Effect of Gas Annealing on the Electrical Properties of Ni/AlN/SiC
Abstract
1. Introduction
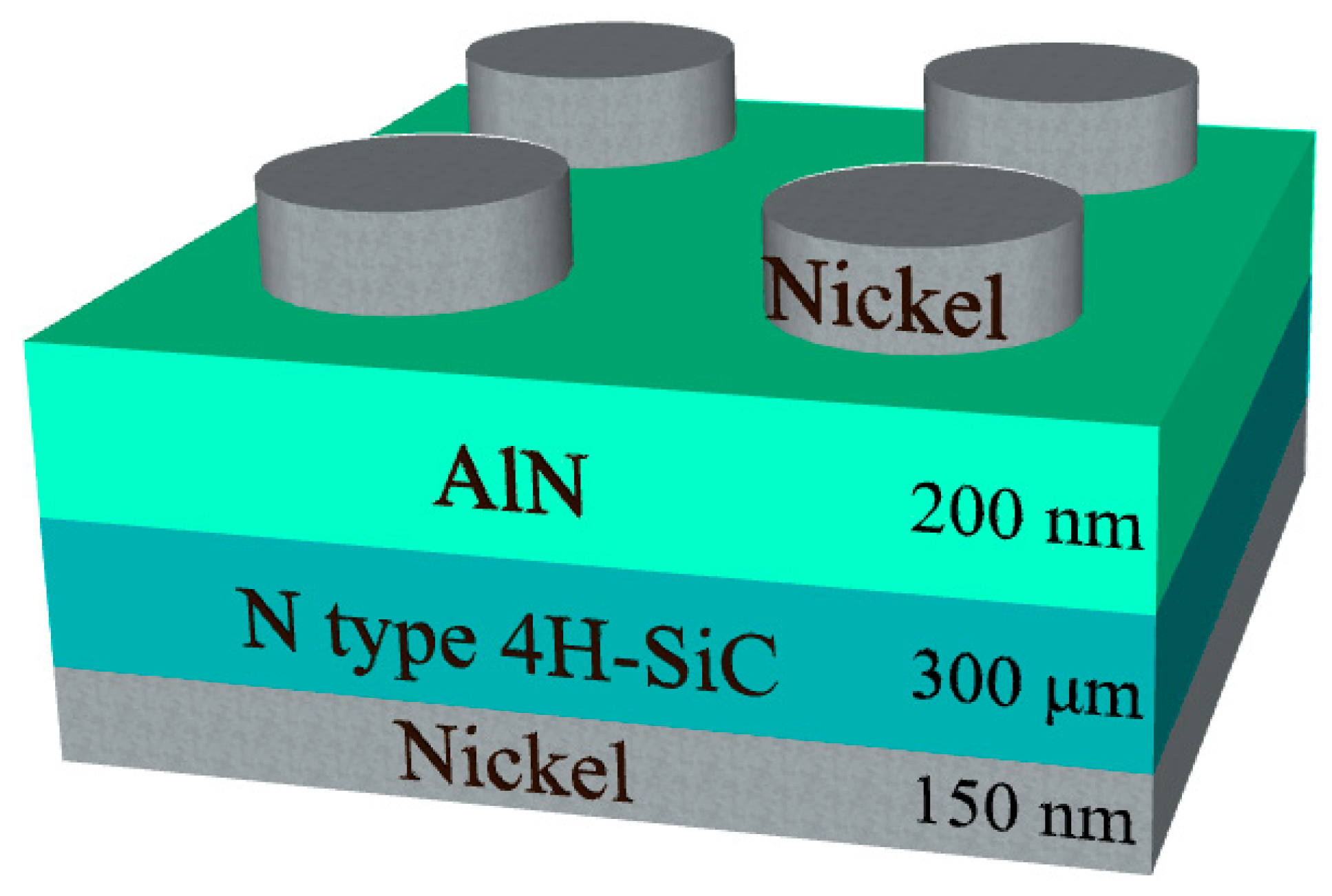
2. Materials and Methods
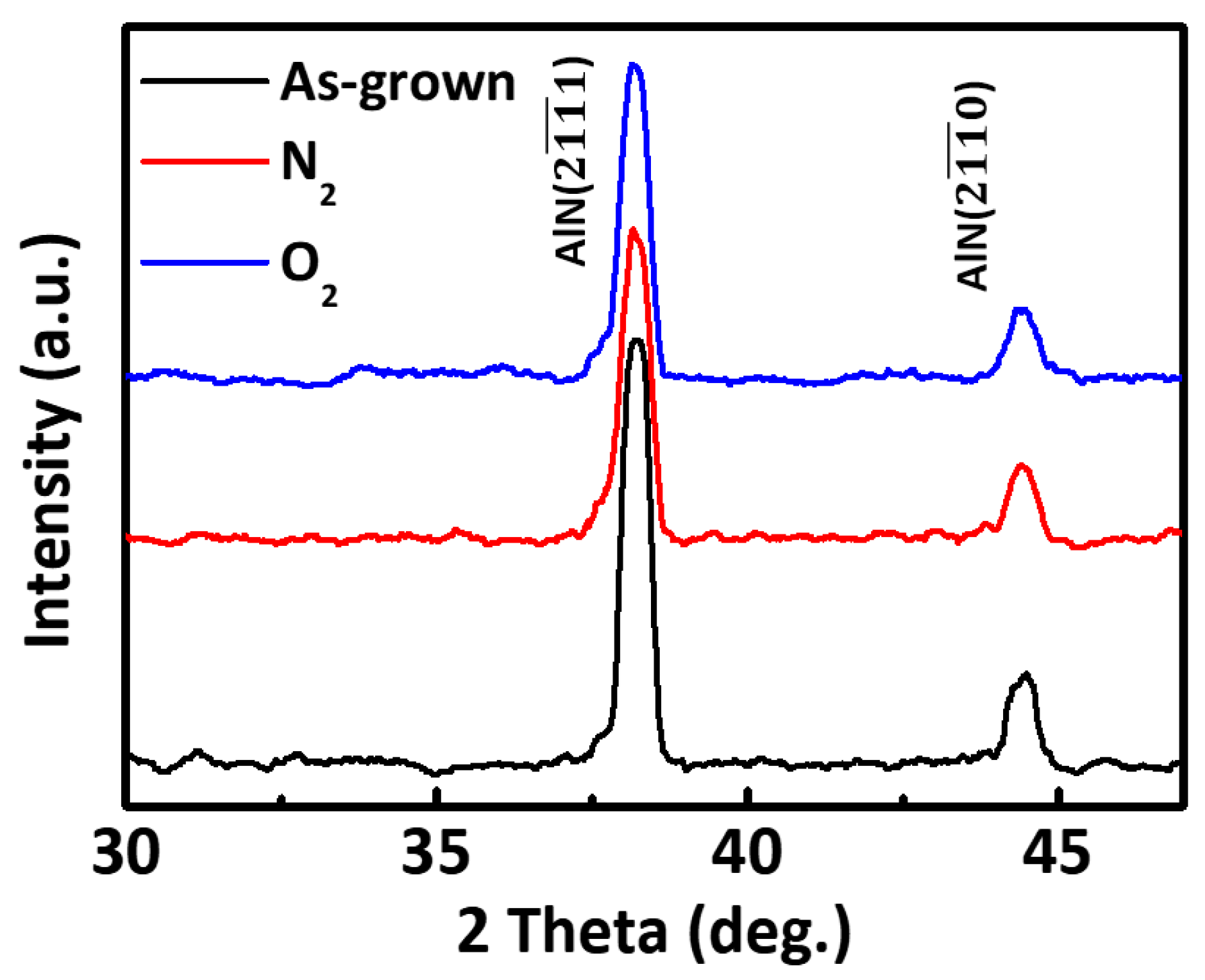
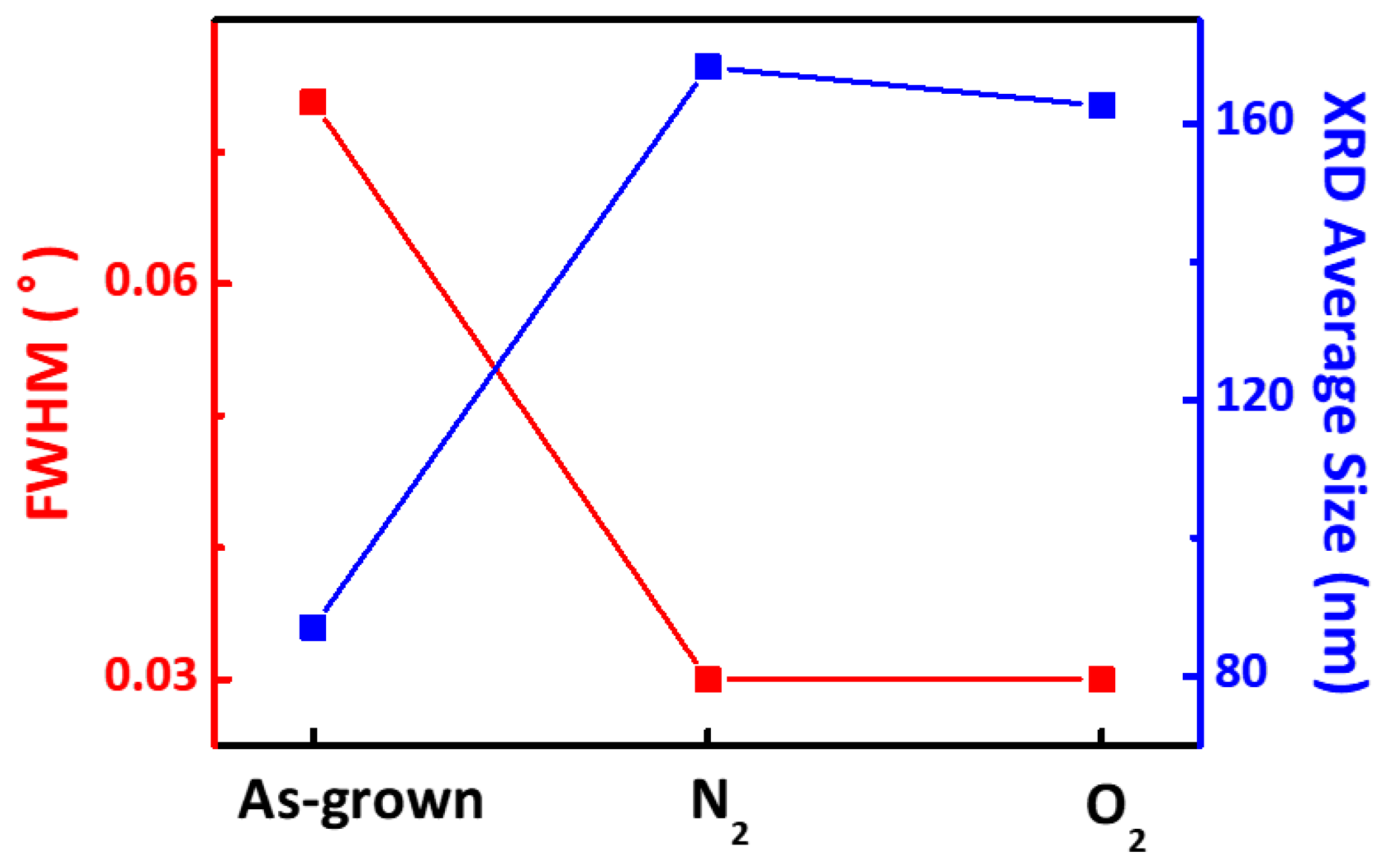
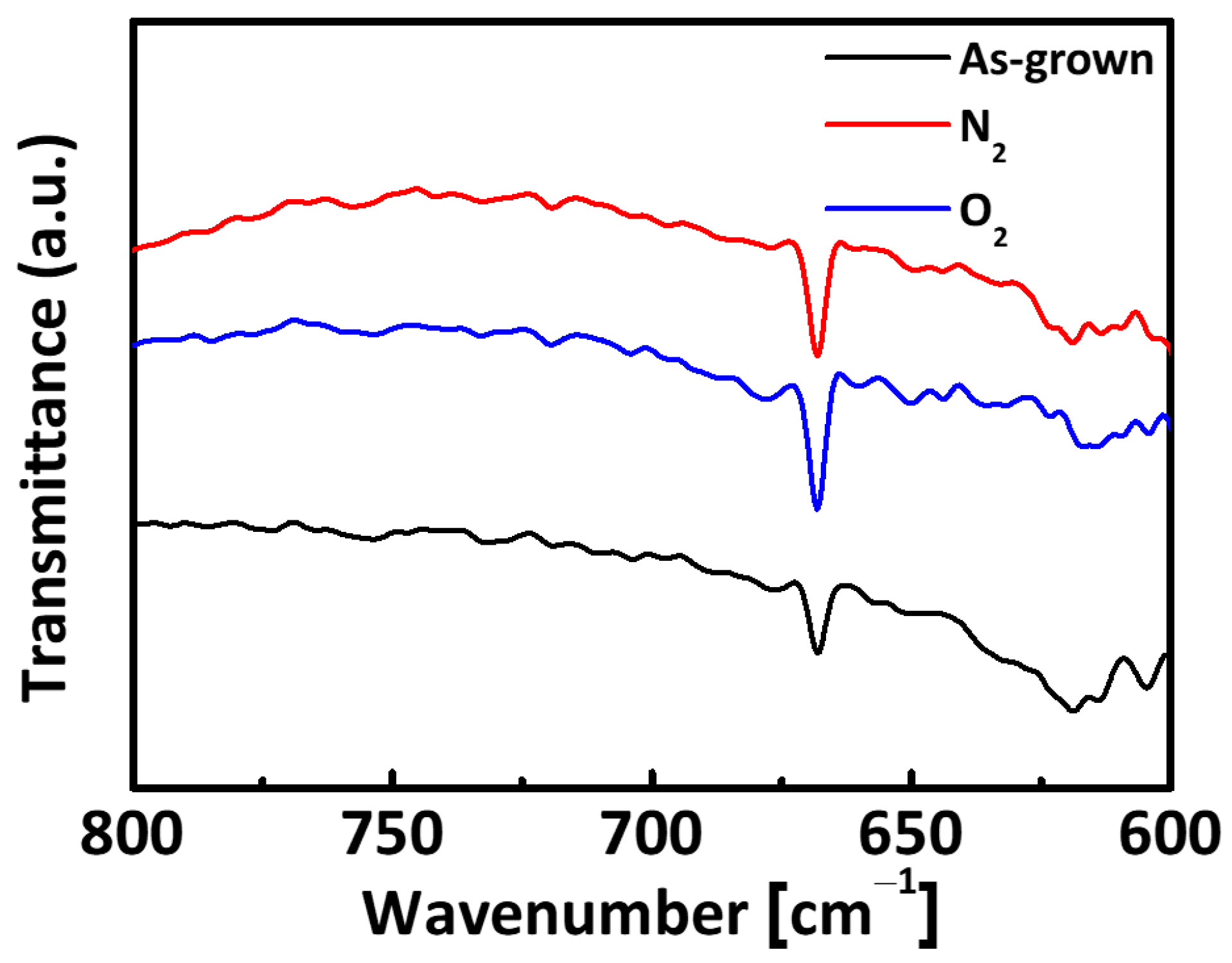
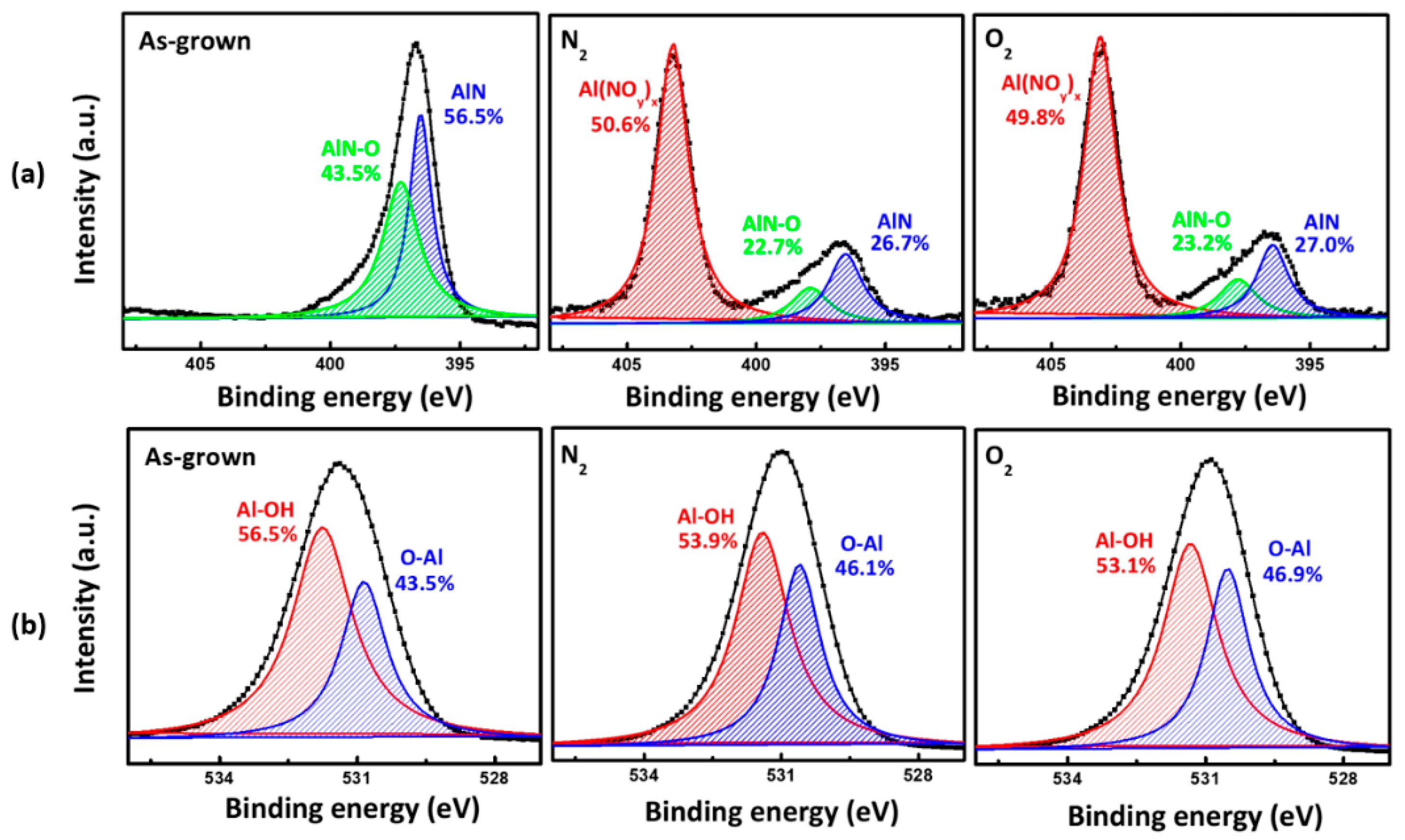
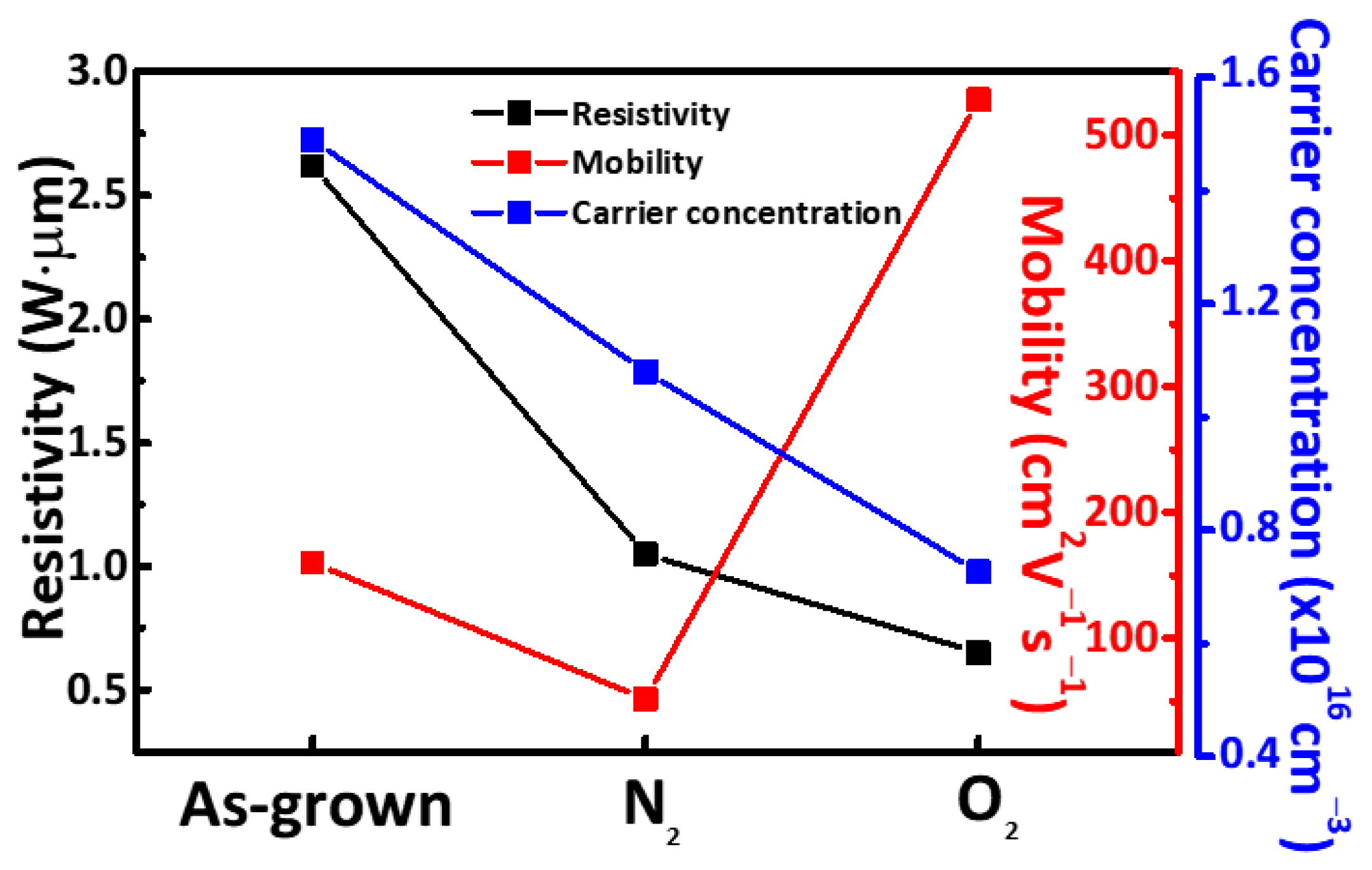
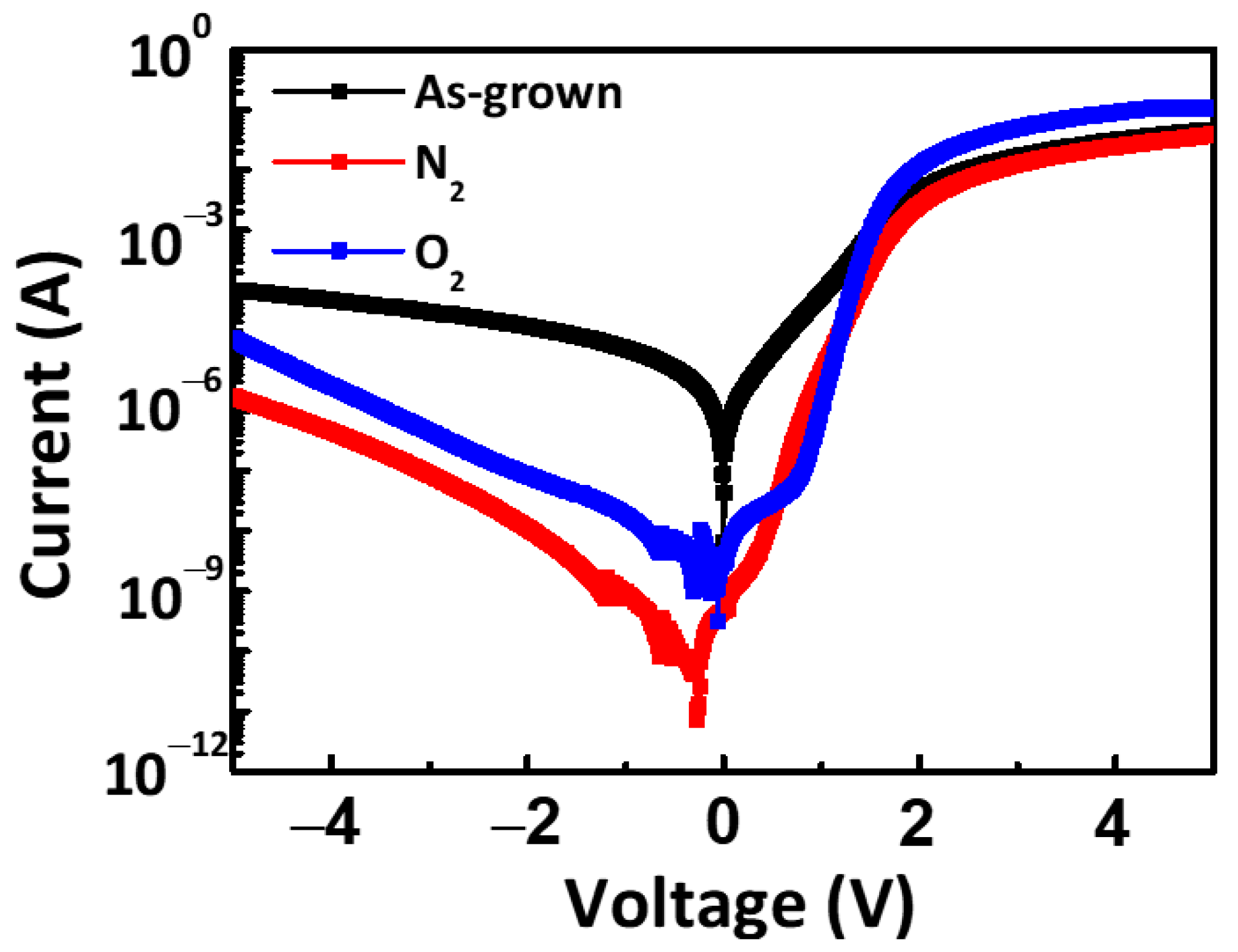
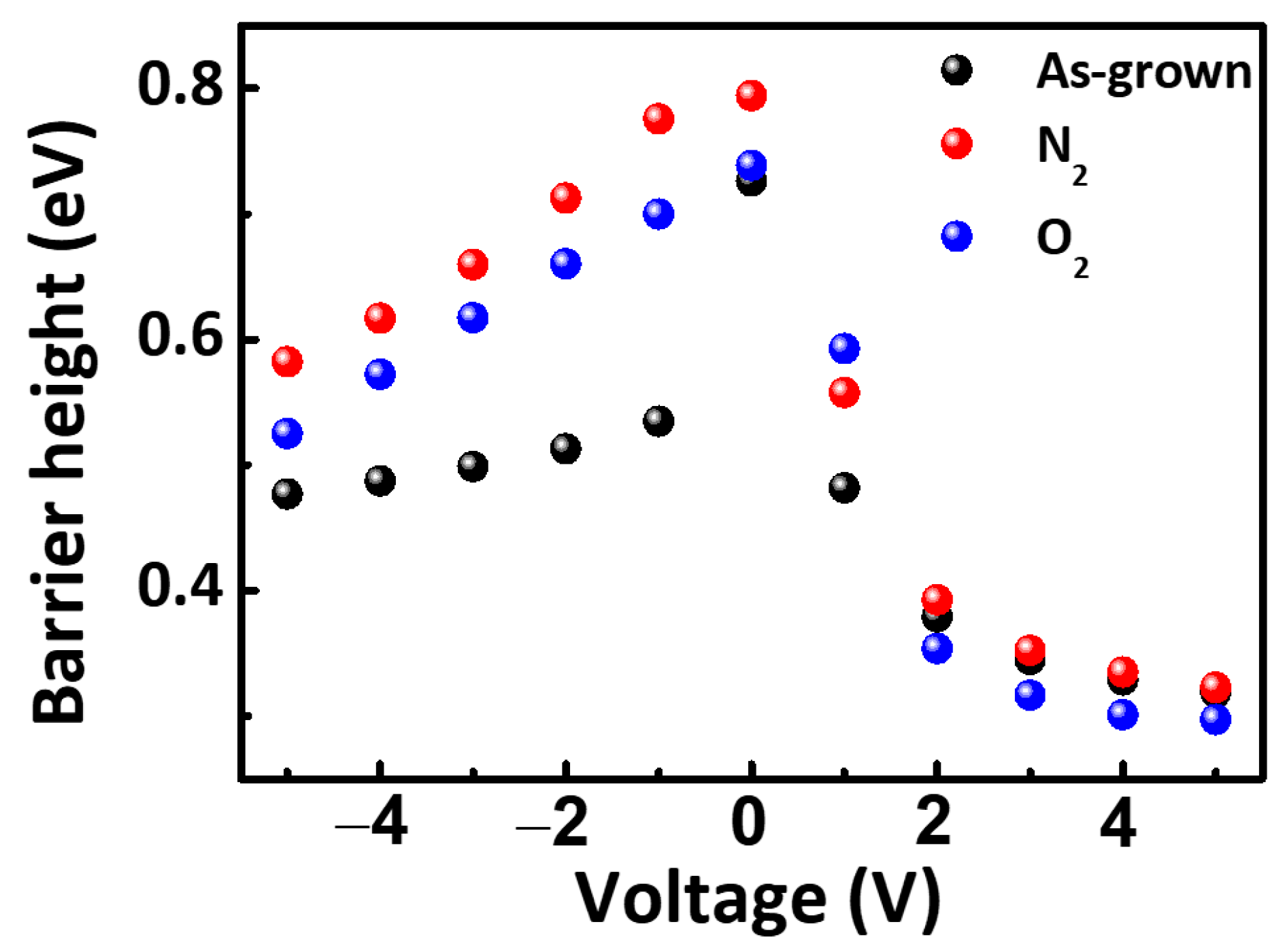
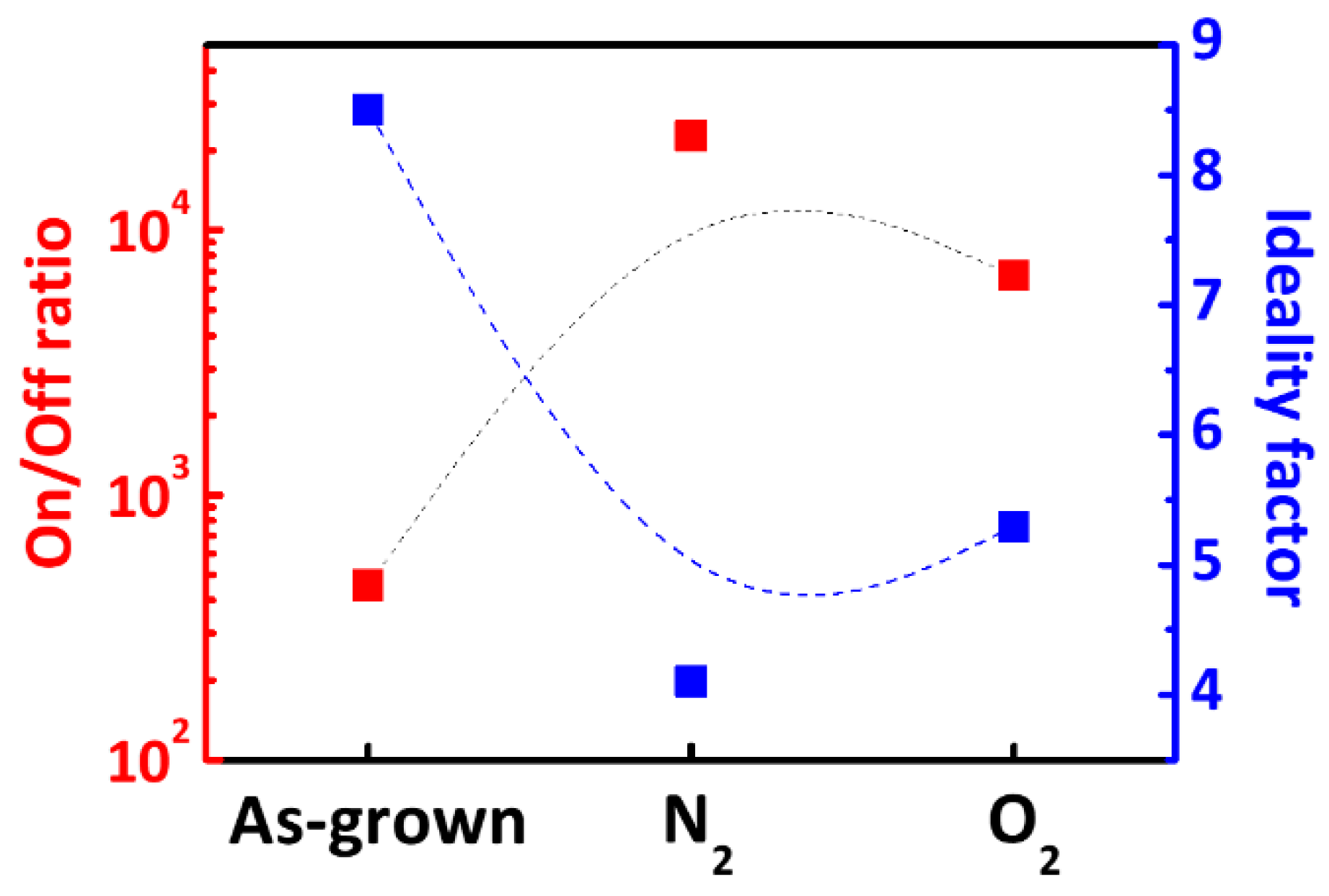
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irokawa, Y.; Víllora, E.A.G.; Shimamura, K. Shottky barrier diodes on AlN free-standing substrates. Jpn. J. Appl. Phys. 2012, 51, 040206. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nagashima, T.; Obata, T.; Takashima, S.; Yamamoto, R.; Togashi, R.; Sitar, Z. Fabrication of vertical Schottky barrier diodes on n-type freestanding AlN substrates grown by hydride vapor phase epitaxy. Appl. Phys. Express 2015, 8, 061003. [Google Scholar] [CrossRef]
- Fu, H.; Baranowski, I.; Huang, X.; Chen, H.; Lu, Z.; Montes, J.; Zhao, Y. Demonstration of AlN Schottky barrier diodes with blocking voltage over 1 kV. IEEE Electron Device Lett. 2017, 38, 1286–1289. [Google Scholar] [CrossRef]
