Public-Health-Driven Microfluidic Technologies: From Separation to Detection
Abstract
1. Introduction
2. Microfluidic Separation Methods
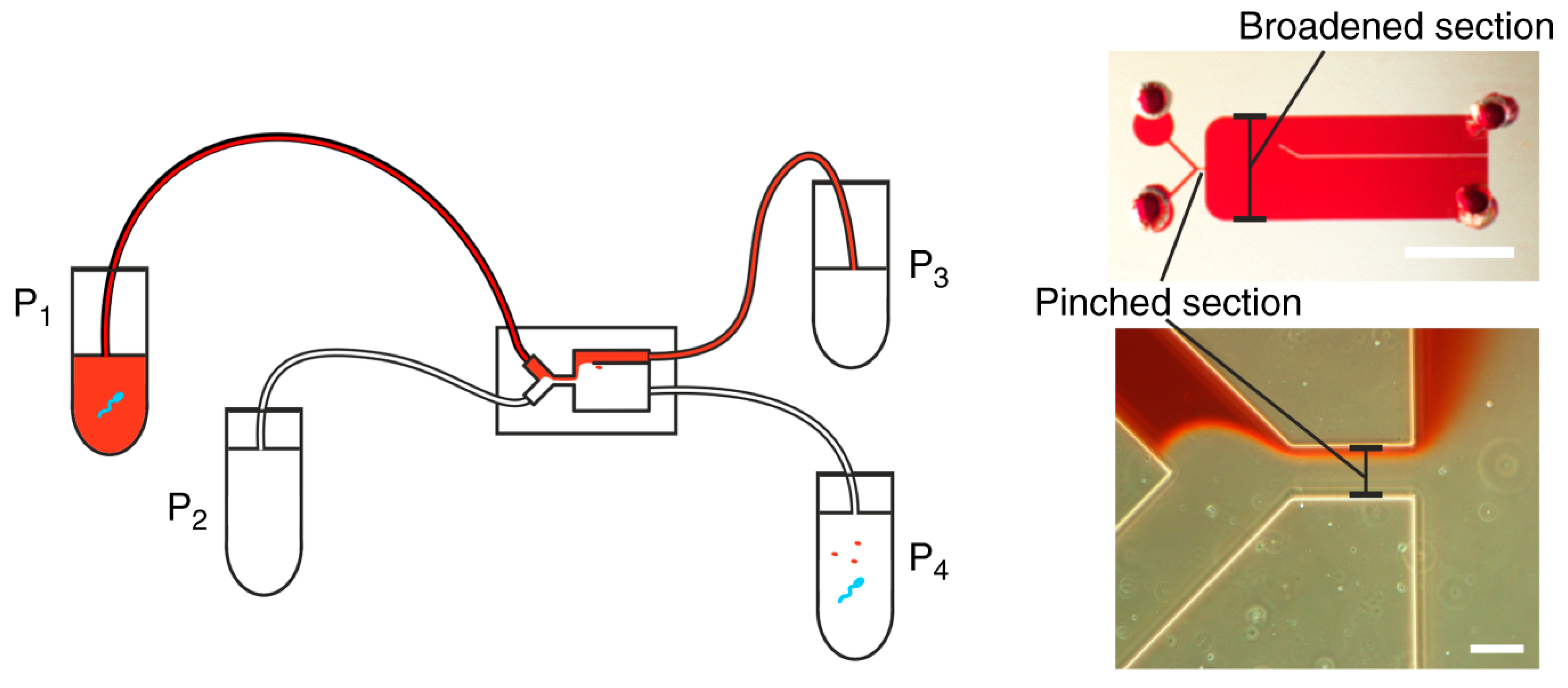
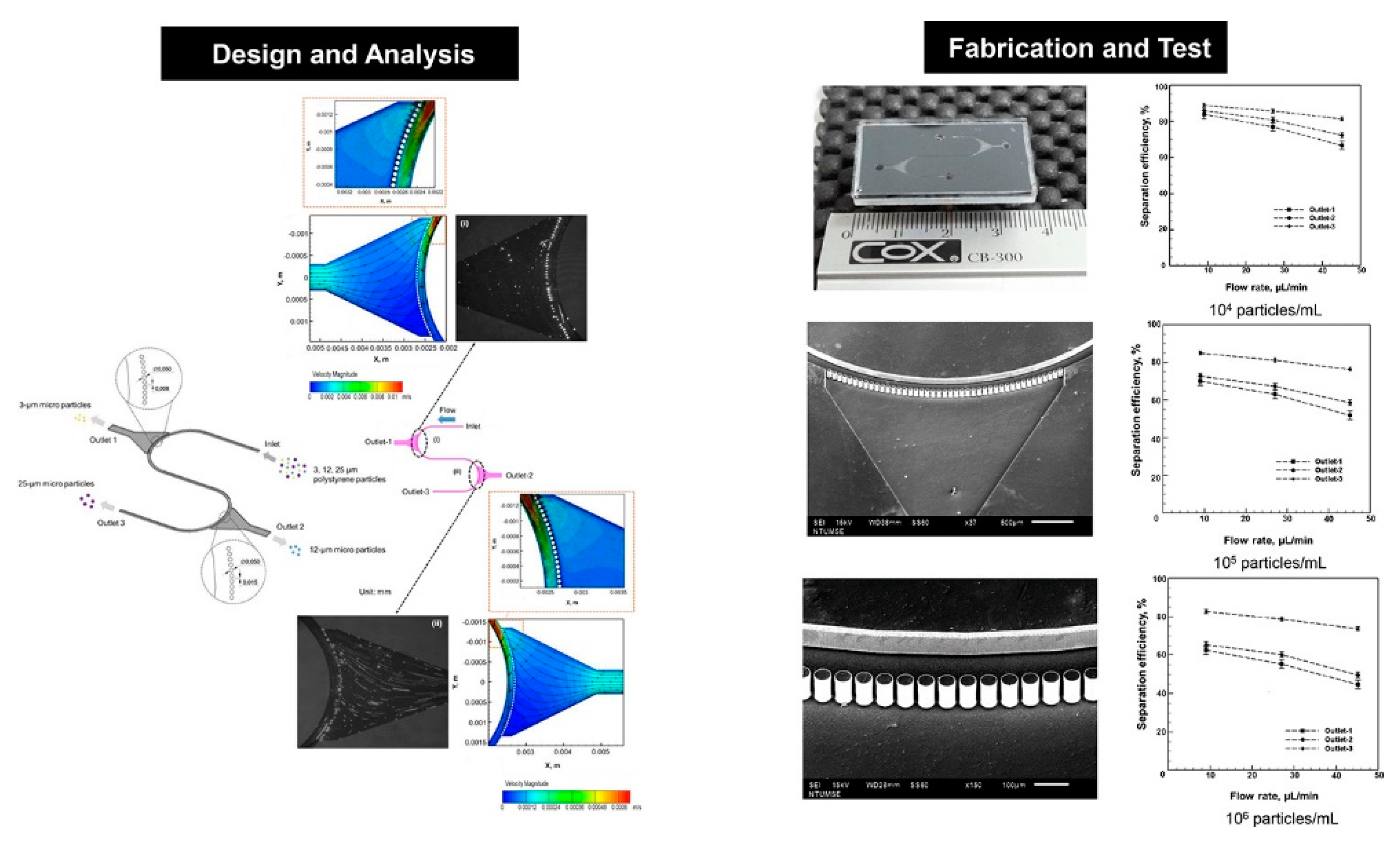
2.1. Pinched-Flow Fractionation (PFF)
2.2. Inertia and Dean Flow
2.3. Deterministic Lateral Displacement (DLD)
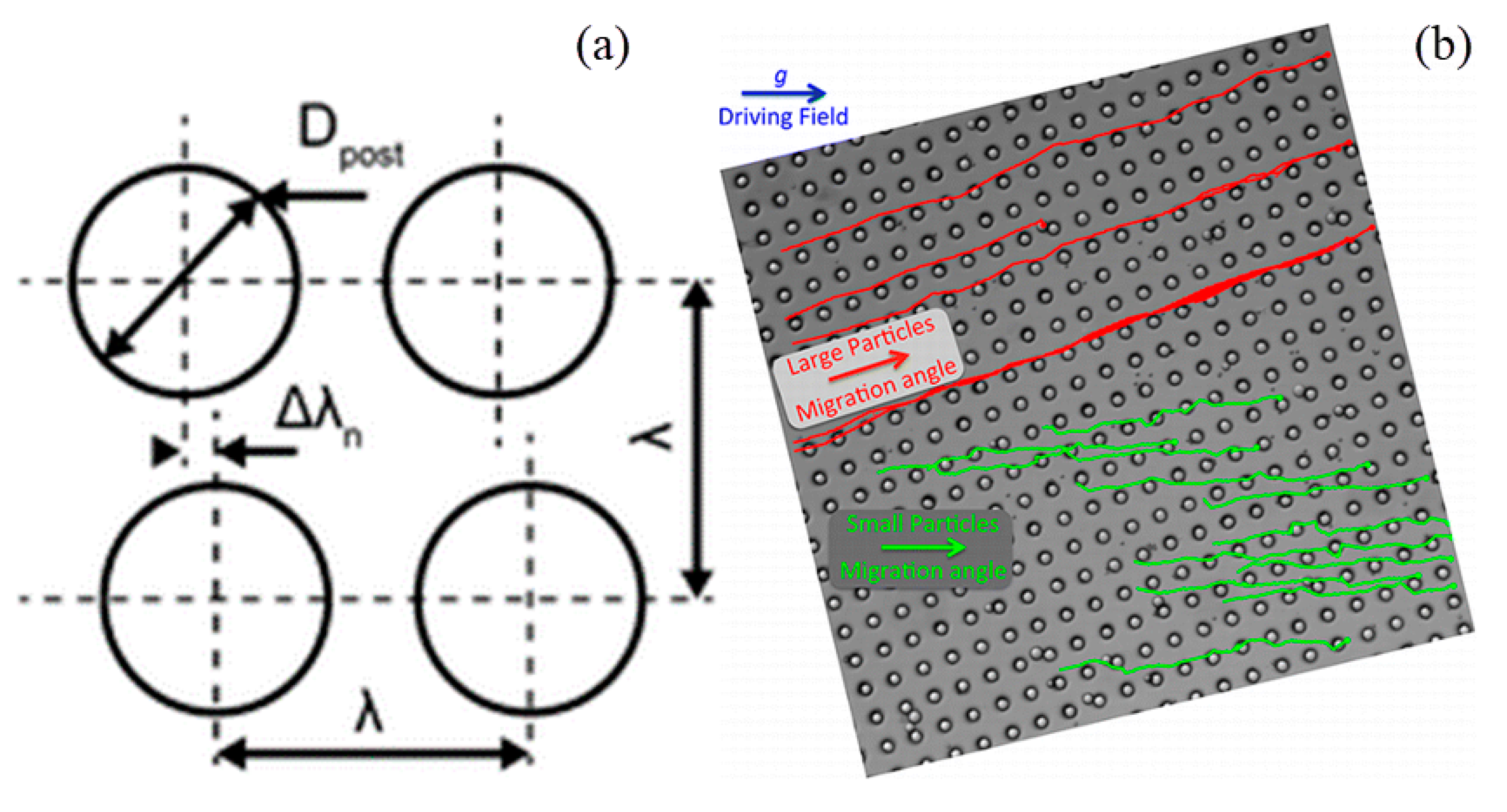
2.4. Microscale Filters
2.5. Other Hydrodynamic Methods
3. Microfluidic Detection Methods
