A Label-Free Liquid Crystal Biosensor Based on Specific DNA Aptamer Probes for Sensitive Detection of Amoxicillin Antibiotic
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
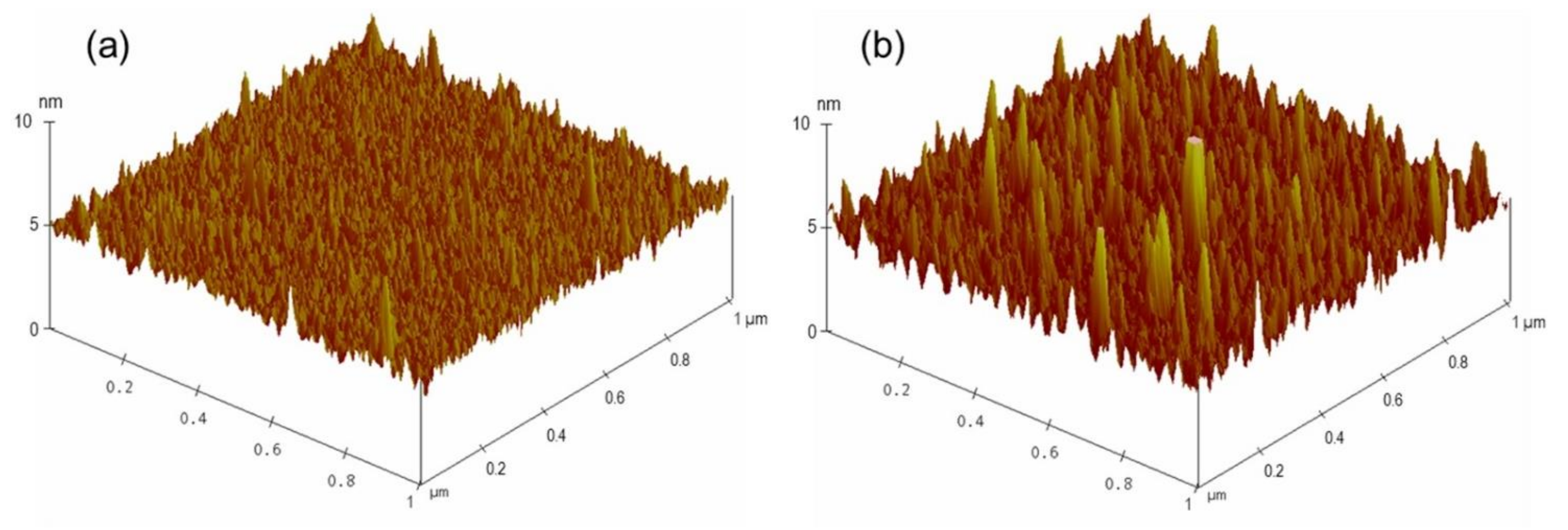
2.2. Preparation of Modified Glass Slides and Fabrication of LC Cells
2.3. Specific Binding of AMX Aptamer and AMX
2.4. Detection of AMX in Real Water Samples
3. Results
3.1. Principle of LC-Based Aptasensor for AMX Detection
3.2. Optimization of the Detection Conditions
3.2.1. Effects of the APTES/DMOAP Ratio
3.2.2. Effects of DMOAP Concentrations on the Upper Glass Slides
3.2.3. Effects of GA Concentration
3.2.4. Effects of AMX Aptamer Concentration
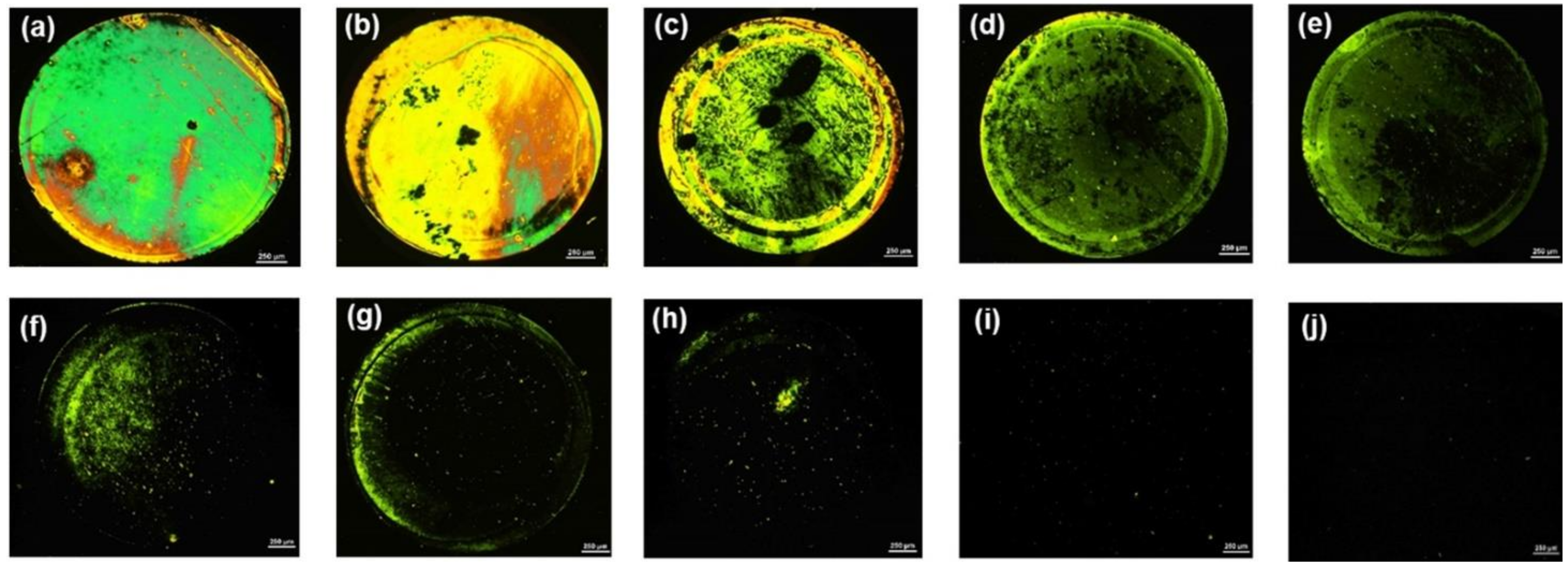
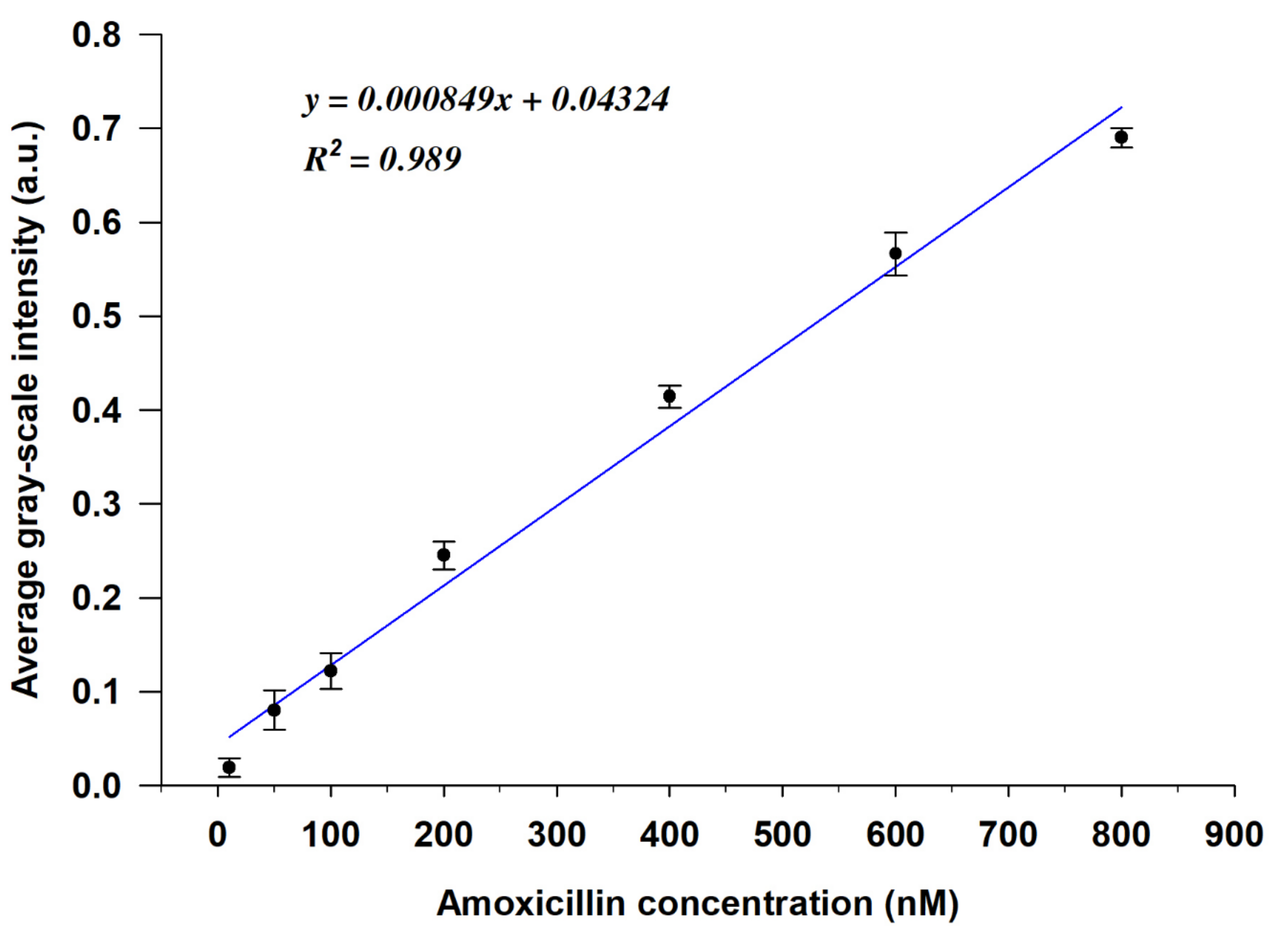
3.3. Feasibility of the LC-Based Aptasensor for AMX Detection
3.4. Sensor Specificity
3.5. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Huang, M.; Jiang, N.; Zheng, S.; Mu, T.; Meng, L.; Liu, Y.; Liu, J.; Chen, G. Ultrasensitive detection of amoxicillin by TiO2-g-C3N4@AuNPs impedimetric aptasensor: Fabrication, optimization, and mechanism. J. Hazard. Mater. 2020, 391, 122024. [Google Scholar] [CrossRef] [PubMed]
- Isla, A.; Troconiz, I.F.; Canut, A.; Labora, A.; Martin-Herrero, J.E.; Pedraz, J.L.; Gascon, A.R. Pharmacokinetic/pharmacodynamic evaluation of amoxicillin, amoxicillin/clavulanate and ceftriaxone in the treatment of paediatric acute otitis media in Spain. Enferm. Infecc. Microbiol. Clin. 2011, 29, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Fuchs, A.; Bielicki, J.; Van Den Anker, J.; Sharland, M. Antibiotic use for community-acquired pneumonia in neonates and children: WHO evidence review. Paediatr. Int. Child Health 2018, 38, S66–S75. [Google Scholar] [CrossRef]
- Fernandez, J.; Ribeiro, I.A.C.; Martin, V.; Martija, O.L.; Zuza, E.; Bettencourt, A.F.; Sarasua, J.R. Release mechanisms of urinary tract antibiotics when mixed with bioabsorbable polyesters. Mater. Sci. Eng. C 2018, 93, 529–538. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef]
