1. Introduction
The neck or back pain resulting from spine disorders, including acute back pain and chronic lower back pain that continues for several months, reaches 60–80% prevalence in humans. Although these kinds of pain would not immediately affect human life, they do seriously influence life quality and retard economic production. Current strategies used for pain control clinically include oral painkillers, Nonsteroidal anti-inflammatory drugs (NSAID), physical therapy, transdermal electrical stimulators, invasive pulsed radiofrequency (PRF) stimulators, implantable spinal cord electrical stimulators (SCS), and intraspinal cord injections [
1,
2,
3,
4]. A population of over 75 million in U.S. are suffering from chronic neuron disease-induced pain, and the costs of therapeutics and medical treatments have reached 2.3 billion with a year-on-year (YOY) of 12.1% [
5,
6].
Pain feeling transmission was impeded to achieve pain relief by the electric field effect released from the implanted PRF neural stimulatory device which stimulated neurons in the transmission pathway. A therapeutic temperature lower than 42 °C of the PRF neural stimulatory device would possess good pain relief and lower the risk of tissue heating damage and nearby neural capability loss. However, the therapeutic duration of the PRF stimulatory device is limited, so patients with chronic back pain need to repeat the identical therapeutic operation 3–6 months later [
7,
8]. Besides, the PRF stimulatory device is single-use and expensive, resulting in the patient’s heavy medical burden. Therefore, the development of the wireless energy transferred PRF neural stimulatory device will possess a high impact on the clinical application as well as largely reduce the risk of patient and health care personnel from exposure to radiology during therapeutic operations [
9,
10].
Generally, neuron stimulatory electrodes can be classified into the rod-shaped type applied on the brain cortex implantation and the patch type for in vivo adhesion through surgery, which all should possess biocompatibility and non-toxicity to humans in compliance with the Food and Drug Administration (FDA) regulation for the medical device. The patched electrode generally adheres onto the dura layer in the spinal cord through microsurgery, however, the drawback is that it is difficult to control the stimulatory site precisely and the stimulus will affect a large area. Therefore, the patch-type stimulation would impact the pain transmission, but also might impede the motor neuron transmission [