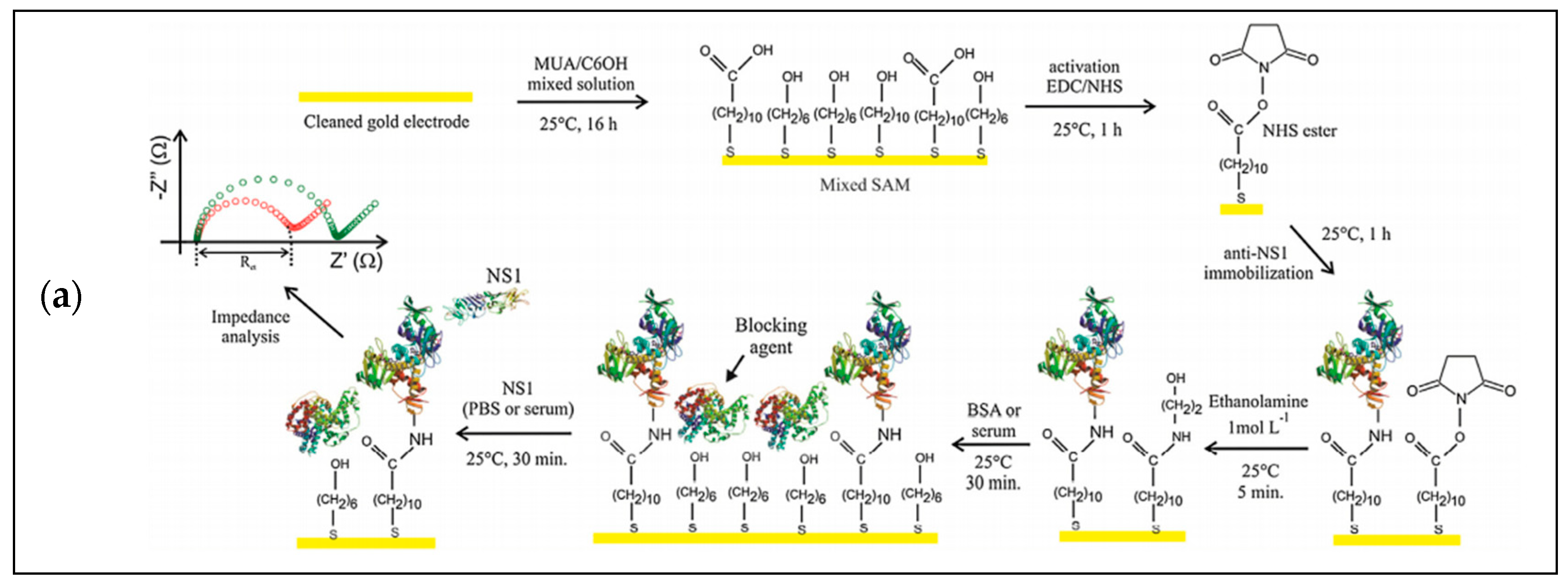
3.1. Electrochemical Detection
3.2. Optical Detection
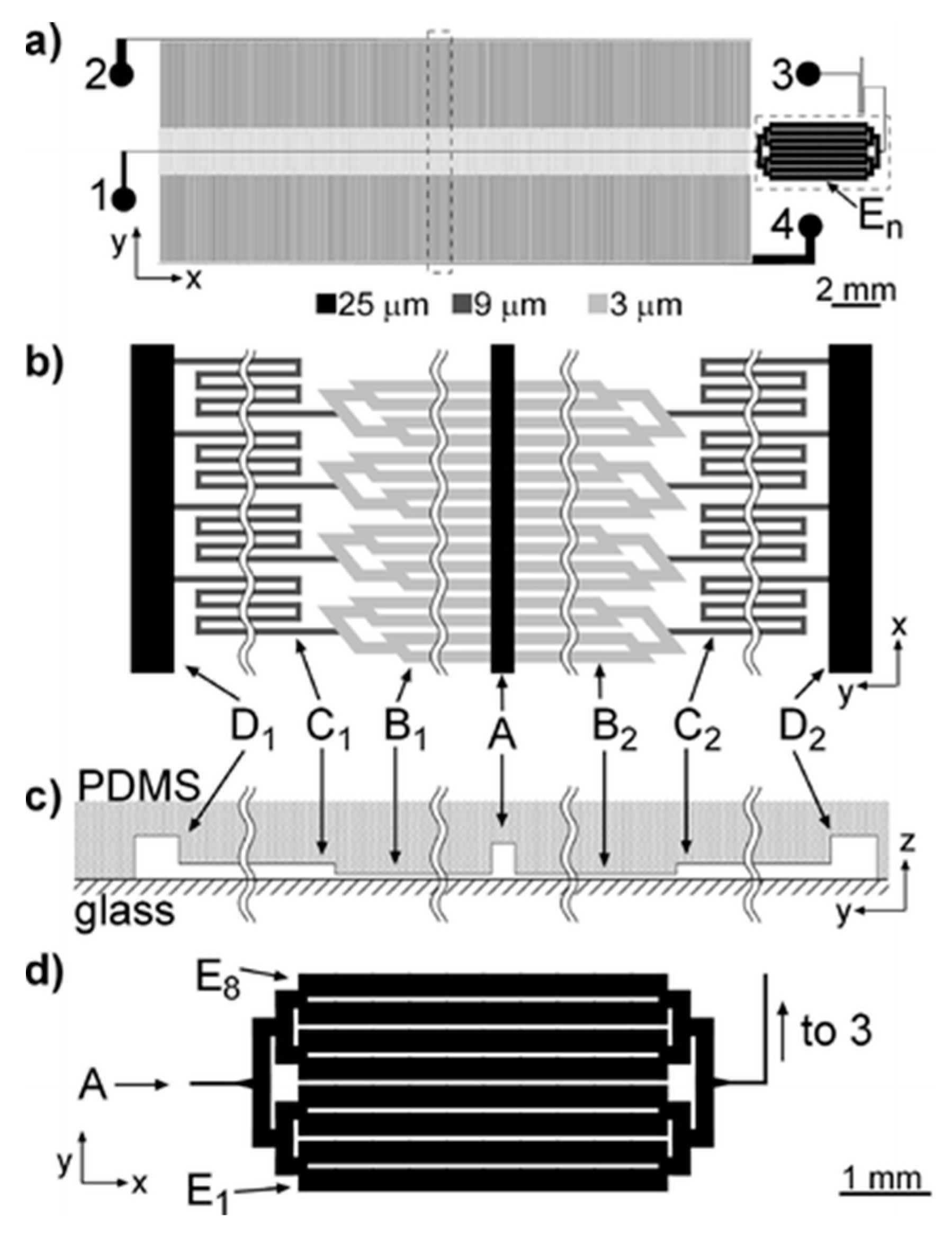
3.3. Magnetic Detection
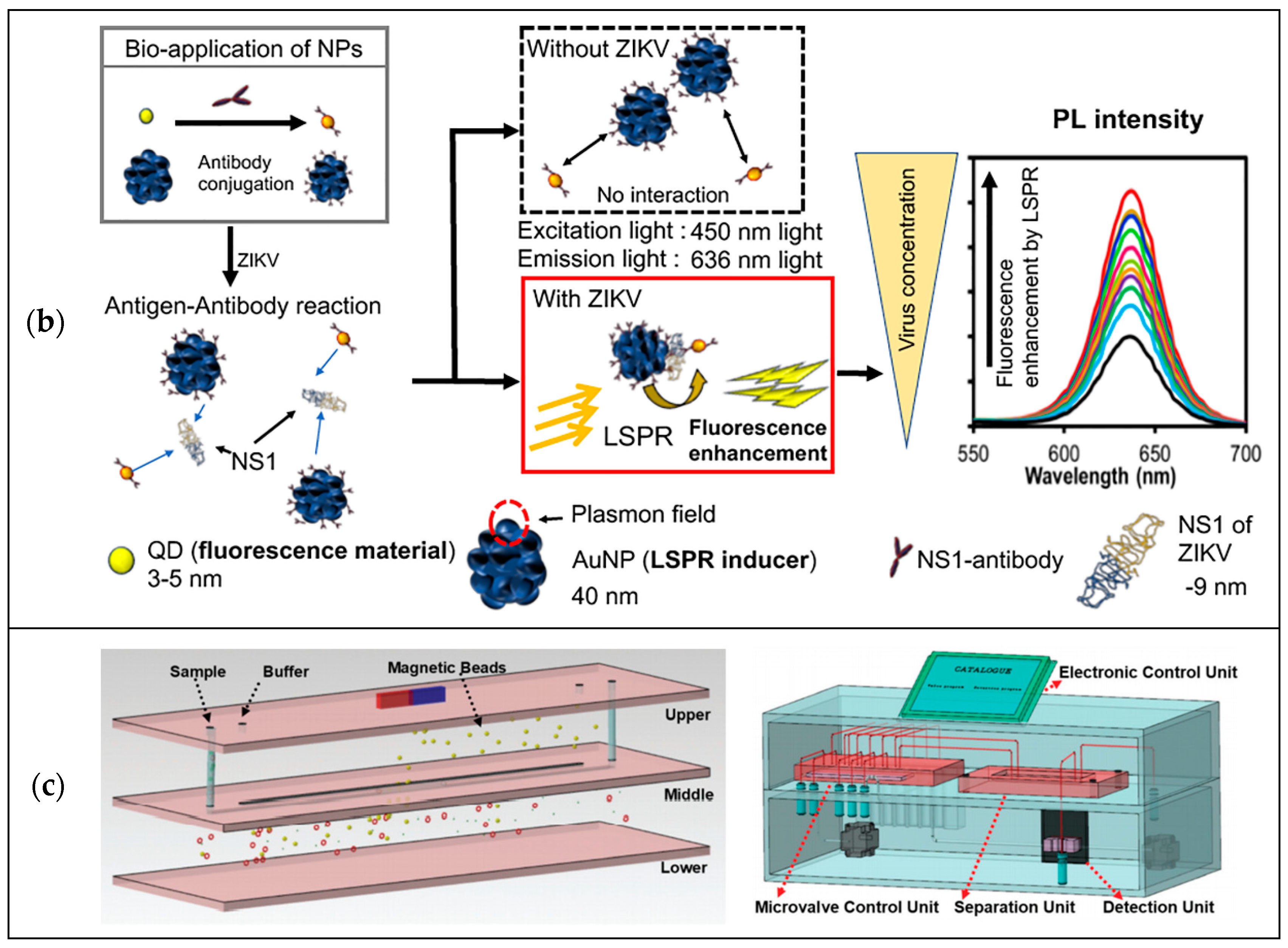
4. Prospects of Microfluidics for Public Health Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowie, B.C.; Dore, G.J. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012, 367, 89. [Google Scholar]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; De Roode, J.C.; Fenton, A. Why infectious disease research needs community ecology. Science 2015, 349, 1259504. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Fauci, A.S. The 1918 influenza pandemic: Insights for the 21st century. J. Infect. Dis. 2007, 195, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.X.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Geneva. 2021. Available online: https://www.who.int/ (accessed on 28 February 2021).