- Sun, L.; Jia, L.; Xie, X.; Xie, K.; Wang, J.; Liu, J.; Cui, L.; Zhang, G.; Dai, G.; Wang, J. Quantitative analysis of amoxicillin, its major metabolites and ampicillin in eggs by liquid chromatography combined with electrospray ionization tandem mass spectrometry. Food Chem. 2016, 192, 313–318. [Google Scholar] [CrossRef]
- Rosy, N.K.; Goyal, R.N. Gold-palladium nanoparticles aided electrochemically reduced graphene oxide sensor for the simultaneous estimation of lomefloxacin and amoxicillin. Sens. Actuators B Chem. 2017, 243, 658–668. [Google Scholar]
- Santos, A.J.D.; Kronka, M.S.; Fortunato, G.V.; Lanza, M.R. Recent advances in electrochemical water technologies for the treatment of antibiotics: A short review. Curr. Opin. Electrochem. 2021, 26, 100674. [Google Scholar] [CrossRef]
- Chullasat, K.; Nurerk, P.; Kanatharana, P.; Davis, F.; Bunkoed, O. A facile optosensing protocol based on molecularly imprinted polymer coated on CdTe quantum dots for highly sensitive and selective amoxicillin detection. Sens. Actuators B Chem. 2018, 254, 255–263. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Ji, Z.; Fan, J. Sensitive detection of amoxicillin in aqueous solution with novel fluorescent probes containing boron-doped carbon quantum dots. J. Mol. Liq. 2020, 311, 113278. [Google Scholar] [CrossRef]
- Rudnicki, K.; Sipa, K.; Brycht, M.; Borgul, P.; Skrzypek, S.; Poltorak, L. Electrochemical sensing of fluoroquinolone antibiotics. Trends Anal. Chem. 2020, 128, 115907. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Cincotto, F.H.; Moraes, F.C.; Fatibello-Filho, O.; Sotomayor, M.D.P.T. A new electrochemical platform based on low cost nanomaterials for sensitive detection of the amoxicillin antibiotic in different matrices. Talanta 2020, 206, 120252. [Google Scholar] [CrossRef]