- Roccaforte, F.; Fiorenza, P.; Greco, G.; Nigro, R.L.; Giannazzo, F.; Iucolano, F.; Saggio, M. Emerging trends in wide band gap semiconductors (SiC and GaN) technology for power devices. Microelectron. Eng. 2018, 187, 66–77. [Google Scholar] [CrossRef]
- O’leary, S.K.; Foutz, B.E.; Shur, M.S.; Eastman, L.F. Steady-state and transient electron transport within the III–V nitride semiconductors, GaN, AlN, and InN: A review. J. Mater. Sci. Mater. Electron. 2006, 17, 87–126. [Google Scholar] [CrossRef]
- Prabaswara, A.; Birch, J.; Junaid, M.; Serban, E.A.; Hultman, L.; Hsiao, C.L. Review of GaN Thin Film and Nanorod Growth Using Magnetron Sputter Epitaxy. Appl. Sci. 2020, 10, 3050. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Zhang, J.X.; Zhang, Y.B.; Yuan, S.; Hing, P. AlN films deposited under various nitrogen concentrations by RF reactive sputtering. J. Cryst. Growth 2003, 254, 46–54. [Google Scholar] [CrossRef]
- Núñez-Cascajero, A.; Valdueza-Felip, S.; Monteagudo-Lerma, L.; Monroy, E.; Taylor-Shaw, E.; Martin, R.W.; Naranjo, F.B. In-rich AlxIn1− xN grown by RF-sputtering on sapphire: From closely-packed columnar to high-surface quality compact layers. J. Phys. D Appl. Phys. 2017, 50, 065101. [Google Scholar] [CrossRef]
