Hemp-Based Microfluidics
Abstract
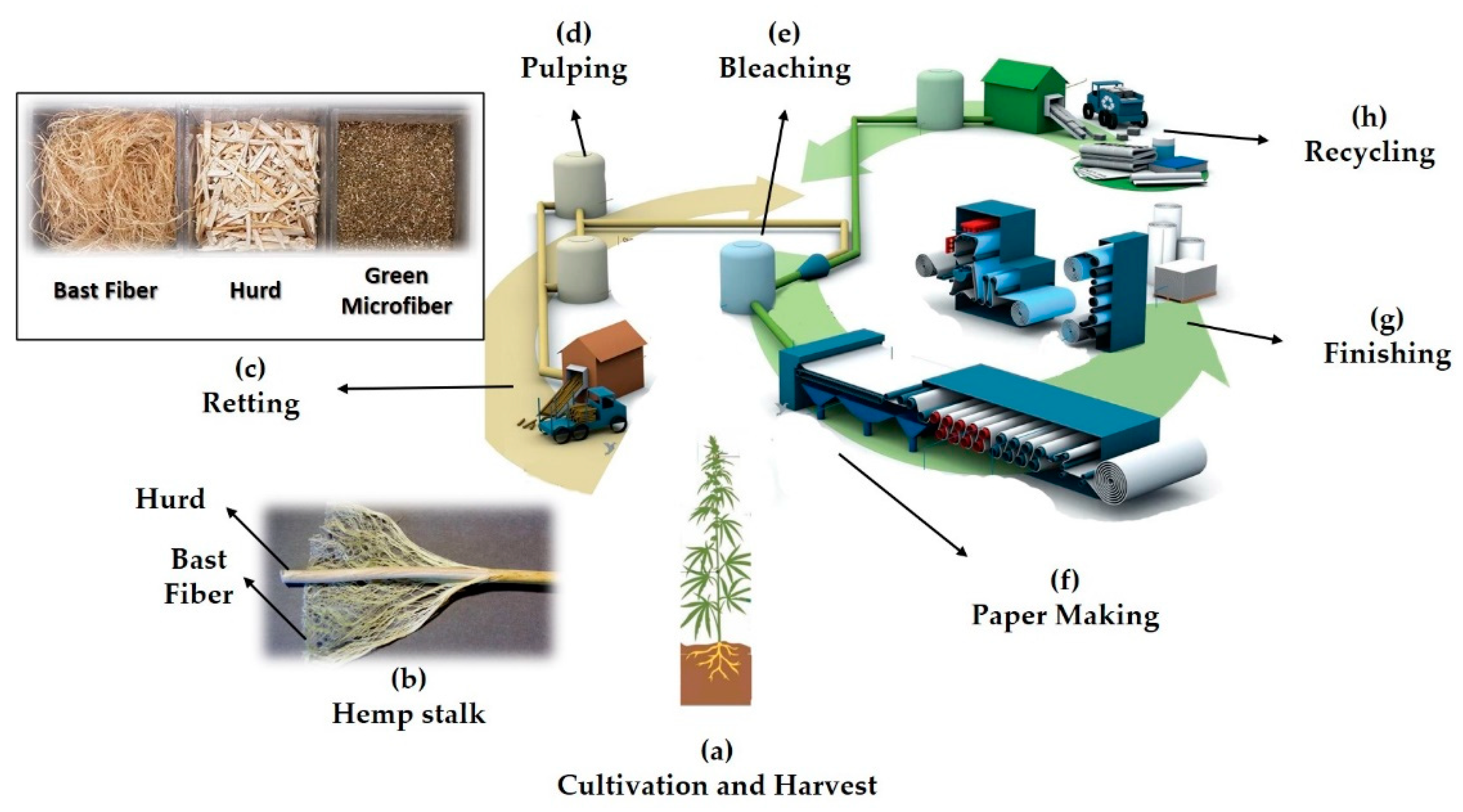
1. Introduction
2. Materials
3. Methods
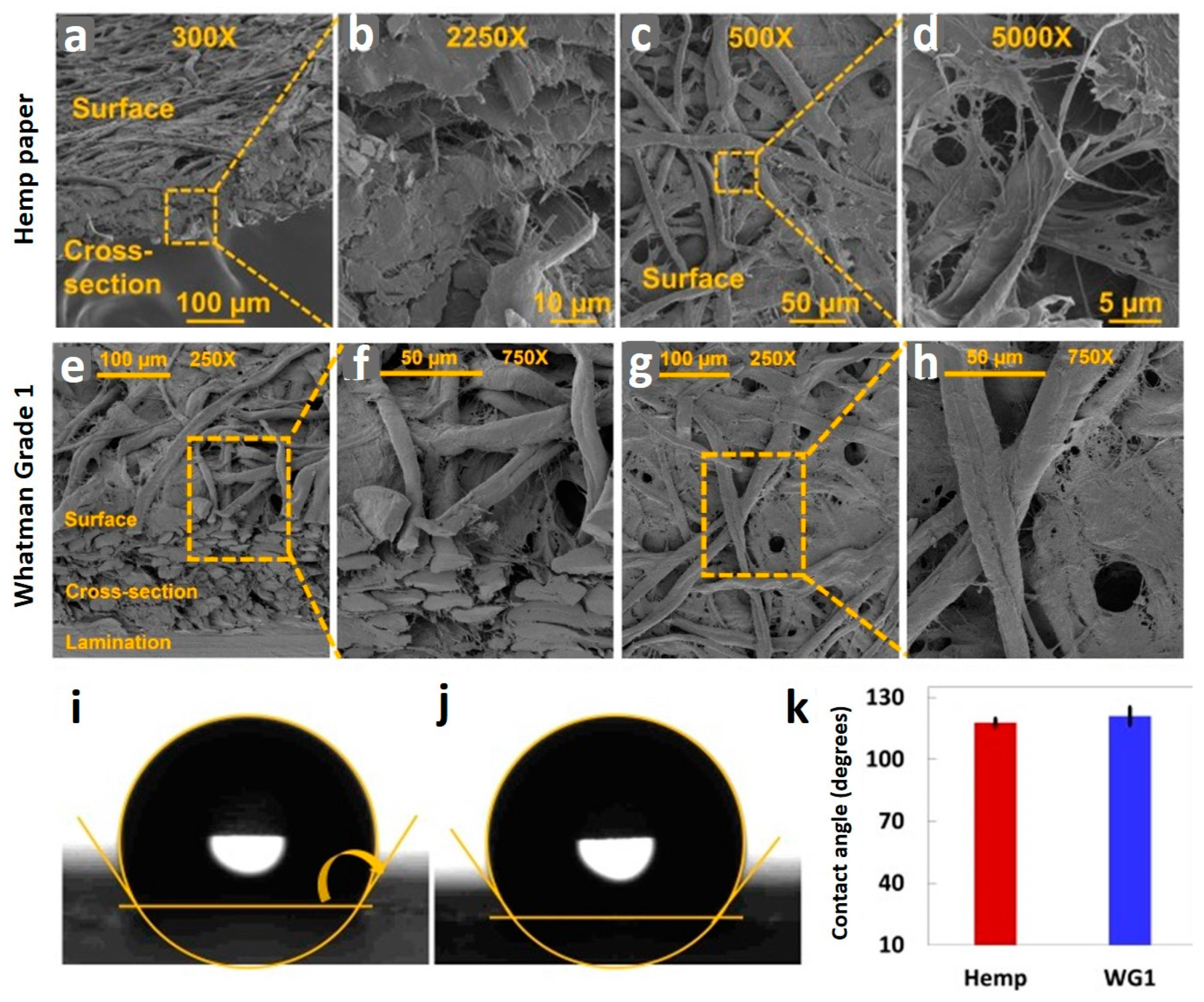
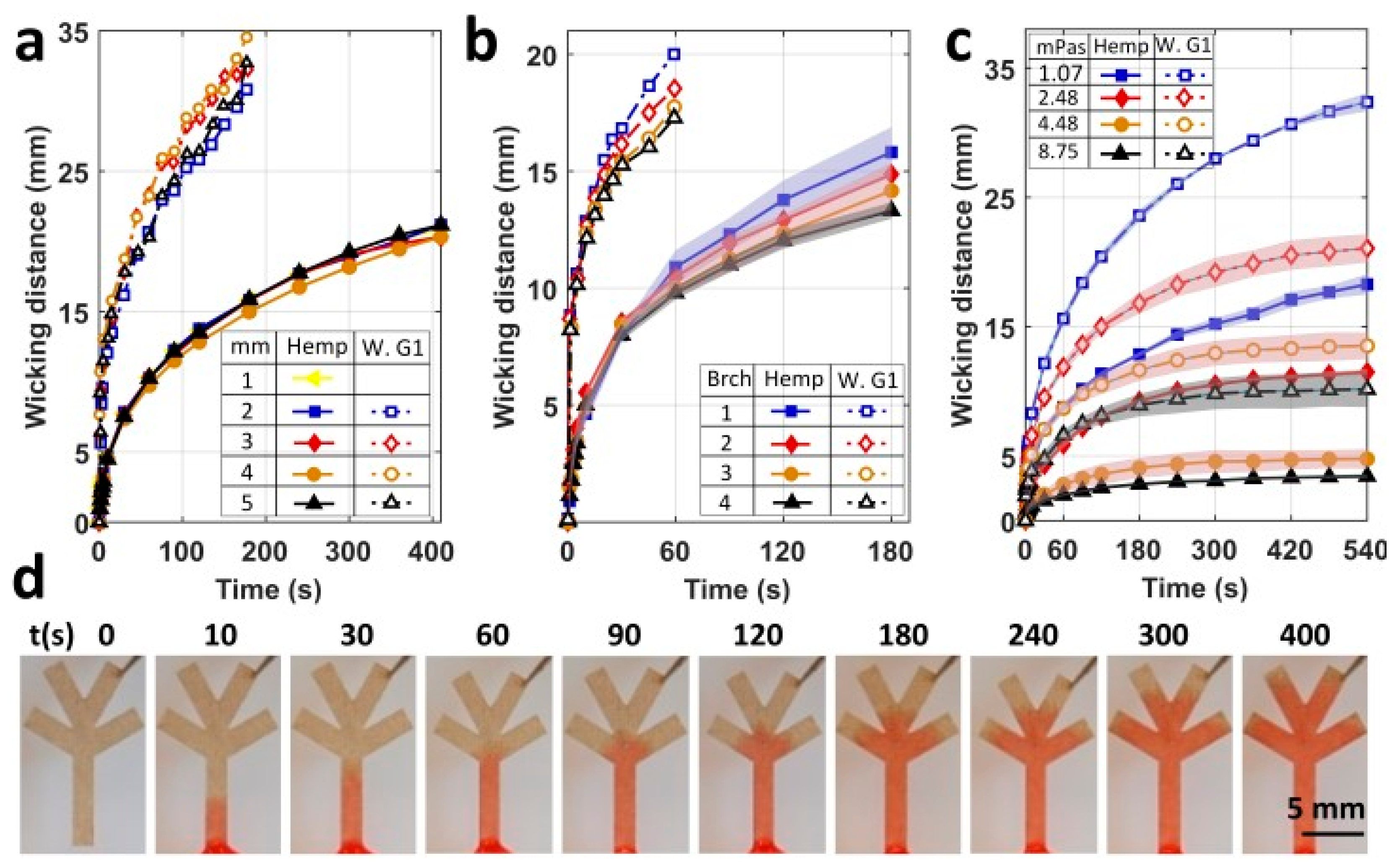
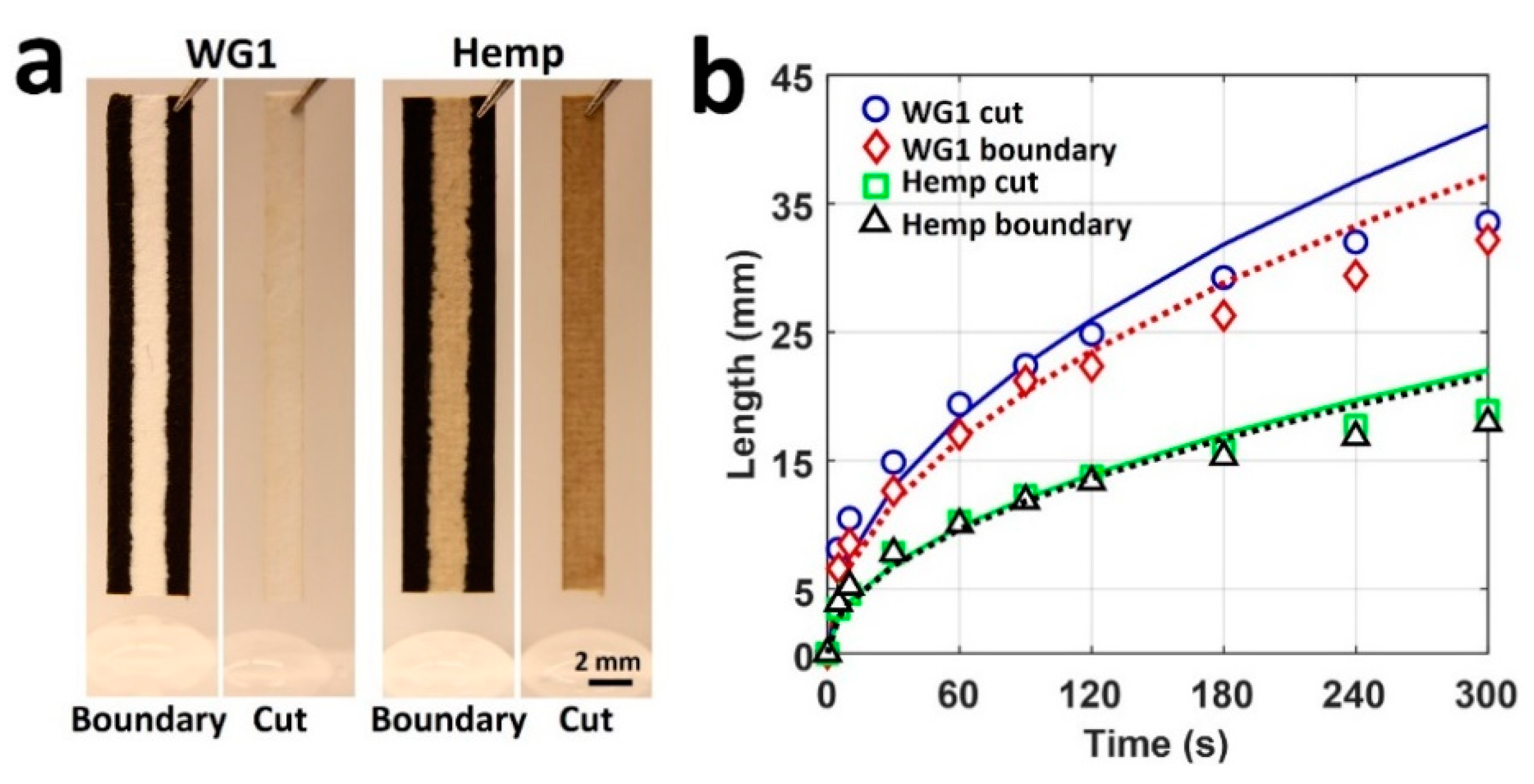
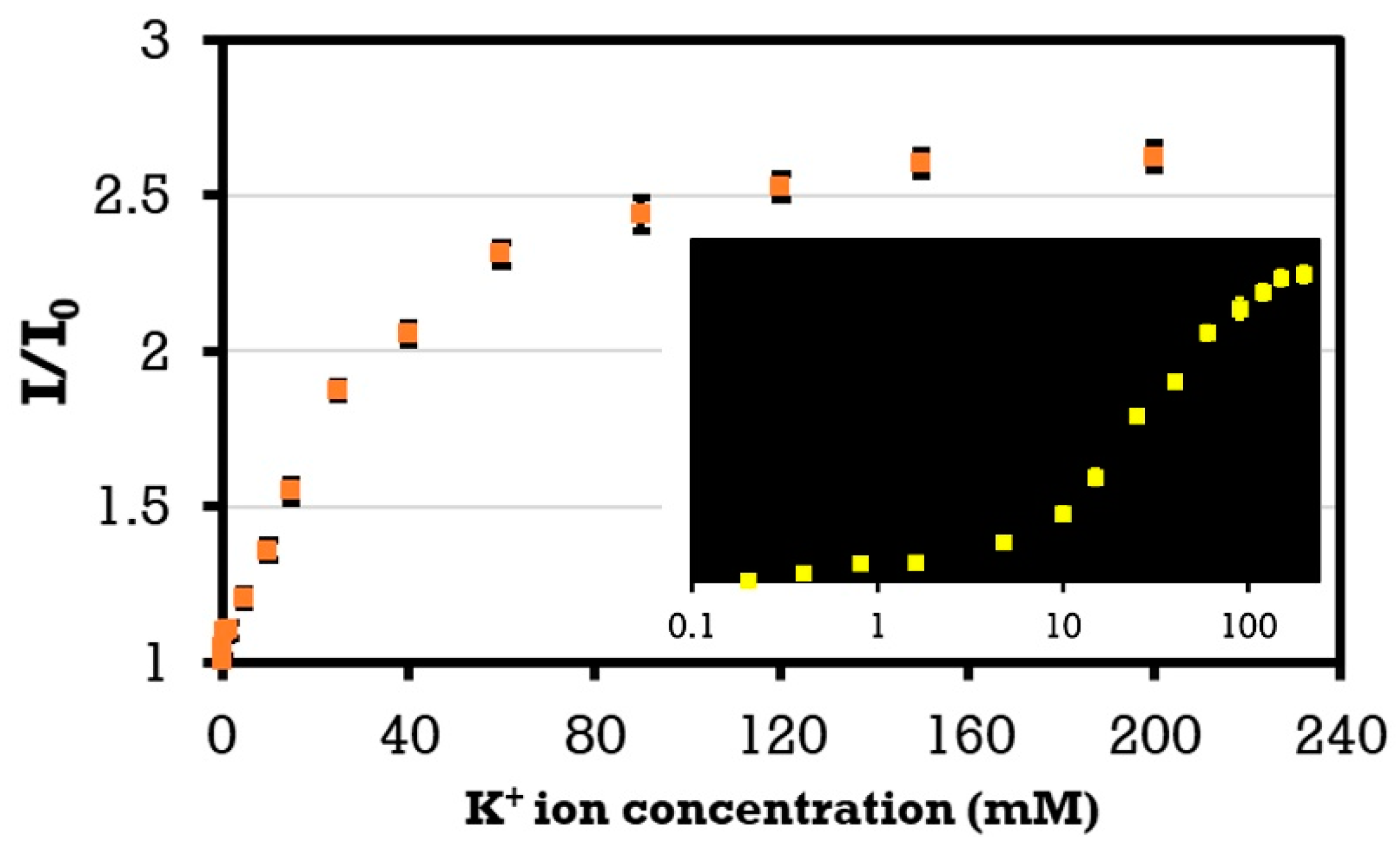
4. Results and Discussion
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oakleaf, J.R.; Kennedy, C.M.; Baruch-Mordo, S.; Gerber, J.S.; West, P.C.; Johnson, J.A.; Kiesecker, J. Mapping global development potential for renewable energy, fossil fuels, mining and agriculture sectors. Sci. Data 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Albouy, C.; Delattre, V.; Donati, G.; Frölicher, T.L.; Albouy-Boyer, S.; Rufino, M.; Pellissier, L.; Mouillot, D.; Leprieur, F. Global vulnerability of marine mammals to global warming. Sci. Rep. 2020, 10, 548. [Google Scholar] [CrossRef]
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Chang. 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.-G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin. J. Chem. Eng. 2019, 27, 1523–1535. [Google Scholar] [CrossRef]
- Kotcher, J.; Maibach, E.; Choi, W.-T. Fossil fuels are harming our brains: Identifying key messages about the health effects of air pollution from fossil fuels. BMC Public Health 2019, 19, 1079. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.; Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Cienc. 2020, 92, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.C.; Lyman, J.M. Warming trends increasingly dominate global ocean. Nat. Clim. Chang. 2020, 10, 757–761. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Herrgård, M.J.; Forster, J.; Hauschild, M.Z.; Fantke, P. Addressing environmental sustainability of biochemicals. Nat. Sustain. 2020, 3, 167–174. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Zegaoui, A.; Nizamani, A.A.; Kiran, S.; Wang, J.; Derradji, M.; Cai, W.-A.; Liu, W.-B. The influence of different chemical treatments on the hemp fiber/polybenzoxazine based green composites: Mechanical, thermal and water absorption properties. Mater. Chem. Phys. 2018, 217, 270–277. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Liu, W.; Chen, T.; Fei, M.