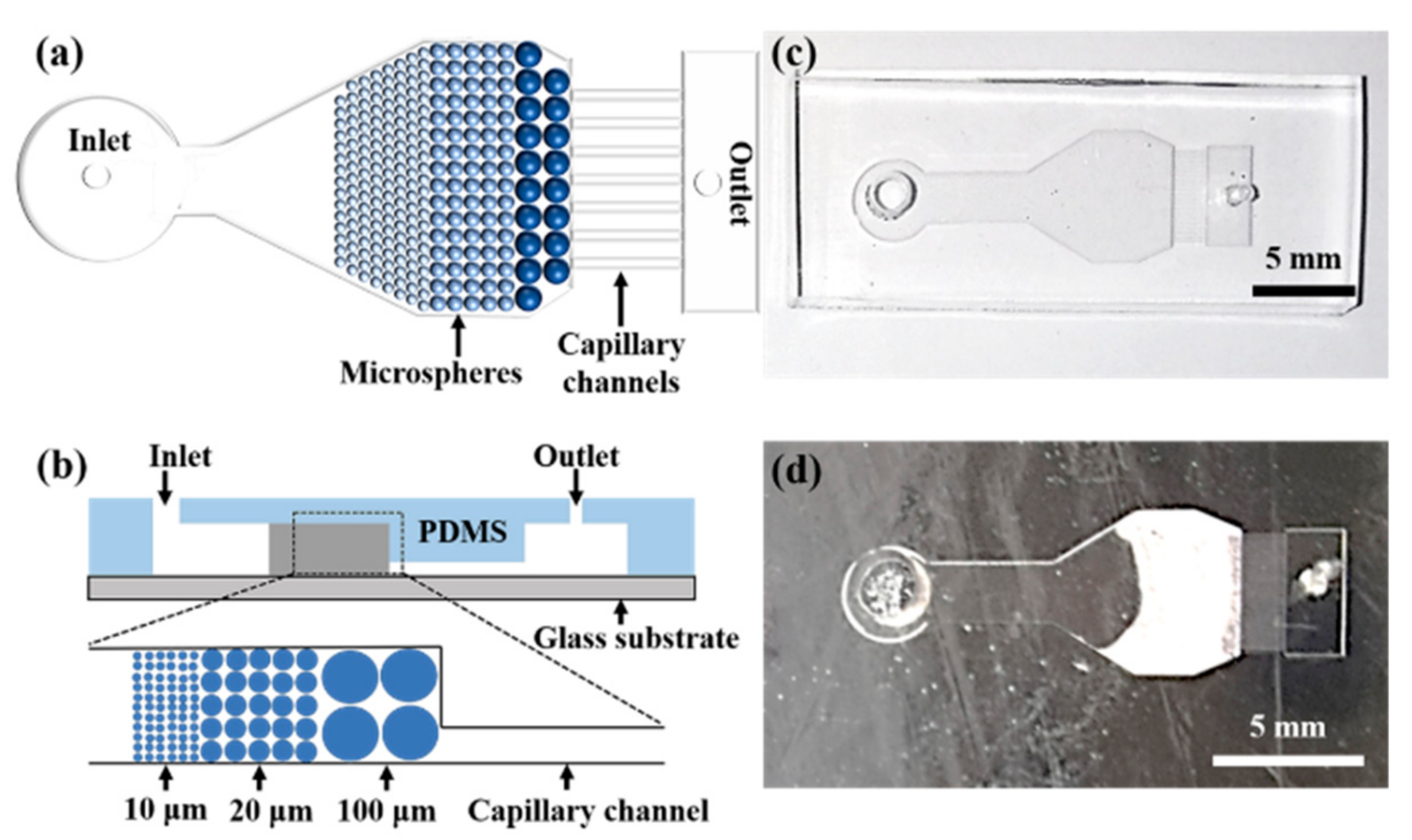
2.2. Microchip Design and Fabrication
2.3. Beads Stacking
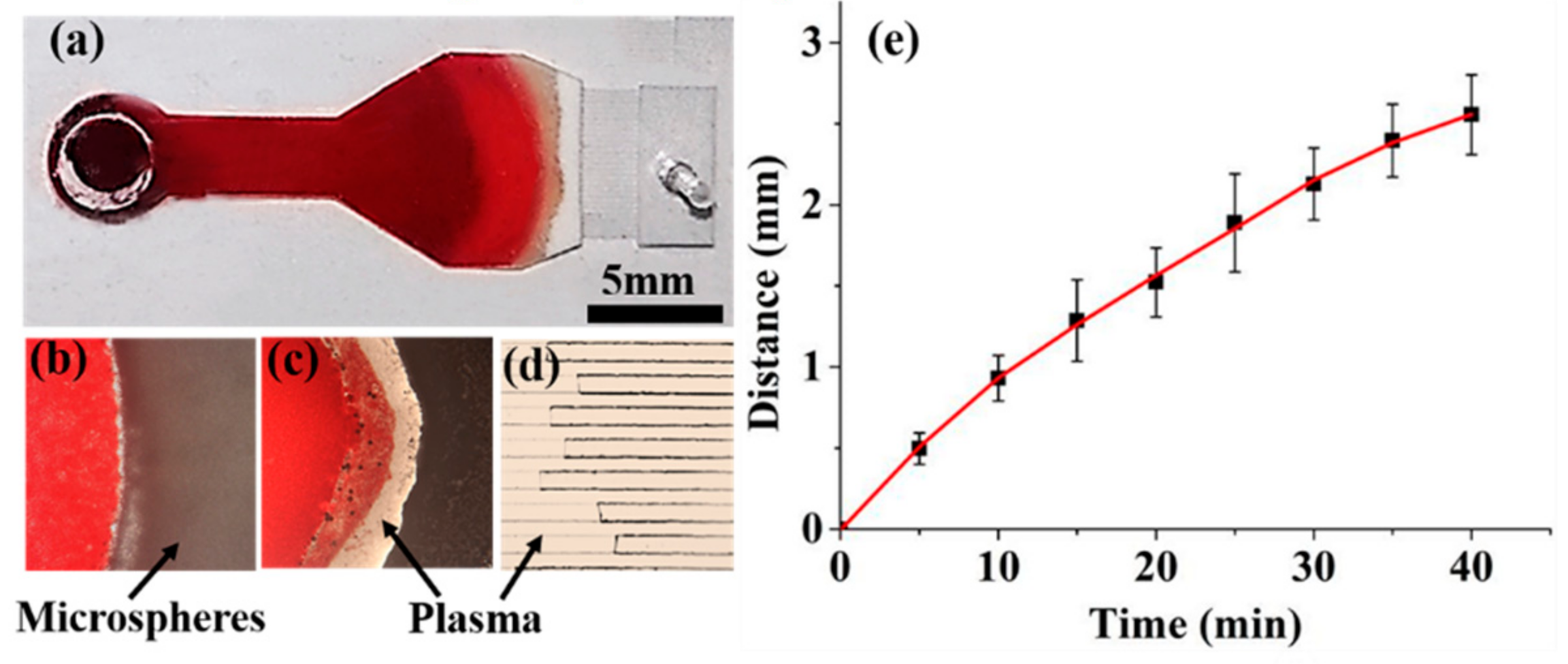
2.4. Plasma Separation
2.5. Plasma Analysis
3. Results and Discussion
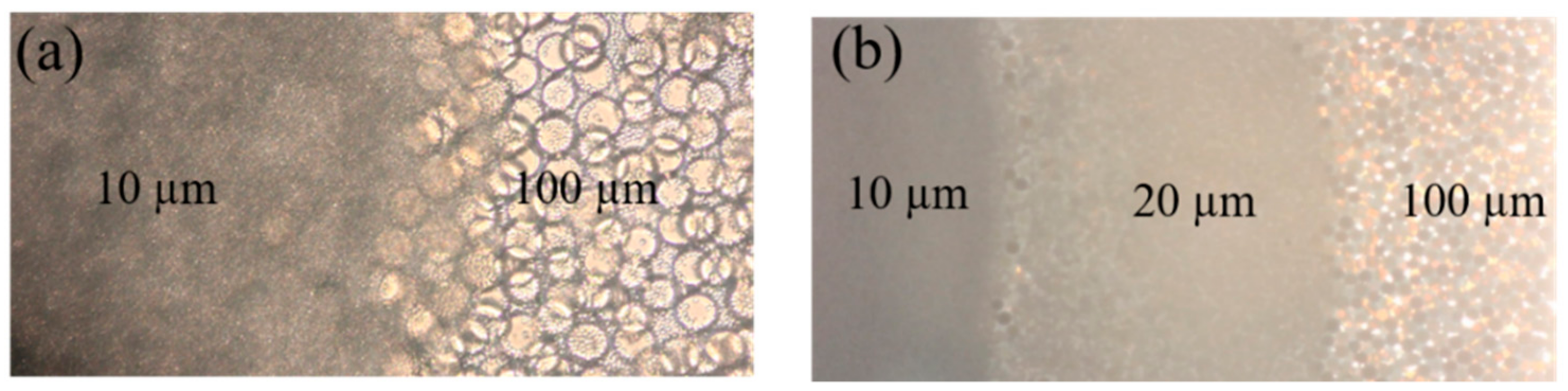
3.1. Microspheres Stacking
3.2. Plasma Separation
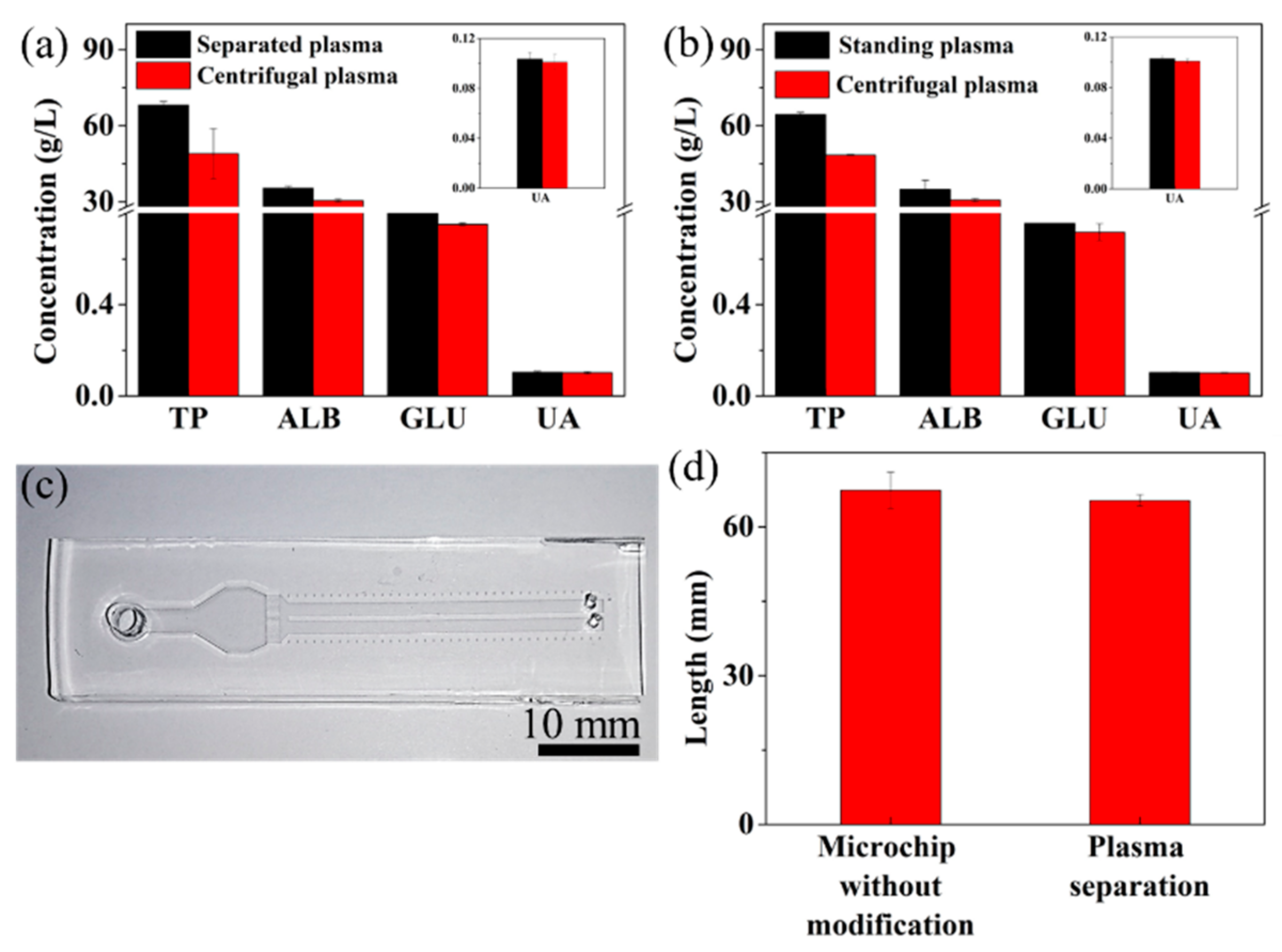
3.3. Plasma Analysis
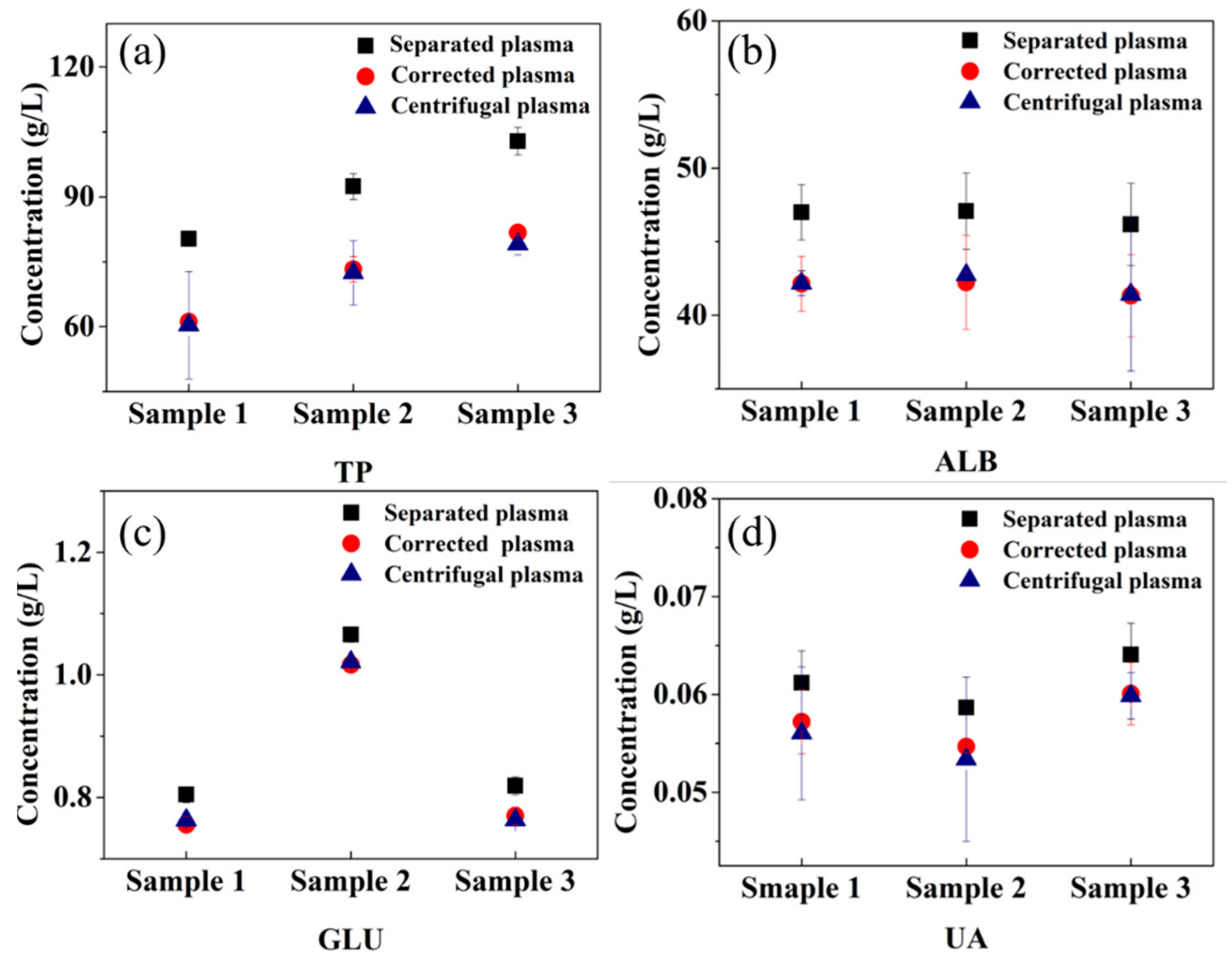
3.4. Clinical Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef]
- Yang, X.; Forouzan, O.; Brown, T.P.; Shevkoplyas, S.S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 2012, 12, 274–280. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. [Google Scholar] [CrossRef]
- Ammerlaan, W.; Trezzi, J.P.; Lescuyer, P.; Mathay, C.; Hiller, K.; Betsou, F. Method validation for preparing serum and plasma samples from human blood for downstream proteomic, metabolomic, and circulating nucleic acid-based applications. Biopreserv. Biobank 2014, 12, 269–280. [Google Scholar] [CrossRef]