- Unutkan, T.; Bakırdere, S.; Keyf, S. Development of an analytical method for the determination of amoxicillin in commercial drugs and wastewater samples, and assessing its stability in simulated gastric digestion. J. Chromatogr. Sci. 2018, 56, 36–40. [Google Scholar] [CrossRef]
- Yola, M.L.; Eren, T.; Atar, N. Molecular imprinted nanosensor based on surface plasmon resonance: Application to the sensitive determination of amoxicillin. Sens. Actuators B Chem. 2014, 195, 28–35. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid molecularly imprinted polymer for amoxicillin detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ain, N.U.; Anis, I.; Ahmed, F.; Shah, M.R.; Parveen, S.; Faizi, S.; Ahmed, S. Colorimetric detection of amoxicillin based on querecetagetin coated silver nanoparticles. Sens. Actuators B Chem. 2018, 265, 617–624. [Google Scholar] [CrossRef]
- Brahman, P.K.; Dar, R.A.; Pitre, K.S. Conducting polymer film based electrochemical sensor for the determination of amoxicillin in micellar media. Sens. Actuators B Chem. 2013, 176, 307–314. [Google Scholar] [CrossRef]
- Du, J.Y.; Jiang, Q.F.; Lu, X.C.; Chen, L.C.; Zhang, Y.; Xiong, X.L. Detection of sulfadimethoxine using optical images of liquid crystals. Analyst 2019, 144, 1761. [Google Scholar] [CrossRef]
- Hussain, A.; Pina, A.S.; Roque, A.C.A. Bio-recognition and detection using liquid crystals. Biosens. Bioelectron. 2009, 25, 1–8. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.H. Liquid crystal-based capillary sensory platform for the detection of bile acids. Chem. Phys. Lipids 2017, 204, 10–14. [Google Scholar] [CrossRef]
- Xia, C.; Zhou, D.; Su, Y.; Zhou, G.; Yao, L.; Sun, W.; Liu, Y. A liquid-crystal-based immunosensor for the detection of cardiac troponin I. Analyst 2020, 145, 4569. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441. [Google Scholar] [CrossRef]