-E.; Qiu, R.; Yu, D.; Fu, T.; Qiu, J. Properties of natural fiber-reinforced biobased thermoset biocomposites: Effects of fiber type and resin composition. Compos. Part B Eng. 2019, 171, 87–95. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Potential of using multiscale corn husk fiber as reinforcing filler in cornstarch-based biocomposites. Int. J. Biol. Macromol. 2019, 139, 596–604. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Ranaivoarisoa, T.O.; Singh, R.; Rengasamy, K.; Guzman, M.S.; Bose, A. Towards sustainable bioplastic production using the photoautotrophic bacterium Rhodopseudomonas palustris TIE-1. J. Ind. Microbiol. Biotechnol. 2019, 46, 1401–1417. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Schumacher, A.G.D.; Pequito, S.; Pazour, J. Industrial hemp fiber: A sustainable and economical alternative to cotton. J. Clean. Prod. 2020, 268, 122180. [Google Scholar] [CrossRef]
- Nguyen, S.; Tran-Le, A.; Vu, M.; To, Q.; Douzane, O.; Langlet, T. Modeling thermal conductivity of hemp insulation material: A multi-scale homogenization approach. Build. Environ. 2016, 107, 127–134. [Google Scholar] [CrossRef]
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2017, 3, 1–9. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Traditional and New Applications of Hemp. In Sustainable Agriculture Reviews 42; Springer: Cham, Switzerland, 2020; pp. 37–87. [Google Scholar]
- Jasinskas, A.; Streikus, D.; Vonžodas, T. Fibrous hemp (Felina 32, USO 31, Finola) and fibrous nettle processing and usage of pressed biofuel for energy purposes. Renew. Energy 2020, 149, 11–21. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Amode, N.S.; Jeetah, P. Paper Production from Mauritian Hemp Fibres. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, D.; Kong, Z.; He, L.; Guan, Q.; Ning, P. Strong Immobilization of Phosphate in Wastewater onto the Surface of MgO-Modified Industrial Hemp-Stem-Driven Biochar by Flowerlike Crystallization. Ind. Eng. Chem. Res. 2020, 59, 14578–14586. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Miriţoiu, C.M.; Stănescu, M.M.; Burada, C.O.; Bolcu, D.; Pădeanu, A.; Bolcu, A. Comparisons between some composite materials reinforced with hemp fibers. Mater. Today Proc. 2019, 12, 499–507. [Google Scholar] [CrossRef]