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.E.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef]
- Libertucci, J.; Young, V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019, 4, 35–45. [Google Scholar] [CrossRef]
- You, M.; Li, Z.; Feng, S.; Gao, B.; Yao, C.; Hu, J.; Xu, F. Ultrafast photonic PCR based on photothermal nanomaterials. Trends Biotechnol. 2020, 38, 637–649. [Google Scholar] [CrossRef]
- Yang, B.; Kong, J.; Fang, X. Bandage-like wearable flexible microfluidic recombinase polymerase amplification sensor for the rapid visual detection of nucleic acids. Talanta 2019, 204, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shang, C.; Ma, H.; You, M. An upconversion nanoparticle-based photostable FRET system for long-chain DNA sequence detection. Nanotechnology 2020, 31, 235501. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Sun, H.H.; Yin, B.C.; Ye, B.C. An RNA-guided Cas9 nickase-based method for universal isothermal DNA amplification. Angew. Chem. Int. Ed. 2019, 58, 5382–5386. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, H.; Liu, F.; Lv, Z.H.; Wu, K.L.; Liu, Y.L.; Feng, Y.; Zhu, C.L. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 2020, 505, 172–175. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vazquez, J.; Courtney, S.; Matias, K.Y.; Andersen, L.E.; Colon, C.; Butler, A.E.; Roulo, R.; Bowzard, J.; Villanueva, J.M.; et al. Performance of the trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat. Commun. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Wu, A.G.; Chen, X.Y. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 2017, 46, 2038–2056. [Google Scholar] [CrossRef]
- Lam, H.M.; Remais, J.; Fung, M.C. Food supply and food safety issues in China. Lancet 2013, 381, 2044–2053. [Google Scholar] [CrossRef]
- Kaptan, G.; Fischer, A.R.H.; Frewer, L.J. Extrapolating understanding of food risk perceptions to emerging food safety cases. J. Risk Res. 2018, 21, 996–1018. [Google Scholar] [CrossRef]
- Chiocchetti, G.D.M.E.; Piedra, C.A.J.; Monedero, V.; Cabrera, M.Z.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 15341545. [Google Scholar] [CrossRef]
- Wu, W.; Yu, C.; Wang, Q.; Zhao, F.; He, H.; Liu, C.; Yang, Q. Research advances of DNA aptasensors for foodborne pathogen detection. Crit. Rev. Food Sci. Nutr. 2019, 60, 1636763. [Google Scholar] [CrossRef]
- Cristina, L.; Elena, A.; Davide, C.; Marzia, G.; Lucia, D.; Cristiano, G.; Marco, A.; Carlo, R.; Laura, C.; Gabriella, G.M. Validation of a mass spectrometry-based method for milk traces detection in baked food. Food Chem. 2016, 199, 119–127. [Google Scholar] [CrossRef]
- Wang, Y.; Duncan, T.V. Nanoscale sensors for assuring the safety of food products. Curr. Opin. Biotechnol. 2017, 44, 74–86. [Google Scholar] [CrossRef]
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Water Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Ohira, S.-I.; Toda, K. Micro gas analysis system for measurement of atmospheric hydrogen sulfide and sulfur dioxide. Lab Chip 2005, 5, 1374–1379. [Google Scholar] [CrossRef]
- Montes, R.J.; Ladd, A.J.C.; Butler, J.E. Transverse migration and microfluidic concentration of DNA using Newtonian buffers. Biomicrofluidics 2019, 13, 044104. [Google Scholar] [CrossRef]
- Tweedie, M.; Sun, D.; Ward, B.; Maguire, P.D. Long-term hydrolytically stable bond formation for future membrane-based deep ocean microfluidic chemical sensors. Lab Chip 2019, 19, 1287–1295. [Google Scholar] [CrossRef]
- An, X.; Zuo, P.; Ye, B.C. A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta 2020, 209, 120571. [Google Scholar] [CrossRef] [PubMed]
- Citartan, M.; Tang, T.H. Recent developments of aptasensors expedient for point-of-care (POC) diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.M.; McCracken, K.E.; Yoon, J.Y. Future developments in biosensors for field-ready Zika virus diagnostics. J. Biol. Eng. 2017, 11, 7. [Google Scholar] [CrossRef]
- Tepeli, Y.; Ülkü, A. Electrochemical biosensors for influenza virus a detection: The potential of adaptation of these devices to POC systems. Sens. Actuators B Chem. 2018, 254, 377–384. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Nordwood, MA, USA, 2019; ISBN 1630813656. [Google Scholar]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R.; Umviligihozo, G. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluid. 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Yan, S.; Tan, S.H.; Li, Y.; Tang, S.; Teo, A.J.T.; Zhang, J.; Zhao, Q.; Yuan, D.; Sluyter, R.; Nguyen, N.-T. A portable, hand-powered microfluidic device for sorting of biological particles. Microfluid. Nanofluid. 2018, 22, 8. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Coluccio, M.L.; D’Attimo, M.A.; Cristiani, C.M.; Candeloro, P.; Parrotta, E.; Dattola, E.; Guzzi, F.; Cuda, G.; Lamanna, E.; Carbone, E. A passive microfluidic device for chemotaxis studies. Micromachines 2019, 10, 551. [Google Scholar] [CrossRef]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Miglierina, R.; Le Coniat, M.; Gendron, M.; Berger, R. Diagnosis of Fanconi’s anemia by flow cytometry. Nouv. Rev. Fr. Hematol. 1990, 32, 391–393. [Google Scholar] [PubMed]
- Cheng, X.; Irimia, D.; Dixon, M.; Sekine, K.; Demirci, U.; Zamir, L.; Tompkins, R.G.; Rodriguez, W.; Toner, M. A microfluidic device for practical label-free CD4+ T cell counting of HIV-infected subjects. Lab Chip 2007, 7, 170–178. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef]
- Situma, C.; Hashimoto, M.; Soper, S.A. Merging microfluidics with microarray-based bioassays. Biomol. Eng. 2006, 23, 213–231. [Google Scholar] [CrossRef]
- Dong, Y.; Skelley, A.M.; Merdek, K.D.; Sprott, K.M.; Jiang, C.; Pierceall, W.E.; Lin, J.; Stocum, M.; Carney, W.P.; Smirnov, D.A. Microfluidics and circulating tumor cells. J. Mol. Diagn. 2013, 15, 149–157. [Google Scholar] [CrossRef] [PubMed]
- VAziri, A.; GopinAth, A. Cell and biomolecular mechanics in silico. Nat. Mater. 2008, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, M.; Metrakos, N.; Juarez Perez, E.; Azer, F.; Yang, F.; Yang, X.; Wang, G. Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics 2013, 7, 11803. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2005, 1, 15–30. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef]
- Cranston, H.A.; Boylan, C.W.; Carroll, G.L.; Sutera, S.P.; Gluzman, I.Y.; Krogstad, D.J. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science 1984, 223, 400–403. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent advances and future perspectives on microfluidic liquid handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef]
- Link, D.R.; Grasland-Mongrain, E.; Duri, A.; Sarrazin, F.; Cheng, Z.; Cristobal, G.; Marquez, M.; Weitz, D.A. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. 2006, 45, 2556–2560. [Google Scholar] [CrossRef]
- Shukla, V.; Ali, N.B.Z.; Hussin, F.A.; Zwolinski, M. On testing of MEDA based digital microfluidics biochips. In Proceedings of the Fifth Asia Symposium on Quality Electronic Design (ASQED 2013), Penang, Malaysia, 26–28 August 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 60–65. [Google Scholar]
- Nguyen, N.-T.; Shaegh, S.A.M.; Kashaninejad, N.; Phan, D.-T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Chan, W.K.; Nguyen, N.-T. Fluid mechanics of flow through rectangular hydrophobic microchannels. In Proceedings of the International Conference on Nanochannels, Microchannels, and Minichannels, Edmonton, AB, Canada, 19–22 June 2011; ASME: New York, NY, USA, 2011; Volume 44632, pp. 647–655. [Google Scholar]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 2017, 38, 238–249. [Google Scholar] [CrossRef]