11].
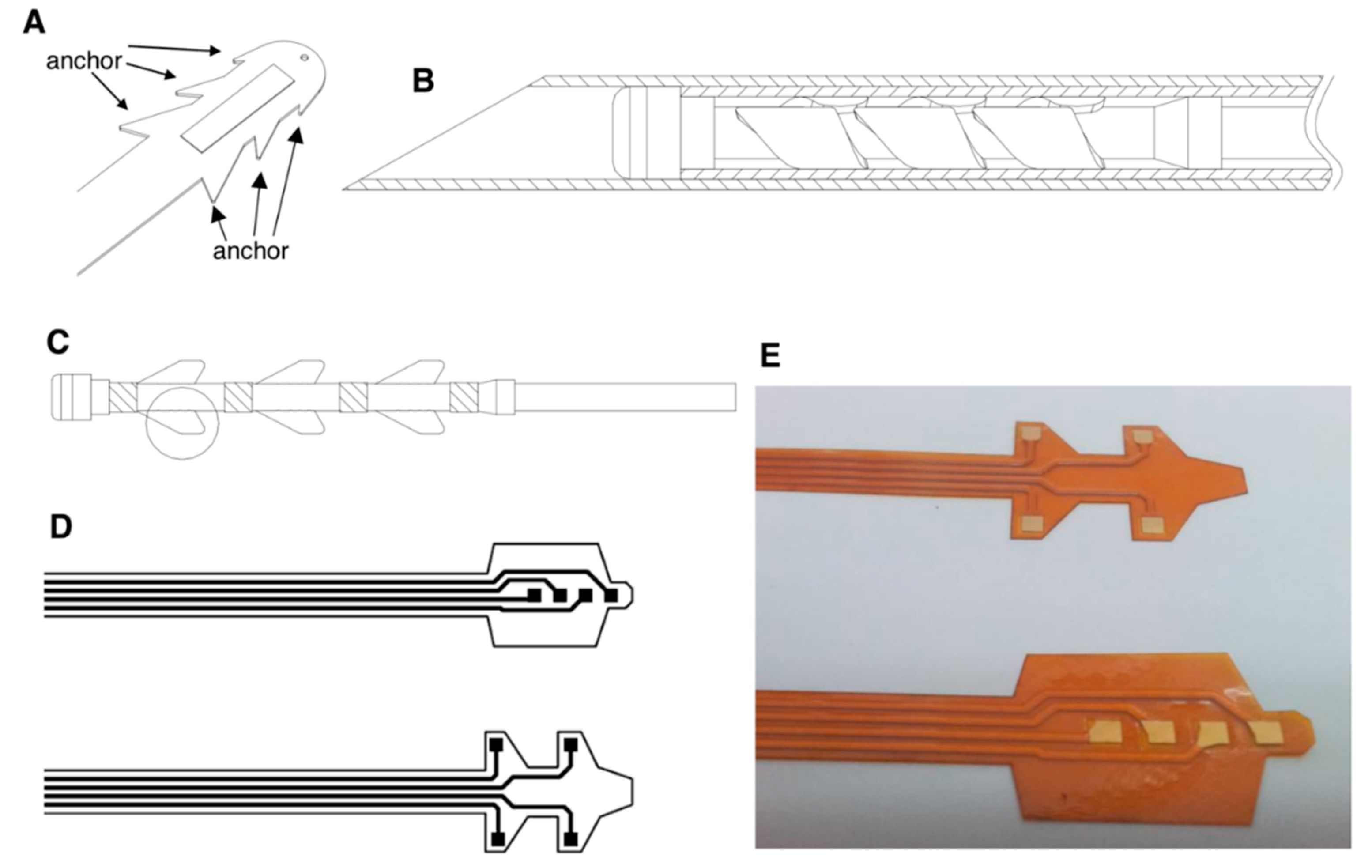
In this study, we would like to design and manufacture a novel PRF neuron stimulatory electrode with stretchable anchors to address pain relief. Through microsurgery, the PRF electrode could penetrate the spinal dura guided by a stainless needle to reach and precisely anchor on the soft tissue around the dorsal root ganglion, then elicit the therapeutic stimulus and block pain feeling. High grade implantable medical devices is an emerging composite industry combined with medicine, chemistry, biomaterials, mechanics, and photo-electronics, creating tremendous research potential and economic value. However, biomedical products are distinct from conventional consumables; they are stringently regulated by the FDA, guaranteeing their safety concerns. Therefore, developing high-grade implantable medical devices would establish several mutual core platforms, such as wireless energy transmission, durable and flexible electrodes, and biocompatible insulation, which could be applied for many distinct implant developments and industrial chain promotion.
2. Materials and Methods
2.1. Wire Loops, Architecture Design, and Simulation of Wireless Power Transmission
The wireless power transmission (WPT) system in this study was designed using a resonant coupling technique for neuron stimulatory electrodes. Mathematical analysis and simulations of various wire loop designs were carried out by two electromagnetic (EM)-simulation tools and a calculating software, ANSYS HFSS (Version 15.0, ANSYS, Inc., Canonsburg, PA, USA), CST STUDIO SUITE® (CST Studio Suite 2019, Dassault Systèmes, Inc., Waltham, MA, USA), and PathWave ADS circuit simulator (W3600B, Keysight Technologies, Inc., Santa Rosa, CA, USA), respectively. ANSYS HFSS was used to set various parameters for modeling the transmitter and the receiver coupling-wire loops to calculate the transmission efficiency, such as wire loops size, shape, quantity factor of coils, the line width of wires, self-inductance, mutual inductance, coupling coefficient, and magnetic flux magnitude. CST STUDIO SUITE® was adopted for designing the resonate dual-loop method for enhancing the WPT distance. PathWave ADS was used to design the matching network of WPT and also calculate the power attenuation and efficiency of the proposed dual-loop design.