- Musio, S.; Müssig, J.; Amaducci, S. Optimizing hemp fiber production for high performance composite applications. Front. Plant Sci. 2018, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crop. Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Ingrao, C.; Giudice, A.L.; Bacenetti, J.; Tricase, C.; Dotelli, G.; Fiala, M.; Siracusa, V.; Mbohwa, C. Energy and environmental assessment of industrial hemp for building applications: A review. Renew. Sustain. Energy Rev. 2015, 51, 29–42. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites–A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Bouloc, P. Hemp: Industrial Production and Uses; CABI: London, UK, 2013. [Google Scholar]
- Malachowska, E.; Przybysz, P.; Dubowik, M.; Kucner, M.; Buzala, K. Comparison of papermaking potential of wood and hemp cellulose pulps. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2015, 91, 134–137. [Google Scholar]
- Rehman, M.S.U.; Rashid, N.; Saif, A.; Mahmood, T.; Han, J.-I. Potential of bioenergy production from industrial hemp (Cannabis sativa) Pakistan perspective. Renew. Sustain. Energy Rev. 2013, 18, 154–164. [Google Scholar] [CrossRef]
- Kaiser, C.; Cassady, C.; Ernst, M. Industrial hemp production. Cent. Crop. Diversif. Univ. Ky. Sept. 2015, 27, 101–106. [Google Scholar]
- Dupeyre, D.; Vignon, M. Fibres from semi-retted hemp bundles by steam explosion treatment. Biomass Bioenergy 1998, 14, 251–260. [Google Scholar]
- Lavrieux, M.; Jacob, J.; Disnar, J.-R.; Bréhéret, J.-G.; Le Milbeau, C.; Miras, Y.; Andrieu-Ponel, V. Sedimentary cannabinol tracks the history of hemp retting. Geology 2013, 41, 751–754. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Y.; Deng, J.; Rodrigue, D. Effect of fiber treatment on the water absorption and mechanical properties of hemp fiber/polyethylene composites. J. Appl. Polym. Sci. 2013, 127, 942–949. [Google Scholar] [CrossRef]
- Jami, T.; Karade, S.; Singh, L. A review of the properties of hemp concrete for green building applications. J. Clean. Prod. 2019, 239, 117852. [Google Scholar] [CrossRef]
- Grubeša, I.N.; Marković, B.; Gojević, A.; Brdarić, J. Effect of hemp fibers on fire resistance of concrete. Constr. Build. Mater. 2018, 184, 473–484. [Google Scholar] [CrossRef]