- Rouhbakhsh, Z.; Verdian, A.; Rajabzadeh, G. Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline. Talanta 2020, 206, 120246. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.T.K.; Jang, C.H. Sensitive and label-free liquid crystal-based optical sensor for the detection of malathion. Anal. Biochem. 2020, 593, 113589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Jang, C.H. Label-free liquid crystal-based biosensor for detection of As(III) ions using ssDNA as a recognition probe. Microchem. J. 2020, 156, 104834. [Google Scholar] [CrossRef]
- Verma, I.; Devi, M.; Sharma, D.; Nandi, R.; Pal, S.K. Liquid crystal based detection of Pb(II) ions using spinach RNA as recognition probe. Langmuir 2019, 35, 7816–7823. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Jang, C.H. Label-free liquid crystal-based biosensor for detection of dopamine using DNA aptamer as a recognition probe. Anal. Biochem. 2020, 605, 113807. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.H. Liquid crystal-based aptasensor for the detection of interferon-γ and its application in the diagnosis of tuberculosis using human blood. Sens. Actuators B Chem. 2019, 282, 574–579. [Google Scholar] [CrossRef]
- Verdian, A.; Rouhbakhsh, Z.; Fooladi, E. An ultrasensitive platform for PCB77 detection: New strategy for liquid crystal-based aptasensor fabrication. J. Hazard. Mater. 2021, 402, 123531. [Google Scholar] [CrossRef]
- Nandi, R.; Pal, S.K. Liquid crystal based sensing device using a smartphone. Analyst 2018, 143, 1046–1052. [Google Scholar] [CrossRef]
- Vergara-Barberán, M.; Lerma-García, M.J.; Moga, A.; Carrasco-Correa, E.J.; Martínez-Pérez-Cejuela, H.; Beneito-Cambra, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Recent advances in aptamer-based miniaturized extraction approaches in food analysis. Trends Anal. Chem. 2021, 138, 116230. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xiong, X.; Deng, S.X. Gold nanoparticle-based signal enhancement of an aptasensor for ractopamine using liquid crystal based optical imaging. Microchim. Acta 2019, 186, 697. [Google Scholar] [CrossRef]