The efficiency attenuation between the wireless power transmitter and the receiver was simulated through the following equation:
2.2. Neuron Stimulatory Electrode Design and Prototyping
To design a PRF neuron stimulatory electrode through microsurgery for implantation, we designed a flexible electrode, which could be wrapped in a guiding needle, penetrating the spinal dura, reaching and anchoring on soft tissue around the dorsal root ganglion which needed therapeutic stimuli. According to human anatomy, the dorsal root ganglion, with a size approximately 3–6 mm in diameter and 4–11 mm in length, is surrounded with soft tissues. The electrode would be delivered by a needle, which might not directly reach the target “dorsal root ganglion”. Therefore, we designed a protective cap on the tip of the electrode with expandable anchors. Distinct from conventional electrodes to release electricity on the tip and heat the tissues orthotopically, the PRF electrode impeded neural transmission through the specified electric signal generated from the microcontroller, which provided power by the wireless power transfer design in a different frequency. Therefore, the electrode wire could be designed on the anchors. For prototyping, we selected polyimide as the base material to coat metal electrodes for biocompatibility. A 250 × 6 × 1 mm supporting glass plate substrate was marked by mechanical engraving. Subsequently, an about 250 μm-thick layer of polyimide BPDA/PDA (derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA) and p-phenylendiamine (PDA)) was applied onto the front side of the glass plate as a liquid precursor by spin coating and cured to polyimide (PI 2611, HD MicroSystems, Parlin, NJ, USA). A 1 μm layer of titanium was applied on the polyimide layer and a 5 μm layer of platinum was applied on the titanium layer by magnetron sputtering sequentially, followed by a final 1 μm titanium layer, yielding a titanium/platinum/titanium thin film stack.
Subsequently, a 10 μm layer of positive photoresist (AZ 1512, Microchemicals GmbH, D-89079 Ulm, Germany) was applied on the titanium layer. The photoresist layer was irradiated by UV light through a mask whereby a wire pattern was created, as shown in
Figure 1D. The irradiated areas of the photoresist layer were removed by a developer solution (AZ 300-MIF, Microchemicals GmbH, D-89079 Ulm, Germany). The remaining areas of the photoresist layer selectively masked the areas where electrode wires were obtained in the further process. In the etching process, the titanium layer was removed by wet etch, 50:1 dilute hydrofluoric acid (HF) (Ashland Chemical, Wilmington, DE, USA), in the exposed areas. The platinum layer was removed by deionized (DI) water:HCl:HNO
3 in 1:3:1 (
v/
v), in the exposed area. Then, the residual photoresist layer was removed with a liquid immersion, with 20 sec in acetone and 20 sec in isopropanol. The areas where titanium/platinum was removed exposed polyimide as the surface layer. The base polyimide surface layer was activated and partially removed by reactive ion etching (RIE) in all areas uncovered by electrode wires. The surface was treated in 100 mTorr, 85% O
2, 15% CF
4 for 2 min at 200 W and 20 °C.
The surface was subsequently treated by KOH-deimidization bath at 25 °C for 5 min with manual agitation of the carrier boat at least every 60 s, followed with cascade rinse bath for 60 s, in triplicate. Then, the surface was dried with filtered nitrogen, and further re-treated in an HCl-deimidization bath at 25 °C for 5 min with mild agitation, followed by an identical cascade rinse bath. Finally, an about 250 μm-thick top layer of a precursor solution was applied by spin coating and cured to polyimide (PI2611, HD MicroSystems, US), which was combined with base polyimide after curing.