- Abdellatef, Y.; Kavgic, M. Thermal, microstructural and numerical analysis of hempcrete-microencapsulated phase change material composites. Appl. Therm. Eng. 2020, 178, 115520. [Google Scholar] [CrossRef]
- Demir, İ.; Doğan, C. Physical and Mechanical Properties of Hempcrete. Open Waste Manag. J. 2020, 13, 26–34. [Google Scholar] [CrossRef]
- Närep, M. Industrial Hemp Based Insulation Materials and Their Properties. Master’s Thesis, Tallinn University of Technology, Tallinn, Estonia, 2017. [Google Scholar]
- Mirski, R.; Boruszewski, P.; Trociński, A.; Dziurka, D. The possibility to use long fibres from fast growing hemp (Cannabis sativa L.) for the production of boards for the building and furniture industry. BioResources 2017, 12, 3521–3529. [Google Scholar] [CrossRef]
- Azeez, M.A. Pulping of non-woody biomass. Pulp Pap. Process. 2018, 55–86. [Google Scholar] [CrossRef]
- Bajpai, P. Basic overview of pulp and paper manufacturing process. In Green Chemistry and Sustainability in Pulp and Paper Industry; Springer: Cham, Switzerland, 2015; pp. 11–39. [Google Scholar]
- Sullins, T.; Pillay, S.; Komus, A.; Ning, H. Hemp fiber reinforced polypropylene composites: The effects of material treatments. Compos. Part B Eng. 2017, 114, 15–22. [Google Scholar] [CrossRef]
- Shahzad, A. Impact and fatigue properties of hemp–glass fiber hybrid biocomposites. J. Reinf. Plast. Compos. 2011, 30, 1389–1398. [Google Scholar] [CrossRef]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable thermal insulation biocomposites from rice husk, wheat husk, wood fibers and textile waste fibers: Elaboration and performances evaluation. Ind. Crop. Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Sain, M. Injection-molded short hemp fiber/glass fiber-reinforced polypropylene hybrid composites—Mechanical, water absorption and thermal properties. J. Appl. Polym. Sci. 2007, 103, 2432–2441. [Google Scholar] [CrossRef]
- Tasoglu, S.; Tekin, H.C.; Inci, F.; Knowlton, S.; Wang, S.; Wang-Johanning, F.; Johanning, G.; Colevas, D.; Demirci, U. Advances in nanotechnology and microfluidics for human papillomavirus diagnostics. Proc. IEEE 2015, 103, 161–178. [Google Scholar] [CrossRef]