- Jiang, F.; Zheng, C.; Changda, W.; Fang, L.; Wenqing, P.; Yong, D.; Dai, J. The growth and properties of ZnO film on Si (111) substrate with an AlN buffer by AP-MOCVD. J. Lumin. 2007, 122, 905–907. [Google Scholar] [CrossRef]
- Xu, X.H.; Wu, H.S.; Zhang, C.J.; Jin, Z.H. Morphological properties of AlN piezoelectric thin films deposited by DC reactive magnetron sputtering. Thin Solid Films 2001, 388, 62–67. [Google Scholar] [CrossRef]
- Mattila, T.; Nieminen, R.M. Ab initio study of oxygen point defects in GaAs, GaN, and AlN. Phys. Rev. B 1996, 54, 16676. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Christiansen, S.H.; Moutanabbir, O.; Gösele, U. The phenomenology of ion implantation-induced blistering and thin-layer splitting in compound semiconductors. J. Electron. Mater. 2010, 39, 2177–2189. [Google Scholar] [CrossRef]
- Liu, H.Y.; Tang, G.S.; Zeng, F.; Pan, F. Influence of sputtering parameters on structures and residual stress of AlN films deposited by DC reactive magnetron sputtering at room temperature. J. Cryst. Growth 2013, 363, 80–85. [Google Scholar] [CrossRef]