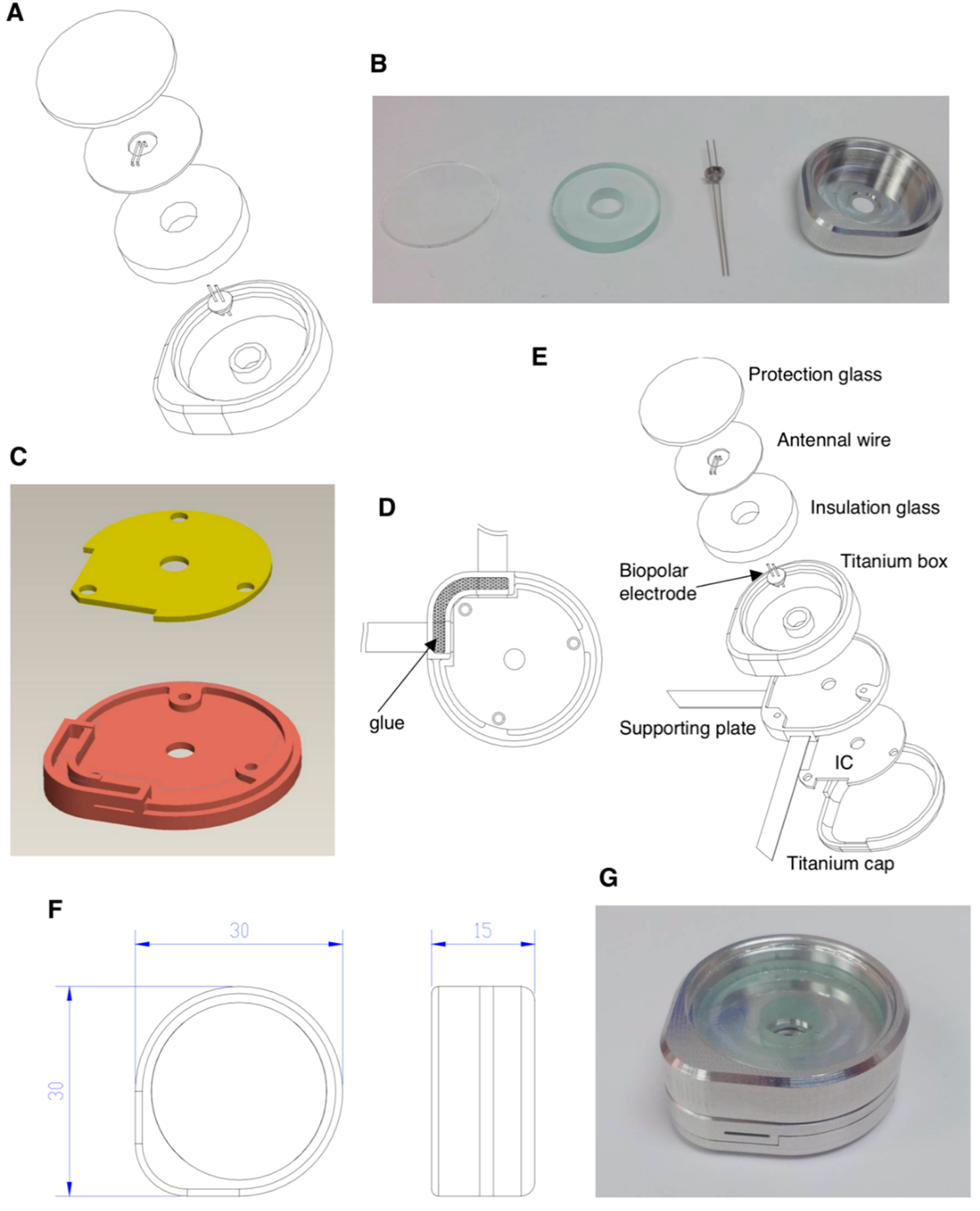
2.3. Protection Box Design for Wire Loops and Controller IC and Prototyping
To design a protection box to compartmentalize the receiver wire loops and controller IC from body fluid invasion after device implantation in vivo, reliable metal and insulation materials are crucial. We selected 6AL-4V ELI medical-grade titanium bulk as the crude material for protection box manufacturing and designed it as a round shape to match the physiological implantation. The prototype was outsourced to INTAI
® Inc., Co. (Taichung, Taiwan) for manufacturing, which sophisticatedly lathed under computer numerical control in compliance with our design, as shown in the Results section. A high-voltage vacuum capacitor amid the receiver wire loops and the passive components that surround the microcontroller integrated circuit (IC) was manufactured with titanium and ceramic composite (P/N: 1034101 Rev A, Greatbatch Medical, Minneapolis, MN, USA) for the consideration of biocompatibility with the shape of the trans-center of the anode and cathode to conduct electricity (
Figure S1). A medical-grade epoxy resin (LOCTITE
® M-21HP
TM, R. S. Hughes Company, Inc., Sunnyvale, CA, USA.) was used as the insulating and adhesive material for both glass and titanium box packaging. Another titanium box under the receiver wire loops was considered for the protection of the controller IC, which was designed as an irregular shape in compliance with the round shape of the protection box and fixed on the bottom titanium plate with three pieces of M2 screw. The flexible polyimide-based circuits were inserted into the bottom titanium plate to connect with the controller IC, the empty slot was filled with LOCTITE
® resin for a complete insulation, and overall, the titanium pieces were welded together to ensure complete packing.