- Knowlton, S.; Sencan, I.; Aytar, Y.; Khoory, J.; Heeney, M.; Ghiran, I.; Tasoglu, S. Sickle cell detection using a smartphone. Sci. Rep. 2015, 5, 15022. [Google Scholar] [CrossRef]
- Knowlton, S.; Joshi, A.; Syrrist, P.; Coskun, A.F.; Tasoglu, S. 3D-printed smartphone-based point of care tool for fluorescence-and magnetophoresis-based cytometry. Lab Chip 2017, 17, 2839–2851. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Jain, N.; Ghiran, I.C.; Tasoglu, S. Smart-phone based magnetic levitation for measuring densities. PLoS ONE 2015, 10, e0134400. [Google Scholar] [CrossRef] [PubMed]
- Yenilmez, B.; Knowlton, S.; Yu, C.H.; Heeney, M.M.; Tasoglu, S. Label-free sickle cell disease diagnosis using a low—cost, handheld platform. Adv. Mater. Technol. 2016, 1, 1600100. [Google Scholar] [CrossRef]
- Yenilmez, B.; Knowlton, S.; Tasoglu, S. Self-contained handheld magnetic platform for point of care cytometry in biological samples. Adv. Mater. Technol. 2016, 1, 1600144. [Google Scholar] [CrossRef]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics based point-of-care diagnostics. Biotechnol. J. 2018, 13, 1700047. [Google Scholar] [CrossRef]
- Lee, W.-B.; Fu, C.-Y.; Chang, W.-H.; You, H.-L.; Wang, C.-H.; Lee, M.S.; Lee, G.-B. A microfluidic device for antimicrobial susceptibility testing based on a broth dilution method. Biosens. Bioelectron. 2017, 87, 669–678. [Google Scholar] [CrossRef]
- Tasoglu, S.; Safaee, H.; Zhang, X.; Kingsley, J.L.; Catalano, P.N.; Gurkan, U.A.; Nureddin, A.; Kayaalp, E.; Anchan, R.M.; Maas, R.L. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small 2013, 9, 3374–3384. [Google Scholar] [CrossRef]
- Knowlton, S.; Tasoglu, S. A bioprinted liver-on-a-chip for drug screening applications. Trends Biotechnol. 2016, 34, 681–682. [Google Scholar] [CrossRef]
- Knowlton, S.M.; Sadasivam, M.; Tasoglu, S. Microfluidics for sperm research. Trends Biotechnol. 2015, 33, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Joshi, A.; Yenilmez, B.; Ozbolat, I.T.; Chua, C.K.; Khademhosseini, A.; Tasoglu, S. Advancing cancer research using bioprinting for tumor-on-a-chip platforms. Int. J. Bioprinting 2016, 2, 3–8. [Google Scholar] [CrossRef]
- Weng, X.; Neethirajan, S. Ensuring food safety: Quality monitoring using microfluidics. Trends Food Sci. Technol. 2017, 65, 10–22. [Google Scholar] [CrossRef]