- Ekpe, S.D.; Jimenez, F.J.; Dew, S.K. Effect of process conditions on the microstructural formation of dc reactively sputter deposited AlN. J. Vac. Sci. Technol. A Vac. Surf. Films 2010, 28, 1210–1214. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Som, S.; Yousif, A.; Singh, N.; Ntwaeaborwa, O.M.; Swart, H.C. Effect of annealing on the structural, morphological and photoluminescence properties of ZnO thin films prepared by spin coating. J. Colloid Interface Sci. 2014, 428, 8–15. [Google Scholar] [CrossRef]
- Fu, B.; Wang, F.; Cao, R.; Han, Y.; Miao, Y.; Feng, Y.; Zhang, K. Optimization of the annealing process and nanoscale piezoelectric properties of (002) AlN thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 9295–9300. [Google Scholar] [CrossRef]
- Kar, J.P.; Bose, G.; Tuli, S. Effect of annealing on DC sputtered aluminum nitride films. Surf. Coat. Technol. 2005, 198, 64–67. [Google Scholar] [CrossRef]
- Jung, S.W.; Shin, M.C.; Schweitz, M.A.; Oh, J.M.; Koo, S.M. Influence of Gas Annealing on Sensitivity of AlN/4H-Sic-Based Temperature Sensors. Materials 2021, 14, 683. [Google Scholar] [CrossRef]
- Fu, H.; Huang, X.; Chen, H.; Lu, Z.; Zhao, Y. Fabrication and characterization of ultra-wide bandgap AlN-based Schottky diodes on sapphire by MOCVD. IEEE J. Electron Devices Soc. 2017, 5, 518–524. [Google Scholar] [CrossRef]