- Lenshof, A.; Laurell, T. Continuous separation of cells and particles in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef]
- Doddabasavana, G.; PadmaPriya, K.; Nagabhushana, K. A review of recent advances in separation and detection of whole blood components. World J. Sci. Technol. 2012, 2, 5–9. [Google Scholar]
- Bayareh, M. An updated review on particle separation in passive microfluidic devices. Chem. Eng. Process. Intensif. 2020, 153, 107984. [Google Scholar] [CrossRef]
- Oakey, J.; Allely, J.; Marr, D.W.M. Laminar-flow-based separations at the microscale. Biotechnol. Prog. 2002, 18, 1439–1442. [Google Scholar] [CrossRef]
- Jain, A.; Posner, J.D. Particle dispersion and separation resolution of pinched flow fractionation. Anal. Chem. 2008, 80, 1641–1648. [Google Scholar] [CrossRef]
- Ma, J.-T.; Xu, Y.-Q.; Tang, X.-Y. A numerical simulation of cell separation by simplified asymmetric pinched flow fractionation. Comput. Math. Methods Med. 2016, 2016, 2564584. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Ouchi, T.; Yamada, M.; Seki, M. Hydrodynamic microparticle separation mechanism using three-dimensional flow profiles in dual-depth and asymmetric lattice-shaped microchannel networks. Micromachines 2019, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, J.T.W.; Eijkel, J.C.T.; Wetzels, A.M.; Segerink, L.I. Separation of spermatozoa from erythrocytes using their tumbling mechanism in a pinch flow fractionation device. Microsyst. Nanoeng. 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maenaka, H.; Yamada, M.; Yasuda, M.; Seki, M. Continuous and size-dependent sorting of emulsion droplets using hydrodynamics in pinched microchannels. Langmuir 2008, 24, 4405–4410. [Google Scholar] [CrossRef]
- Morijiri, T.; Sunahiro, S.; Senaha, M.; Yamada, M.; Seki, M. Sedimentation pinched-flow fractionation for size-and density-based particle sorting in microchannels. Microfluid. Nanofluid. 2011, 11, 105–110. [Google Scholar] [CrossRef]
- Sai, Y.; Yamada, M.; Yasuda, M.; Seki, M. Continuous separation of particles using a microfluidic device equipped with flow rate control valves. J. Chromatogr. A 2006, 1127, 214–220. [Google Scholar] [CrossRef]
- Vig, A.L.; Kristensen, A. Separation enhancement in pinched flow fractionation. Appl. Phys. Lett. 2008, 93, 203507. [Google Scholar] [CrossRef]
- Stoecklein, D.; Wu, C.-Y.; Owsley, K.; Xie, Y.; Di Carlo, D.; Ganapathysubramanian, B. Micropillar sequence designs for fundamental inertial flow transformations. Lab Chip 2014, 14, 4197–4204. [Google Scholar] [CrossRef]
- Di Carlo, D.; Edd, J.F.; Humphry, K.J.; Stone, H.A.; Toner, M. Particle segregation and dynamics in confined flows. Phys. Rev. Lett. 2009, 102, 94503. [Google Scholar] [CrossRef]
- Park, J.-S.; Jung, H.-I. Multiorifice flow fractionation: Continuous size-based separation of microspheres using a series of contraction/expansion microchannels. Anal. Chem. 2009, 81, 8280–8288. [Google Scholar] [CrossRef]
- Schaaf, C.; Stark, H. Inertial migration and axial control of deformable capsules. Soft Matter 2017, 13, 3544–3555. [Google Scholar] [CrossRef] [PubMed]
- Bayareh, M.; Mortazavi, S. Numerical simulation of the motion of a single drop in a shear flow at finite Reynolds numbers. Iran. J. Sci. Technol. Trans. B Eng. 2009, 33, 441–452. [Google Scholar]
- Bayareh, M.; Mortazavi, S. Binary collision of drops in simple shear flow at finite Reynolds numbers: Geometry and viscosity ratio effects. Adv. Eng. Softw. 2011, 42, 604–611. [Google Scholar] [CrossRef]
- Mortazavi, S.; Bayareh, M. Geometry effects on the interaction of two equal-sized drops in simple shear flow at finite Reynolds numbers. WIT Trans. Eng. Sci. 2009, 63, 379–388. [Google Scholar]
- Liu, L.; Han, L.; Shi, X.; Tan, W.; Cao, W.; Zhu, G. Hydrodynamic separation by changing equilibrium positions in contraction–expansion array channels. Microfluid. Nanofluid. 2019, 23, 52. [Google Scholar] [CrossRef]
- Zeng, L.; Balachandar, S.; Fischer, P. Wall-induced forces on a rigid sphere at finite Reynolds number. J. Fluid Mech. 2005, 536, 1–25. [Google Scholar] [CrossRef]
- Kim, Y.W.; Yoo, J.Y. The lateral migration of neutrally-buoyant spheres transported through square microchannels. J. Micromech. Microeng. 2008, 18, 65015. [Google Scholar] [CrossRef]
- Shao, X.; Yu, Z.; Sun, B. Inertial migration of spherical particles in circular Poiseuille flow at moderately high Reynolds numbers. Phys. Fluids 2008, 20, 103307. [Google Scholar] [CrossRef]
- Doddi, S.K.; Bagchi, P. Lateral migration of a capsule in a plane Poiseuille flow in a channel. Int. J. Multiph. Flow 2008, 34, 966–986. [Google Scholar] [CrossRef]
- Abkarian, M.; Viallat, A. Dynamics of vesicles in a wall-bounded shear flow. Biophys. J. 2005, 89, 1055–1066. [Google Scholar] [CrossRef]
- Hur, S.C.; Henderson-MacLennan, N.K.; McCabe, E.R.B.; Di Carlo, D. Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip 2011, 11, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Ha, J.B.; Bahk, Y.K.; Arakawa, T.; Shoji, S.; Go, J.S. Size-selective separation of micro beads by utilizing secondary flow in a curved rectangular microchannel. Lab Chip 2009, 9, 87–90. [Google Scholar] [CrossRef]
- Russom, A.; Gupta, A.K.; Nagrath, S.; Di Carlo, D.; Edd, J.F.; Toner, M. Differential inertial focusing of particles in curved low-aspect-ratio microchannels. New J. Phys. 2009, 11, 75025. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef]
- Di Carlo, D.; Edd, J.F.; Irimia, D.; Tompkins, R.G.; Toner, M. Equilibrium separation and filtration of particles using differential inertial focusing. Anal. Chem. 2008, 80, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 2007, 7, 1644–1659. [Google Scholar] [CrossRef]
- Chatterjee, A. Size-Dependant Separation of Multiple Particles in Spiral Microchannels. Ph.D. Thesis, University of Cincinnati, Cincinnati, OH, USA, 2011. [Google Scholar]
- Hood, K.; Lee, S.; Roper, M. Inertial migration of a rigid sphere in three-dimensional Poiseuille flow. J. Fluid Mech. 2015, 765, 452–479. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef]
- Lee, W.C.; Bhagat, A.A.S.; Huang, S.; Van Vliet, K.J.; Han, J.; Lim, C.T. High-throughput cell cycle synchronization using inertial forces in spiral microchannels. Lab Chip 2011, 11, 1359–1367. [Google Scholar] [CrossRef]
- Mohamed Yousuff, C.; Hamid, N.H.B.; Kamal Basha, I.H.; Wei Ho, E.T. Output channel design for collecting closely-spaced particle streams from spiral inertial separation devices. AIP Adv. 2017, 7, 85004. [Google Scholar] [CrossRef]
- Ghadami, S.; Kowsari-Esfahan, R.; Saidi, M.S.; Firoozbakhsh, K. Spiral microchannel with stair-like cross section for size-based particle separation. Microfluid. Nanofluid. 2017, 21, 115. [Google Scholar] [CrossRef]
- Chen, H. A triplet parallelizing spiral microfluidic chip for continuous separation of tumor cells. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Son, J.; Jafek, A.R.; Carrell, D.T.; Hotaling, J.M.; Gale, B.K. Sperm-like-particle (SLP) behavior in curved microfluidic channels. Microfluid. Nanofluid. 2019, 23, 4. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, D.; Yan, S.; Zhang, J.; Du, H.; Alici, G.; Li, W. Flow rate-insensitive microparticle separation and filtration using a microchannel with arc-shaped groove arrays. Microfluid. Nanofluid. 2017, 21, 55. [Google Scholar] [CrossRef]
- Özbey, A.; Karimzadehkhouei, M.; Bayrak, Ö.; Koşar, A. Inertial focusing of microparticles in curvilinear microchannels with different curvature angles. Microfluid. Nanofluid. 2018, 22, 62. [Google Scholar] [CrossRef]
- Lee, M.G.; Choi, S.; Park, J.-K. Inertial separation in a contraction-expansion array microchannel. J. Chromatogr. A 2011, 1218, 4138–4143. [Google Scholar] [CrossRef]