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic lab-on-a-chip platforms for environmental monitoring. TrAC Trends Anal. Chem. 2017, 95, 62–68. [Google Scholar] [CrossRef]
- Ozdalgic, B.; Ustun, M.; Dabbagh, S.R.; Haznedaroglu, B.Z.; Kiraz, A.; Tasoglu, S. Microfluidics for Microalgal Biotechnology. Biotechnol. Bioeng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Panchal, J.H.; Kent, N.J.; Knox, A.J.; Harris, L.F. Microfluidics in haemostasis: A review. Molecules 2020, 25, 833. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Knowlton, S.; Yenilmez, B.; Hart, A.; Joshi, A.; Tasoglu, S. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Adv. 2016, 6, 93922–93931. [Google Scholar] [CrossRef]
- Wlodarczyk, K.L.; Carter, R.M.; Jahanbakhsh, A.; Lopes, A.A.; Mackenzie, M.D.; Maier, R.R.; Hand, D.P.; Maroto-Valer, M.M. Rapid laser manufacturing of microfluidic devices from glass substrates. Micromachines 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, J.; Mandenius, C.-F. Fabrication of a microfluidic cell culture device using photolithographic and soft lithographic techniques. In Cell-Based Assays Using iPSCs for Drug Development and Testing; Springer: New York, NY, USA, 2019; pp. 227–233. [Google Scholar]
- Luo, Z.; Güven, S.; Gozen, I.; Chen, P.; Tasoglu, S.; Anchan, R.M.; Bai, B.; Demirci, U. Deformation of a single mouse oocyte in a constricted microfluidic channel. Microfluid. Nanofluid. 2015, 19, 883–890. [Google Scholar] [CrossRef]
- Mészáros, B.; Járvás, G.; Hajba, L.; Szigeti, M.; Dallos, A.; Guttman, A. Quantitative characterization of plasma treated PDMS microfluidic substrates by inverse gas chromatography. Sens. Actuators B Chem. 2018, 258, 1184–1190. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.-Y.; Yang, C.; Restaino, M.; Lin, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-Printed Microneedles in Biomedical Applications. iScience 2020, 24, 102012. [Google Scholar] [CrossRef]
- Knowlton, S.; Yenilmez, B.; Tasoglu, S. Towards single-step biofabrication of organs on a chip via 3D printing. Trends Biotechnol. 2016, 34, 685–688. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016, 8, 025019. [Google Scholar] [CrossRef]