- Jung, J.C.; Koo, S.M. The Effect of Catalytic Metal Work Functions and Interface States on the High Temperature SiC-based Gas Sensors. J. Korean Inst. Electr. Electron. Mater. Eng. 2011, 24, 280–284. [Google Scholar] [CrossRef]
- Clement, M.; Vergara, L.; Sangrador, J.; Iborra, E.; Sanz-Hervás, A. SAW characteristics of AlN films sputtered on silicon substrates. Ultrasonics 2004, 42, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Xu, F.J.; Xie, N.; Sun, Y.H.; Liu, B.Y.; Qin, Z.X.; Shen, B. Crystal quality evolution of AlN films via high-temperature annealing under ambient N2 conditions. CrystEngComm 2018, 20, 6613–6617. [Google Scholar] [CrossRef]
- Chen, F.H.; Hung, M.N.; Yang, J.F.; Kuo, S.Y.; Her, J.L.; Matsuda, Y.H.; Pan, T.M. Effect of surface roughness on electrical characteristics in amorphous InGaZnO thin-film transistors with high-κ Sm2O3 dielectrics. J. Phys. Chem. Solids 2013, 74, 570–574. [Google Scholar] [CrossRef]
- Hajakbari, F.; Hojabri, A.; Mojtahedzadeh Larijani, M. Effect of Thickness on Structural and Morphological Properties of AlN Films Prepared Using Single Ion Beam Sputtering. J. Appl. Chem. Res. 2014, 8, 45–52. [Google Scholar]