- Jung, W.E.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Varun Kumar, Y.V.B.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromechanics Microengineering 2015, 25. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Pauletti, G.M.; Hegener, M.A.; Steckl, A.J. Correcting the effect of hematocrit in whole blood coagulation analysis on paper-based lateral flow device. Anal. Methods 2018, 10, 2869–2874. [Google Scholar] [CrossRef]
- Sista, R.S.; Ng, R.; Nuffer, M.; Basmajian, M.; Coyne, J.; Elderbroom, J.; Hull, D.; Kay, K.; Krishnamurthy, M.; Roberts, C.; et al. Digital Microfluidic Platform to Maximize Diagnostic Tests with Low Sample Volumes from Newborns and Pediatric Patients. Diagnostics 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, D.F.; Liu, C.C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Crowley, T.A.; Pizziconi, V. Isolation of plasma from whole blood using planar microfilters for lab-on-a-chip applications. Lab Chip 2005, 5, 922–929. [Google Scholar] [CrossRef]
- Han, J.Y.; DeVoe, D.L. Plasma isolation in a syringe by conformal integration of inertial microfluidics. Ann. Biomed. Eng. 2021, 49, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Marks, H.; Hinsdale, T.; Maitland, K.; Cote, G. Rapid isolation of blood plasma using a cascaded inertial microfluidic device. Biomicrofluidics 2017, 11. [Google Scholar] [CrossRef]
- Rafeie, M.; Zhang, J.; Asadnia, M.; Li, W.; Warkiani, M.E. Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 2016, 16, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.-H.; Chiang, P.-H.; Lin, Z.-T.; Yang, H.-C.; Song, H.-L.; Li, B.-R. Rapid purification of lung cancer cells in pleural effusion through spiral microfluidic channels for diagnosis improvement. Lab Chip 2020, 20, 4007–4015. [Google Scholar] [CrossRef]
- Yang, S.; Undar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef]
- Maria, M.S.; Kumar, B.S.; Chandra, T.S.; Sen, A.K. Development of a microfluidic device for cell concentration and blood cell-plasma separation. Biomed. Microdevices 2015, 17. [Google Scholar] [CrossRef]