4. Discussion
Electrical stimulation or heated energy was generally used to act on neurons in the spinal cord to block or interfere pain signaling to the brain. The pain signal transmission was generally prevented by the conventional continuous radiofrequency (CRF), which would generate a high temperature reaching 70–85 °C on the electrode tip to destroy the neural transmission and achieve pain relief [
14,
15,
16,
17].
Our PRF electrode designing concept is based on the spinal cord tissue structure, spread with many types of sensory neurons which are responsible for pain feeling transmission. The conventional therapy of administrating bulk and continuous stimulations on large areas of the spinal cord, including the dorsal root ganglion neurons, would be accompanied by significant side effects, such as paralysis, contraction, and a slightly uncomfortable feeling. Therefore, we aimed to design a tiny, stretchable and sophisticated neural stimulatory electrode possessing the capability to precisely fix adjacent to the dorsal root ganglion. Here, we explain the design concept and show the hands-on prototype.
Our first design of a wireless power transmission system possessed at least 80% transmission efficiency, providing very sufficient power for a general system-on-chip (SoC) to work in a common circumstance [
18] while working with a low power 0.5 W radiofrequency transmitter. The power generated from this wireless energy transmission produced at least 300 mW for neuron stimulation (
Figure 1), and fulfilled the requirement for the misalignment of receiver and transmitter (
Figure 2), making this wireless energy transmission design more robust and practical.
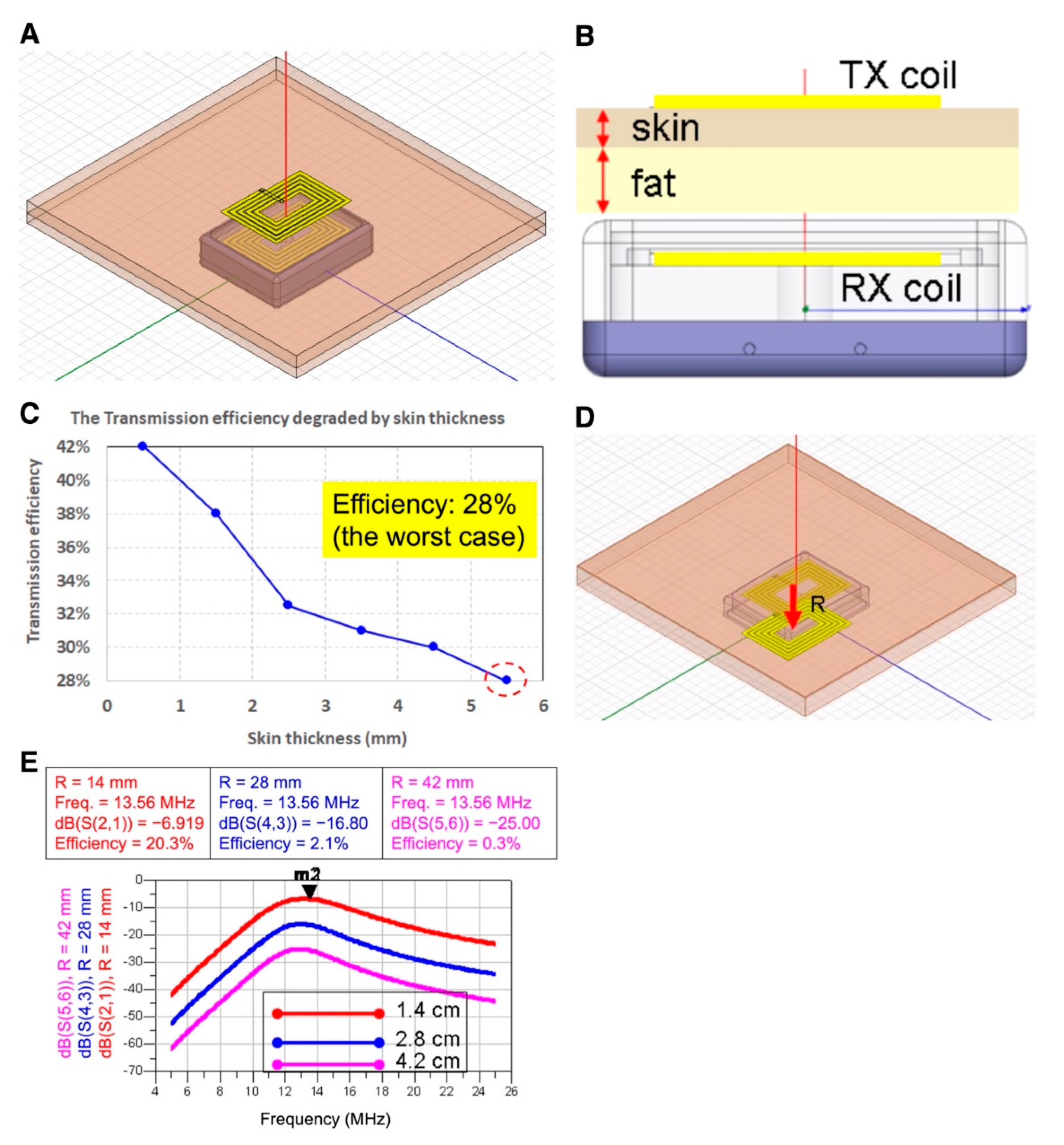
In terms of the wireless energy transmission efficiency influenced by skin thickness, simulation results indicated that overall energy transmission efficiency maintained >28% at 5.5 mm skin thickness setting, which is approximately equal to footpad skin thickness. While the transmitter was set at 13.56 MHz, 0.5 W, overall transmission efficiency could reach ≥100 mW, which was sufficient for the power supply for most of the neuron stimulatory SoC (
Figure 2). In the misalignment simulation of transmitter and receiver wire loops, the overall transmission efficiency decreased to 9.9 mW in the circumstance of 0.5 W transmitter power, and 28 mm misalignment in both wire loop centers, approaching the minimum requirement of power supply for SoC in our other studies (data not shown). Therefore, the largest misalignment of receiver and transmitter should be set ≤28 mm for therapy.