- Khan, S.; Shahid, M.; Mahmood, A.; Shah, A.; Ahmed, I.; Mehmood, M.; Alam, M. Texture of the nano-crystalline AlN thin films and the growth conditions in DC magnetron sputtering. Prog. Nat. Sci. Mater. Int. 2015, 25, 282–290. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 2009, 11, 8295–8305. [Google Scholar] [CrossRef]
- Rosenberger, L.; Baird, R.; McCullen, E.; Auner, G.; Shreve, G. XPS analysis of aluminum nitride films deposited by plasma source molecular beam epitaxy. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Films 2008, 40, 1254–1261. [Google Scholar] [CrossRef]
- Bourlier, Y.; Bouttemy, M.; Patard, O.; Gamarra, P.; Piotrowicz, S.; Vigneron, J.; Etcheberry, A. Investigation of InAlN Layers Surface Reactivity after Thermal Annealings: A Complete XPS Study for HEMT. ECS J. Solid State Sci. Technol. 2018, 7, P329. [Google Scholar] [CrossRef]
- Cao, D.; Cheng, X.; Xie, Y.H.; Zheng, L.; Wang, Z.; Yu, X.; Yu, Y. Effects of rapid thermal annealing on the properties of AlN films deposited by PEALD on AlGaN/GaN heterostructures. RSC Adv. 2015, 5, 37881–37886. [Google Scholar] [CrossRef]