- Ghaderinezhad, F.; Amin, R.; Temirel, M.; Yenilmez, B.; Wentworth, A.; Tasoglu, S. High-throughput rapid-prototyping of low-cost paper-based microfluidics. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.I.; Kong, D.S.; Murthy, S.K.; Carr, P.A. Enabling Microfluidics: From Clean Rooms to Makerspaces. Trends Biotechnol. 2017, 35, 383–392. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef]
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Becher, E.; Ghaderinezhad, F.; Havlucu, H.; Ozcan, O.; Ozkan, M.; Yetisen, A.K.; Tasoglu, S. Increasing the Packing Density of Assays in Paper-Based Microfluidic Devices. Biomicrofluidics 2021, 15, 88–94. [Google Scholar] [CrossRef]
- Nilghaz, A.; Wicaksono, D.H.; Gustiono, D.; Majid, F.A.A.; Supriyanto, E.; Kadir, M.R.A. Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. Lab Chip 2012, 12, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, C. Low-cost, high-throughput fabrication of cloth-based microfluidic devices using a photolithographical patterning technique. Lab Chip 2015, 15, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Berthier, J.; Brakke, K.; Gosselin, D.; Berthier, E.; Navarro, F. Thread-based microfluidics: Flow patterns in homogeneous and heterogeneous microfiber bundles. Med. Eng. Phys. 2017, 48, 55–61. [Google Scholar] [CrossRef]
- Guan, W.; Liu, M.; Zhang, C. Electrochemiluminescence detection in microfluidic cloth-based analytical devices. Biosens. Bioelectron. 2016, 75, 247–253. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Hwang, K.-H.; Lee, J.S.; Boo, J.-H.; Yun, S.H. Plasma treatments of wool fiber surface for microfluidic applications. Mater. Res. Bull. 2015, 69, 65–70. [Google Scholar] [CrossRef]
- Amin, R.; Ghaderinezhad, F.; Li, L.; Lepowsky, E.; Yenilmez, B.; Knowlton, S.; Tasoglu, S. Continuous-ink, multiplexed pen-plotter approach for low-cost, high-throughput fabrication of paper-based microfluidics. Anal. Chem. 2017, 89, 6351–6357. [Google Scholar] [CrossRef]
- Hong, S.; Kim, W. Dynamics of water imbibition through paper channels with wax boundaries. Microfluid. Nanofluid. 2015, 19, 845–853. [Google Scholar] [CrossRef]