- Lee, M.G.; Choi, S.; Kim, H.-J.; Lim, H.K.; Kim, J.-H.; Huh, N.; Park, J.-K. High-yield blood plasma separation by modulating inertial migration in a contraction-expansion array microchannel. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 258–261. [Google Scholar]
- Kwak, B.; Lee, S.; Lee, J.; Lee, J.; Cho, J.; Woo, H.; Heo, Y.S. Hydrodynamic blood cell separation using fishbone shaped microchannel for circulating tumor cells enrichment. Sens. Actuators B Chem. 2018, 261, 38–43. [Google Scholar] [CrossRef]
- Kwon, K.; Sim, T.; Moon, H.-S.; Lee, J.-G.; Park, J.C.; Jung, H.-I. A novel particle separation method using multi-stage multi-orifice flow fractionation (MS-MOFF). In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherland, 3–7 October 2010. [Google Scholar]
- Hyun, K.-A.; Koo, G.-B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.-I.; Jung, H.-I.; Kim, Y.-S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677. [Google Scholar] [CrossRef]
- Yuan, D.; Sluyter, R.; Zhao, Q.; Tang, S.; Yan, S.; Yun, G.; Li, M.; Zhang, J.; Li, W. Dean-flow-coupled elasto-inertial particle and cell focusing in symmetric serpentine microchannels. Microfluid. Nanofluid. 2019, 23, 41. [Google Scholar] [CrossRef]
- Balvin, M.; Sohn, E.; Iracki, T.; Drazer, G.; Frechette, J. Directional locking and the role of irreversible interactions in deterministic hydrodynamics separations in microfluidic devices. Phys. Rev. Lett. 2009, 103, 78301. [Google Scholar] [CrossRef]
- Frechette, J.; Drazer, G. Directional locking and deterministic separation in periodic arrays. J. Fluid Mech. 2009, 627, 379. [Google Scholar] [CrossRef]
- Holm, S.H.; Beech, J.P.; Barrett, M.P.; Tegenfeldt, J.O. Separation of parasites from human blood using deterministic lateral displacement. Lab Chip 2011, 11, 1326–1332. [Google Scholar] [CrossRef]
- Long, B.R.; Heller, M.; Beech, J.P.; Linke, H.; Bruus, H.; Tegenfeldt, J.O. Multidirectional sorting modes in deterministic lateral displacement devices. Phys. Rev. E 2008, 78, 46304. [Google Scholar] [CrossRef]
- Inglis, D.W.; Davis, J.A.; Austin, R.H.; Sturm, J.C. Critical particle size for fractionation by deterministic lateral displacement. Lab Chip 2006, 6, 655–658. [Google Scholar] [CrossRef]
- Davis, J.A.; Inglis, D.W.; Morton, K.J.; Lawrence, D.A.; Huang, L.R.; Chou, S.Y.; Sturm, J.C.; Austin, R.H. Deterministic hydrodynamics: Taking blood apart. Proc. Natl. Acad. Sci. USA 2006, 103, 14779–14784. [Google Scholar] [CrossRef] [PubMed]
- Beech, J.P.; Holm, S.H.; Adolfsson, K.; Tegenfeldt, J.O. Sorting cells by size, shape and deformability. Lab Chip 2012, 12, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.W.; Lord, M.; Nordon, R.E. Scaling deterministic lateral displacement arrays for high throughput and dilution-free enrichment of leukocytes. J. Micromech. Microeng. 2011, 21, 54024. [Google Scholar] [CrossRef]
- Loutherback, K.; Chou, K.S.; Newman, J.; Puchalla, J.; Austin, R.H.; Sturm, J.C. Improved performance of deterministic lateral displacement arrays with triangular posts. Microfluid. Nanofluid. 2010, 9, 1143–1149. [Google Scholar] [CrossRef]
- Dincau, B.M.; Aghilinejad, A.; Chen, X.; Moon, S.Y.; Kim, J.-H. Vortex-free high-Reynolds deterministic lateral displacement (DLD) via airfoil pillars. Microfluid. Nanofluid. 2018, 22, 137. [Google Scholar] [CrossRef]
- Zeming, K.K.; Ranjan, S.; Zhang, Y. Rotational separation of non-spherical bioparticles using I-shaped pillar arrays in a microfluidic device. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Ranjan, S.; Zeming, K.K.; Jureen, R.; Fisher, D.; Zhang, Y. DLD pillar shape design for efficient separation of spherical and non-spherical bioparticles. Lab Chip 2014, 14, 4250–4262. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Hyun, J.; Wang, S.; Yang, S. Improved pillar shape for deterministic lateral displacement separation method to maintain separation efficiency over a long period of time. Sep. Purif. Technol. 2017, 172, 258–267. [Google Scholar] [CrossRef]
- Beech, J.P.; Tegenfeldt, J.O. Tuneable separation in elastomeric microfluidics devices. Lab Chip 2008, 8, 657–659. [Google Scholar] [CrossRef]
- Bowman, T.; Frechette, J.; Drazer, G. Force driven separation of drops by deterministic lateral displacement. Lab Chip 2012, 12, 2903–2908. [Google Scholar] [CrossRef]
- Herrmann, J.; Karweit, M.; Drazer, G. Separation of suspended particles in microfluidic systems by directional locking in periodic fields. Phys. Rev. E 2009, 79, 61404. [Google Scholar] [CrossRef]
- Devendra, R.; Drazer, G. Gravity driven deterministic lateral displacement for particle separation in microfluidic devices. Anal. Chem. 2012, 84, 10621–10627. [Google Scholar] [CrossRef]
- Lubbersen, Y.S.; Dijkshoorn, J.P.; Schutyser, M.A.I.; Boom, R.M. Visualization of inertial flow in deterministic ratchets. Sep. Purif. Technol. 2013, 109, 33–39. [Google Scholar] [CrossRef]
- Loutherback, K.; D’Silva, J.; Liu, L.; Wu, A.; Austin, R.H.; Sturm, J.C. Deterministic separation of cancer cells from blood at 10 mL/min. AIP Adv. 2012, 2, 42107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Henry, E.; Gompper, G.; Fedosov, D.A. Behavior of rigid and deformable particles in deterministic lateral displacement devices with different post shapes. J. Chem. Phys. 2015, 143, 243145. [Google Scholar] [CrossRef]
- Quek, R.; Le, D.V.; Chiam, K.-H. Separation of deformable particles in deterministic lateral displacement devices. Phys. Rev. E 2011, 83, 56301. [Google Scholar] [CrossRef]
- Ghasemi, M.; Holm, S.H.; Beech, J.P.; Björnmalm, M.; Tegenfeldt, J.O. Separation of deformable hydrogel microparticles in deterministic lateral displacement devices. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2012, Okinawa, Japan, 28 October–1 November 2012; Chemical and Biological Microsystems Society: Washington, DC, USA, 2012; pp. 1672–1674. [Google Scholar]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Deterministic lateral displacement device for droplet separation by size—Towards rapid clonal selection based on droplet shrinking. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 3–7 October 2010. [Google Scholar]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Droplet size based separation by deterministic lateral displacement—Separating droplets by cell-induced shrinking. Lab Chip 2011, 11, 1305–1310. [Google Scholar] [CrossRef]
- Inglis, D.W.; Herman, N.; Vesey, G. Highly accurate deterministic lateral displacement device and its application to purification of fungal spores. Biomicrofluidics 2010, 4, 24109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Lin, H.; Liu, J.-Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.-C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef]
- Nam, Y.-H.; Lee, S.-K.; Kim, J.-H.; Park, J.-H. PDMS membrane filter with nano-slit array fabricated using three-dimensional silicon mold for the concentration of particles with bacterial size range. Microelectron. Eng. 2019, 215, 111008. [Google Scholar] [CrossRef]
- Crowley, T.A.; Pizziconi, V. Isolation of plasma from whole blood using planar microfilters for lab-on-a-chip applications. Lab Chip 2005, 5, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.-C.; Sul, O.; Kim, C.G.; Kim, W.-Y. Continuous separation of circulating tumor cells from whole blood using a slanted weir microfluidic device. Cancers 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Indhu, R.; Mercy, A.S.; Shreemathi, K.M.; Radha, S.; Kirubaveni, S.; Sreeja, B.S. Design of a Filter Using Array of Pillar for Particle Separation. Mater. Today Proc. 2018, 5, 10889–10894. [Google Scholar] [CrossRef]