- Raja, J.; Nguyen, C.P.T.; Lee, C.; Balaji, N.; Chatterjee, S.; Jang, K.; Yi, J. Improved data retention of InSnZnO nonvolatile memory by H2O2 treated Al2O3 tunneling layer: A cost-effective method. IEEE Electron Device Lett. 2016, 37, 1272–1275. [Google Scholar] [CrossRef]
- Taniyasu, Y.; Kasu, M.; Makimoto, T. Increased electron mobility in n-type Si-doped AlN by reducing dislocation density. Appl. Phys. Lett. 2006, 89, 182112. [Google Scholar] [CrossRef]
- Vladimirov, I.; Kühn, M.; Geßner, T.; May, F.; Weitz, R.T. Energy barriers at grain boundaries dominate charge carrier transport in an electron-conductive organic semiconductor. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, G.; Hajlaoui, M.E. Grain size dependent mobility in polycrystalline organic field-effect transistors. Synth. Met. 2001, 122, 185–189. [Google Scholar] [CrossRef]
- Bozack, M.J. Surface studies on SiC as related to contacts. Phys. Status Solidi 1997, 202, 549–580. [Google Scholar] [CrossRef]
- Han, S.M.; Takasawa, T.; Yasutomi, K.; Aoyama, S.; Kagawa, K.; Kawahito, S. A time-of-flight range image sensor with background canceling lock-in pixels based on lateral electric field charge modulation. IEEE J. Electron Devices Soc. 2014, 3, 267–275. [Google Scholar] [CrossRef]