- Lu, X.; Song, X.; Du, J.; Zhang, Y.; Zhang, L.; Xiong, X. Preparation of DNA-functionalized surfaces for simultaneous homeotropic orientation of liquid crystals and optical recognition of analytes: Application to the determination of progesterone. Microchim. Acta 2019, 186, 443. [Google Scholar] [CrossRef]
- Kim, H.; An, Z.; Jang, C.H. Label-free optical detection of thrombin using a liquid crystal-based aptasensor. Microchem. J. 2018, 141, 71–79. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Shen, J.; Xiong, X.L.; Deng, S.X. Aptamer based bare eye detection of kanamycin by using a liquid crystal film on a glass support. Microchim. Acta 2017, 184, 3765–3771. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, Q.; Zhang, T.; Xiong, X.; Chen, P. Liquid crystal biosensor based on Cd2+ inducing the bending of PS-oligo for the detection of cadmium. Health 2015, 7, 986–993. [Google Scholar] [CrossRef][Green Version]
- Yang, S.; Wu, C.; Tan, H.; Wu, Y.; Liao, S.; Wu, Z.; Shen, G.; Yu, R. Label-free liquid crystal biosensor based on specific oligonucleotide probes for heavy metal ions. Anal. Chem. 2013, 85, 14–18. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Jang, C.H. Simple and label-free liquid crystal-based optical sensor for highly sensitive and selective endotoxin detection by aptamer binding and separation. ChemistrySelect 2019, 4, 1416–1422. [Google Scholar] [CrossRef]
- Bala, R.; Dhingra, S.; Kumar, M.; Bansal, K.; Mittal, S.; Sharma, R.K.; Wangoo, N. Detection of organophosphorus pesticide—Malathion in environmental samples using peptide and aptamer based nanoprobes. Chem. Eng. J. 2017, 311, 111–116. [Google Scholar] [CrossRef]
- Bala, R.; Kumar, M.; Bansal, K.; Sharma, R.K.; Wangoo, N. Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens. Bioelectron. 2016, 85, 445–449. [Google Scholar] [CrossRef]



| Methods (Materials) | Linear Ranges | Detection Limit | Reference |