- Wu, C.-C.; Hong, L.-Z.; Ou, C.-T. Blood cell-free plasma separated from blood samples with a cascading weir-type microfilter using dead-end filtration. J. Med. Biol. Eng. 2012, 32, 163–168. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Dang, C.; Hong, S.; Yang, A.-S.; Su, T.-L.; Yang, Y.-C. Microfluidics with new multi-stage arc-unit structures for size-based cross-flow separation of microparticles. Microelectron. Eng. 2019, 207, 37–49. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes. J. Memb. Sci. 1981, 9, 121–189. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, M.; Chen, Y.; He, G.; Wu, M.; Wang, J. Preparation and performance of cellulose acetate/polyethyleneimine blend microfiltration membranes and their applications. J. Membr. Sci. 2004, 235, 73–86. [Google Scholar] [CrossRef]
- Aussawasathien, D.; Teerawattananon, C.; Vongachariya, A. Separation of micron to sub-micron particles from water: Electrospun nylon-6 nanofibrous membranes as pre-filters. J. Membr. Sci. 2008, 315, 11–19. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Du, M.; Wang, W.; Zhang, W.; Wang, Z.; Jiang, X. Accelerating microfluidic immunoassays on filter membranes by applying vacuum. Biomed. Microdevices 2012, 14, 17–23. [Google Scholar] [CrossRef]
- Shao, S.; Liu, Y.; Shi, D.; Qing, W.; Fu, W.; Li, J.; Fang, Z.; Chen, Y. Control of organic and surfactant fouling using dynamic membranes in the separation of oil-in-water emulsions. J. Colloid Interface Sci. 2020, 560, 787–794. [Google Scholar] [CrossRef]
- Ng, T.C.A.; Lyu, Z.; Gu, Q.; Zhang, L.; Poh, W.J.; Zhang, Z.; Wang, J.; Ng, H.Y. Effect of gradient profile in ceramic membranes on filtration characteristics: Implications for membrane development. J. Membr. Sci. 2020, 595, 117576. [Google Scholar] [CrossRef]
- Murthy, S.K.; Sethu, P.; Vunjak-Novakovic, G.; Toner, M.; Radisic, M. Size-based microfluidic enrichment of neonatal rat cardiac cell populations. Biomed. Microdevices 2006, 8, 231–237. [Google Scholar] [CrossRef]
- Ripperger, S.; Altmann, J. Crossflow microfiltration–state of the art. Sep. Purif. Technol. 2002, 26, 19–31. [Google Scholar] [CrossRef]
- VanDelinder, V.; Groisman, A. Perfusion in microfluidic cross-flow: Separation of white blood cells from whole blood and exchange of medium in a continuous flow. Anal. Chem. 2007, 79, 2023–2030. [Google Scholar] [CrossRef]
- Ji, H.M.; Samper, V.; Chen, Y.; Heng, C.K.; Lim, T.M.; Yobas, L. Silicon-based microfilters for whole blood cell separation. Biomed. Microdevices 2008, 10, 251–257. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Zhang, L. Isolation of plasma from whole blood using a microfludic chip in a continuous cross-flow. Chin. Sci. Bull. 2009, 54, 324–327. [Google Scholar] [CrossRef]
- Sethu, P.; Sin, A.; Toner, M. Microfluidic diffusive filter for apheresis (leukapheresis). Lab Chip 2006, 6, 83–89. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Liu, C.; Li, H.; Chen, J. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal. Chim. Acta 2007, 584, 237–243. [Google Scholar] [CrossRef]
- Mielnik, M.M.; Ekatpure, R.P.; Sætran, L.R.; Schönfeld, F. Sinusoidal crossflow microfiltration device—Experimental and computational flowfield analysis. Lab Chip 2005, 5, 897–903. [Google Scholar] [CrossRef]
- Moorthy, J.; Beebe, D.J. In situ fabricated porous filters for microsystems. Lab Chip 2003, 3, 62–66. [Google Scholar] [CrossRef]
- Aran, K.; Fok, A.; Sasso, L.A.; Kamdar, N.; Guan, Y.; Sun, Q.; Ündar, A.; Zahn, J.D. Microfiltration platform for continuous blood plasma protein extraction from whole blood during cardiac surgery. Lab Chip 2011, 11, 2858–2868. [Google Scholar] [CrossRef]
- Lo, M.; Zahn, J.D. Development of a multi-compartment microfiltration device for particle fractionation. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Okinawa, Japan, 28 October–1 November 2012. [Google Scholar]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef]
- Matsuda, M.; Yamada, M.; Seki, M. Blood cell classification utilizing hydrodynamic filtration. Electron. Commun. Jpn. 2011, 94, 1–6. [Google Scholar] [CrossRef]
- Yamada, M.; Seki, M. Microfluidic particle sorter employing flow splitting and recombining. Anal. Chem. 2006, 78, 1357–1362. [Google Scholar] [CrossRef]
- Chiu, Y.-Y.; Huang, C.-K.; Lu, Y.-W. Enhancement of microfluidic particle separation using cross-flow filters with hydrodynamic focusing. Biomicrofluidics 2016, 10, 11906. [Google Scholar] [CrossRef]
- Yang, S.; Ündar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Dhariwal, R.; Desmulliez, M.P.Y.; Jouvet, L. Hydrodynamic blood plasma separation in microfluidic channels. Microfluid. Nanofluid. 2010, 8, 105. [Google Scholar] [CrossRef]
- Jäggi, R.D.; Sandoz, R.; Effenhauser, C.S. Microfluidic depletion of red blood cells from whole blood in high-aspect-ratio microchannels. Microfluid. Nanofluid. 2007, 3, 47–53. [Google Scholar] [CrossRef]
- Wei Hou, H.; Gan, H.Y.; Bhagat, A.A.S.; Li, L.D.; Lim, C.T.; Han, J. A microfluidics approach towards high-throughput pathogen removal from blood using margination. Biomicrofluidics 2012, 6, 24115. [Google Scholar] [CrossRef]
- Fåhraeus, R. The suspension stability of the blood. Physiol. Rev. 1929, 9, 241–274. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, L.; Ju, Y.; Wang, W.; Li, Z. A plasma separation device based on centrifugal effect and Zweifach-Fung effect. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011; pp. 224–226. [Google Scholar]
- Dong, T.; Yang, Z.; Su, Q.; Tran, N.M.; Egeland, E.B.; Karlsen, F.; Zhang, Y.; Kapiris, M.J.; Jakobsen, H. Integratable non-clogging microconcentrator based on counter-flow principle for continuous enrichment of CaSki cells sample. Microfluid. Nanofluid. 2011, 10, 855–865. [Google Scholar] [CrossRef]
- Hønsvall, B.K.; Altin, D.; Robertson, L.J. Continuous harvesting of microalgae by new microfluidic technology for particle separation. Bioresour. Technol. 2016, 200, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Mossige, E.J.; Jensen, A.; Mielnik, M.M. An experimental characterization of a tunable separation device. Microfluid. Nanofluid. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Mossige, E.J.; Jensen, A.; Mielnik, M.M. Separation and concentration without clogging using a high-throughput tunable filter. Phys. Rev. Appl. 2018, 9, 54007. [Google Scholar] [CrossRef]
- Mossige, E.J.; Edvardsen, B.; Jensen, A.; Mielnik, M.M. A tunable, microfluidic filter for clog-free concentration and separation of complex algal cells. Microfluid. Nanofluid. 2019, 23, 56. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Di Carlo, D.; Chen, C.; Irimia, D.; Toner, M. Microvortex for focusing, guiding and sorting of particles. Lab Chip 2008, 8, 2128–2134. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Bagdi, P.; Sen, A.K. Microfluidic device based on a micro-hydrocyclone for particle–liquid separation. Lab Chip 2011, 11, 4012–4021. [Google Scholar] [CrossRef]
- Chand, R.; Ramalingam, S.; Neethirajan, S. A 2D tran sition-metal dichalcogenide MoS2 based novel nanocomposite and nanocarrier for multiplex miRNA detection. Nanoscale 2018, 10, 8217–8225. [Google Scholar] [CrossRef]
- Martins, G.V.; Marques, A.C.; Fortunato, E.; Sales, M.G.F. Wax-printed paper-based device for direct electrochemical detection of 3-nitrotyrosine. Electrochim. Acta 2018, 284, 60–68. [Google Scholar] [CrossRef]
- Reich, P.; Preuss, J.A.; Bahner, N.; Bahnemann, J. Impedimetric aptamer-based biosensors: Principles and techniques. Adv. Biochem. Eng. Biotechnol. 2020, 174, 17–41. [Google Scholar]
- Preuss, J.A.; Reich, P.; Bahner, N.; Bahnemann, J. Impedimetric aptamer-based biosensors: Applications. Adv. Biochem. Eng. Biotechnol. 2020, 174, 43–91. [Google Scholar]