- Shim, J.S.; Browne, A.W.; Ahn, C.H. An on-chip whole blood/plasma separator with bead-packed microchannel on COC polymer. Biomed. Microdevices 2010, 12, 949–957. [Google Scholar] [CrossRef]
- Shim, J.S.; Ahn, C.H. An on-chip whole blood/plasma separator using hetero-packed beads at the inlet of a microchannel. Lab Chip 2012, 12, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, C.; Xu, Z.; Li, J. A power-free deposited microbead plug-based microfluidc chip for whole-blood immunoassay. Microfluid. Nanofluidics 2012, 12, 829–834. [Google Scholar] [CrossRef]
- Chen, M.-D.; Yang, Y.-T.; Deng, Z.-Y.; Xu, H.-Y.; Deng, J.-N.; Yang, Z.; Yang, J. Microchannel with stacked microbeads for separation of plasma from whole blood. Chin. J. Anal. Chem. 2019, 47, 661–668. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Synthetic Paper Separates Plasma from Whole Blood with Low Protein Loss. Anal. Chem. 2020, 92, 6194–6199. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.; Lamanna, J.; Wheeler, A.R. Direct loading of blood for plasma separation and diagnostic assays on a digital microfluidic device. Lab Chip 2020, 20, 1845–1855. [Google Scholar] [CrossRef]