- Cetin, H.; Ayyildiz, E.N.İ.S.E. On barrier height inhomogeneities of Au and Cu/n-InP Schottky contacts. Phys. B Condens. Matter 2010, 405, 559–563. [Google Scholar] [CrossRef]
- Bouzid, F.; Pezzimenti, F.; Dehimi, L.; Megherbi, M.L.; Della Corte, F.G. Numerical simulations of the electrical transport characteristics of a Pt/n-GaN Schottky diode. Jpn. J. Appl. Phys. 2017, 56, 094301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Schweitz, M.A.; Koo, S.-M. Effect of Gas Annealing on the Electrical Properties of Ni/AlN/SiC. Micromachines 2021, 12, 283. https://doi.org/10.3390/mi12030283
Kim D-H, Schweitz MA, Koo S-M. Effect of Gas Annealing on the Electrical Properties of Ni/AlN/SiC. Micromachines. 2021; 12(3):283. https://doi.org/10.3390/mi12030283
Chicago/Turabian StyleKim, Dong-Hyeon, Michael A. Schweitz, and Sang-Mo Koo. 2021. "Effect of Gas Annealing on the Electrical Properties of Ni/AlN/SiC" Micromachines 12, no. 3: 283. https://doi.org/10.3390/mi12030283
APA StyleKim, D.-H., Schweitz, M. A., & Koo, S.-M. (2021). Effect of Gas Annealing on the Electrical Properties of Ni/AlN/SiC. Micromachines, 12(3), 283. https://doi.org/10.3390/mi12030283