|---|---|---|---|
| Electrochemistry (TiO2-g-C3N4@Au NPs) a | 0.5–3 nM | 0.2 nM | [1] |
| Electrochemistry (AuNP-PdNP-ErGO) b | 30–350 μM | 9 μM | [7] |
| Photoluminescence (MIP-CdTeQDs) c | 0.547–163.8 μM | 0.383 nM | [8] |
| Fluorescence (B-CQDs) d | 1.43–429.12 μM | 825 nM | [10] |
| Electrochemistry (P6LC/CdTe QDs) e | 0.9–69 µM | 50 nM | [12] |
| HPLC f | 0.137–1368 µM | 43.8 nM | [13] |
| Chemosensor (hybrid AMO-MIP) g | 100 pM–8 µM | 73 pM | [15] |
| Colorimetry (Qt AgNPs) h | 10–95 µM | 4.46 µM | [16] |
| Liquid crystal-based aptasensor | 10–800 nM | 3.5 nM | Present study |
| Samples | Spiked AMX (nM) | Found AMX (nM) | Recovery (%) | Relative Standard Deviation (RSD) (%) |
|---|---|---|---|---|
| Tap water 1 | 50 | 46.175 | 92.35 | 4.71 |
| Tap water 2 | 400 | 430.35 | 107.58 | 2.37 |
| Tap water 3 | 800 | 758.45 | 94.8 | 2.18 |
| River water 1 | 50 | 45.58 | 91.16 | 4.96 |
| River water 2 | 400 | 386.76 | 96.69 | 7.91 |
| River water 3 | 800 | 754.11 | 94.26 | 1.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.K.; Jang, C.-H. A Label-Free Liquid Crystal Biosensor Based on Specific DNA Aptamer Probes for Sensitive Detection of Amoxicillin Antibiotic. Micromachines 2021, 12, 370. https://doi.org/10.3390/mi12040370
Nguyen DK, Jang C-H. A Label-Free Liquid Crystal Biosensor Based on Specific DNA Aptamer Probes for Sensitive Detection of Amoxicillin Antibiotic. Micromachines. 2021; 12(4):370. https://doi.org/10.3390/mi12040370
Chicago/Turabian StyleNguyen, Duy Khiem, and Chang-Hyun Jang. 2021. "A Label-Free Liquid Crystal Biosensor Based on Specific DNA Aptamer Probes for Sensitive Detection of Amoxicillin Antibiotic" Micromachines 12, no. 4: 370. https://doi.org/10.3390/mi12040370
APA StyleNguyen, D. K., & Jang, C.-H. (2021). A Label-Free Liquid Crystal Biosensor Based on Specific DNA Aptamer Probes for Sensitive Detection of Amoxicillin Antibiotic. Micromachines, 12(4), 370. https://doi.org/10.3390/mi12040370