- Dimov, I.K.; Basabe-Desmonts, L.; Garcia-Cordero, J.L.; Ross, B.M.; Park, Y.; Ricco, A.J.; Lee, L.P. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 2011, 11, 845–850. [Google Scholar] [CrossRef]
- Kim, J.H.; Woenker, T.; Adamec, J.; Regnier, F.E. Simple, miniaturized blood plasma extraction method. Anal. Chem. 2013, 85, 11501–11508. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef]
- Kumar, R.; Nandhini, L.P.; Kamalanathan, S.; Sahoo, J.; Vivekanadan, M. Evidence for current diagnostic criteria of diabetes mellitus. World J. Diabetes 2016, 7, 396–405. [Google Scholar] [CrossRef]
- Association Diabetes American. Updates to the standards of medical care in diabetes-2018. Diabetes Care 2018, 41, 2045–2047. [Google Scholar] [CrossRef]
| Types of Components in Plasma | Correction Factor (g/L) | Standard Deviation |
|---|---|---|
| TP | 19.133 | 8.58 |
| ALB | 4.854 | 0.199 |
| GLU | 0.049 | 0.004 |
| UA | 0.004 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wu, Z.; Deng, J.; Qiu, J.; Hu, N.; Gao, L.; Yang, J. Microsphere-Based Microfluidic Device for Plasma Separation and Potential Biochemistry Analysis Applications. Micromachines 2021, 12, 487. https://doi.org/10.3390/mi12050487
Xu H, Wu Z, Deng J, Qiu J, Hu N, Gao L, Yang J. Microsphere-Based Microfluidic Device for Plasma Separation and Potential Biochemistry Analysis Applications. Micromachines. 2021; 12(5):487. https://doi.org/10.3390/mi12050487
Chicago/Turabian StyleXu, Hongyan, Zhangying Wu, Jinan Deng, Jun Qiu, Ning Hu, Lihong Gao, and Jun Yang. 2021. "Microsphere-Based Microfluidic Device for Plasma Separation and Potential Biochemistry Analysis Applications" Micromachines 12, no. 5: 487. https://doi.org/10.3390/mi12050487
APA StyleXu, H., Wu, Z., Deng, J., Qiu, J., Hu, N., Gao, L., & Yang, J. (2021). Microsphere-Based Microfluidic Device for Plasma Separation and Potential Biochemistry Analysis Applications. Micromachines, 12(5), 487. https://doi.org/10.3390/mi12050487