- Mousavi, M.P.S.; Ainla, A.; Tan, E.K.W.; Abd El-Rahman, M.K.; Yoshida, Y.; Yuan, L.; Sigurslid, H.H.; Arkan, N.; Yip, M.C.; Abrahamsson, C.K.; et al. Ion sensing with thread-based potentiometric electrodes. Lab Chip 2018, 18, 2279–2290. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Park, H.; Shim, Y.-B. Direct Analysis of Trace Phenolics with a Microchip: In-Channel Sample Preconcentration, Separation, and Electrochemical Detection. Anal. Chem. 2006, 78, 6809–6817. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, M.; Kim, J.H.; Lee, H.B.; Inoue, S.; Becker, A.L.; Weigel, K.M.; Cangelosi, G.A.; Lee, K.H.; Chung, J.H. Amperometric immunosensor for rapid detection of Mycobacterium tuberculosis. J. Micromech. Microeng. 2015, 25, 055013. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, R.; Tlili, C.; Abulrob, A.; Tavares, A.C.; Zourob, M. Label-free impedimetric immunosensor for ultrasensitive detection of cancer marker Murine double minute 2 in brain tissue. Biosens. Bioelectron. 2013, 39, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, J.; Carvalho, F.C.; Santos, A.; Fernandes, F.C.B.; Bueno, P.R. An impedimetric biosensor to test neat serum for dengue diagnosis. Sens. Actuators B Chem. 2015, 213, 150–154. [Google Scholar] [CrossRef]
- Ding, J.W.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Luo, X.L.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 5944–5962. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-J.; Zou, X.U.; Hamedi, M.M.; Hu, J.; Parolo, C.; Maxwell, E.J.; Bühlmann, P.; Whitesides, G.M. Paper-Based Potentiometric Ion Sensing. Anal. Chem. 2014, 86, 9548–9553. [Google Scholar] [CrossRef]
- Han, Y.X.; Chen, J.; Li, Z.; Chen, H.L.; Qiu, H.D. Recent progress and prospects of alkaline phosphatase biosensor based on fluorescence strategy. Biosens. Bioelectron. 2020, 148, 111811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.H.; Wang, J.; Li, J.H.; Lin, Y.H. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Takemura, K.; Adegoke, O.; Suzuki, T.; Park, E.Y. A localized surface plasmon resonance-amplified immunofluorescence biosensor for ultrasensitive and rapid detection of nonstructural protein 1 of Zika virus. PLoS ONE 2019, 14, e0211517. [Google Scholar] [CrossRef]
- Sieben, V.J.; Floquet, C.F.A.; Ogilvie, I.R.G.; Mowlem, M.C.; Morgan, H. Microfluidic colourimetric chemical analysis system: Application to nitrite detection. Anal. Methods 2010, 2, 484–491. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Beitler, J.J.; Chen, Z.G.; Khuri, F.R.; Lewis, M.M.; Shin, H.J.C.; Nie, S.; Shin, D.M. Detection of Circulating Tumor Cells in Human Peripheral Blood Using Surface-Enhanced Raman Scattering Nanoparticles. Cancer Res. 2011, 71, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, Y.; Huang, Y.; Li, J.; Ruan, H.; Luo, L.; Yang, S.; Shen, Z.; Wu, A. Improved SERS-Active Nanoparticles with Various Shapes for CTC Detection without Enrichment Process with Supersensitivity and High Specificity. ACS Appl. Mater. Interfaces 2016, 8, 19928–19938. [Google Scholar] [CrossRef]
- Quang, L.X.; Lim, C.; Seong, G.H.; Choo, J.; Do, K.J.; Yoo, S.-K. A portable surface-enhanced Raman scattering sensor integrated with a lab-on-a-chip for field analysis. Lab Chip 2008, 8, 2214–2219. [Google Scholar] [CrossRef]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Puhler, A.; Reiss, G.; Bruckl, H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Santiesteban, O.J.; Kaittanis, C.; Perez, J.M. Identification of toxin inhibitors using a magnetic nanosensor-based assay. Small 2014, 10, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Sideris, C.; Khial, P.P.; Hajimiri, A. Design and implementation of reference-free drift-cancelling CMOS magnetic sensors for biosensing applications. IEEE J. Solid-State Circuits 2018, 53, 3065–3075. [Google Scholar] [CrossRef]
- Hong, S.L.; Zhang, N.; Qin, L.; Tang, M.; Ai, Z.; Chen, A.; Wang, S.; Liu, K. An automated detection of influenza virus based on 3-D magnetophoretic separation and magnetic label. Analyst 2021, 146, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Klein, T.; Krishna, V.D.; Su, D.Q.; Perez, A.M.; Wang, J.P. Portable GMR handheld platform for the detection of influenza A virus. ACS Sens. 2017, 21594–21601. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, J.M.; Saha, R.; Su, D.Q.; Krishna, V.D.; Cheeran, M.C.J.; Wang, J.P. Magnetic particle spectroscopy for detection of influenza A virus subtype H1N1. ACS Appl. Mater. Interfaces 2020, 12, 13686–13697. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: Recent progress, applications, and future perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Jiang, P.J.; Guo, Z.J. Fluorescent detection of zinc in biological systems: Recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 2004, 248, 205–229. [Google Scholar] [CrossRef]
- Xianyu, Y.L.; Wang, Q.L.; Chen, Y.P. Magnetic particles-enabled biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Leong, W.; Wang, D.-A. Cell-laden polymeric microspheres for biomedical applications. Trends Biotechnol. 2015, 33, 653–666. [Google Scholar] [CrossRef]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Van Riel, D.; de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef]
- Rytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008, 5, 629–639. [Google Scholar] [CrossRef]


| Categories | Examples | References |
|---|---|---|
| Pinched-flow fractionation (PFF) | Symmetric PFF | [65] |
| AsPFF | [67,68,70,73] | |
| Tumbling mechanism in PFF | [66] | |
| Sedimentation PFF | [71] | |
| Tunable PFF | [72] | |
| Inertia and Dean flow | Inertial and Dean flow fractionation | [40,79,89,93] |
| Spiral microchannel | [97,98,99,100] | |
| Curvature angles | [90,101,102] | |
| CEA | [83,103,104,105] | |
| Multiorifice | [66,106,107] | |
| Serpentine microchannel | [108] | |
| Deterministic lateral displacement (DLD) | DLD | [109,110,111,112,113,114,115,127,128,129,130,131,134] |
| Disposable parallel DLD | [116] | |
| Optimized shape | [117,118,119,120,121,122] | |
| Tunable DLD | [123] | |
| Force-driven DLD | [124,125,126] | |
| Droplet shrinking | [132,133] | |
| Membrane | [136,144,145,147,148] | |
| Microscale filter | Vacuum-accelerated microfluidic immunoassay (VAMI) | [146] |
| Planar microfilter | [137,139] | |
| Weir microfluidic device | [138,140] | |
| Crossflow microfilter | [150,151,152,153,154,155,156] | |
| Porous filter | [156,158] | |
| Multicompartment | [159] | |
| Other hydrodynamic methods | Hydrodynamic filtration | [160,161,162,163] |
| Zweifach–Fung effect | [164,165,166,167,168,169] | |
| Trilobite separator | [170,171,172,173,174] | |
| Microvortex | [175] | |
| Microhydrocyclone | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, X.; Wang, J.; Wang, C.; Yan, Y.; Wu, A.; Ren, Y. Public-Health-Driven Microfluidic Technologies: From Separation to Detection. Micromachines 2021, 12, 391. https://doi.org/10.3390/mi12040391
Zhang X, Xu X, Wang J, Wang C, Yan Y, Wu A, Ren Y. Public-Health-Driven Microfluidic Technologies: From Separation to Detection. Micromachines. 2021; 12(4):391. https://doi.org/10.3390/mi12040391
Chicago/Turabian StyleZhang, Xiangzhi, Xiawei Xu, Jing Wang, Chengbo Wang, Yuying Yan, Aiguo Wu, and Yong Ren. 2021. "Public-Health-Driven Microfluidic Technologies: From Separation to Detection" Micromachines 12, no. 4: 391. https://doi.org/10.3390/mi12040391
APA StyleZhang, X., Xu, X., Wang, J., Wang, C., Yan, Y., Wu, A., & Ren, Y. (2021). Public-Health-Driven Microfluidic Technologies: From Separation to Detection. Micromachines, 12(4), 391. https://doi.org/10.3390/mi12040391