Skin thickness apparently interferes with the transmission efficiency of the wireless power transfer. The conductivity of physiological tissue increased with the blood content in tissues, resulting in diminished wireless power transmission efficiency [
19]. Therefore, we intended to utilize a resonant wire loop design for the wireless power transfer and termed as the “second wire loop” design, which increased the transmission distance in the identical wire loop area. In the “first wire loop” design, when the distance between transmitter and receiver was 10 mm, the transmission efficiency could reach 80%. However, when the distance increased to 30 mm, the transmission efficiency dropped significantly to be 20%. However, in the resonant wire loop design (i.e., second wire loop design), the transmission efficiency of an identical wire loop area 4 × 4 cm as the first wire loop design could reach 97% in an operation frequency set to 13 MHz (
Figure 3).
The transmission efficiency of the resonant wire loop designed in an octahedral shape holds superior transmission efficiency than that of the rectangular shape. However, the angle of the octahedral wire loop would be very close to the inner wall of the receiver case manufactured in titanium, leading to significant energy loss on the receiver side. Therefore, we modified the wire loop design to a round shape in the second wire loop design, in which the radius of the receiver and transmitter wire loop was 12 mm and 15 mm [
20], respectively (
Figure 3). The overall transmission efficiency reached 30%, providing 120 mW power transfer which is sufficient for most SoC power requirements [
18,
21]. In this study, the development of wireless power transmission for implantable neuron electrode power supply has finished the fine-tuned wire loop design simulation and efficiency evaluation, while the resonant wire loop module design provided longer distance energy transmission and higher efficiency, reaching a maximum of 30 mm in the distance and 96% in efficiency, respectively. Therefore, by applying the wireless power transmission design to the field of integrated implantable medical devices, such as neuron stimulatory electrodes, pain administrable chips, or retinal prosthesis, the recipient could avoid the drawbacks of repeated devices recharging [
22].
The physical locations of pain sensory neurons are distinct between individual patients. Therefore, the neurosurgeons examine the exact sensory neuron location assisted by an in vitro monitor system and patients’ self-feeling, and decide the sites that need electrode stimuli. Due to the conventional electrode design without a fixing anchor on the electrode tip, the stimulus would affect a large area accompanied with significant inevitable side effects [
14,
15,
16,
17]. When the therapeutic effects diminished, doctors generally increased the stimulatory dose to compensate for the lower therapeutic efficacy, which would increase the risk of safety concerns. Therefore, this study aimed to design a PRF electrode possessing the capability to precisely administer the stimuli on the spinal cord ganglion neurons which need therapeutic stimuli (the overall architecture is illustrated in
Figure S3A).
The manufacturing features of the neuron stimulatory electrode must possess not only biocompatibility for the recipient, but also bending flexibility for surgeons to conduct micro-surgical implantation conveniently and reduce the consequent complications. The electrode materials are conventionally manufactured with silicon rubber or polydimethylsiloxane (PDMS) and coated with reliable metals such as gold, silver or platinum, to serve as conducting wires to fulfill the requirement for biocompatibility and flexibility.
When aiming for life-long implantation of the pain-administrable device in the human body, it is inevitable that a metallic box will be used to protect the controller IC, which administrates wire loops to receive energy wirelessly, meanwhile releasing energy to the electrode for neural stimulation. Besides, the metal shielded effect retards the wireless energy transfer, and the electrode should be placed adjacent to the ganglion which needs stimulation. Both antenna wire loops and electrodes should be installed and connected to the outer surface of the metallic protection box (the implantation scenario is illustrated in
Figure S4B). Therefore, reliable metallic material and excellent insulation, such as titanium and silicone medical adhesive, all fulfilling the requirement of biocompatibility should be considered (
Figure 5). In terms of experimental prototyping, we selected the resonant coupling architecture design as illustrated (
Figure 3G) for prototype manufacture. The prototype was composed of multi resonant wire loops and provided long-distance transmission efficiency, reaching 30 mm in maximum and ≥96% transmission efficiency, which was sufficient for lots of implanted medical applications.
To avoid the metallic box interfering with the energy transmission, the metallic box bottom should be separated from receiver wire loops ≥4 mm using thick glass plate protection (
Figure 5A), and top-covered with a thin glass plate. The energy received from antenna wire loops is transferred to the microcontroller IC through the high-voltage vacuum capacitor (
Figure S1) with a medical-grade titanium and ceramic composite materials manufactured anode and cathode, which provides biocompatibility. Besides, the insulation package is designed to protect the internal electronic chips of the implantable PRF neuron stimulatory electrode from water, body fluid, vapor, chemical invasion, and mechanical force loading; meanwhile, it also functions to prevent leaking out of any biohazard component in the electronic chips. The medical-grade epoxy resin is considered for the insulating and adhesive material for both glass and titanium box packaging, which is expected to provide a biocompatible water-proof capability. Furthermore, the design and material of the insulation package of an implantable medical device are crucial when considering the biocompatibility. Another critical consideration for the PRF neural electrode is the wireless power transmission and signal-controlled module, which receives the power and control signals transmitted by wire loop magnetic coupling constructed in the outside part to achieve the wireless energy transmission. Our study intended to finetune the design of the wire loop to optimize the transmission efficiency through simulation and prototype manufacturing. Collectively, our study designed an implantable PRF neuron stimulatory device with a titanium box package and excellent biocompatible glue to provide a water-proof capability.