- Amin, R.; Ghaderinezhad, F.; Bridge, C.; Temirel, M.; Jones, S.; Toloueinia, P.; Tasoglu, S. Pushing the Limits of Spatial Assay Resolution for Paper-Based Microfluidics Using Low-Cost and High-Throughput Pen Plotter Approach. Micromachines 2020, 11, 611. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Telis-Romero, J.; Mazzotti, H.; Gabas, A.L. Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. Int. J. Food Prop. 2007, 10, 185–195. [Google Scholar] [CrossRef]
- Ghaderinezhad, F.; Koydemir, H.C.; Tseng, D.; Karinca, D.; Liang, K.; Ozcan, A.; Tasoglu, S. Sensing of electrolytes in urine using a miniaturized paper-based device. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Jiang, N.; Tamayol, A.; Ruiz-Esparza, G.U.; Zhang, Y.S.; Medina-Pando, S.; Gupta, A.; Wolffsohn, J.S.; Butt, H.; Khademhosseini, A. Paper-based microfluidic system for tear electrolyte analysis. Lab Chip 2017, 17, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, S.; Miao, C.; Li, L.; Shi, W.; Liu, X.; Luo, Y.; Liu, T.; Lin, B.; Wu, W. Paper microfluidics for cell analysis. Adv. Healthc. Mater. 2019, 8, 1801084. [Google Scholar] [CrossRef]
- Deiss, F.; Matochko, W.L.; Govindasamy, N.; Lin, E.Y.; Derda, R. Flow-through synthesis on teflon—patterned paper to produce peptide arrays for cell—based assays. Angew. Chem. 2014, 126, 6492–6495. [Google Scholar] [CrossRef]
- Hong, B.; Xue, P.; Wu, Y.; Bao, J.; Chuah, Y.J.; Kang, Y. A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed. Microdevices 2016, 18, 21. [Google Scholar] [CrossRef]
- Lei, K.F.; Chang, C.-H.; Chen, M.-J. Paper/PMMA hybrid 3D cell culture microfluidic platform for the study of cellular crosstalk. ACS Appl. Mater. Interfaces 2017, 9, 13092–13101. [Google Scholar] [CrossRef]
- Liu, J.; Qiang, Y.; Alvarez, O.; Du, E. Electrical impedance characterization of erythrocyte response to cyclic hypoxia in sickle cell disease. ACS Sens. 2019, 4, 1783–1790. [Google Scholar] [CrossRef]
- Jenkins, G.; Wang, Y.; Xie, Y.L.; Wu, Q.; Huang, W.; Wang, L.; Yang, X. Printed electronics integrated with paper-based microfluidics: New methodologies for next-generation health care. Microfluid. Nanofluid. 2015, 19, 251–261. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Ainla, A.; Güder, F.; Christodouleas, D.C.; Fernández-Abedul, M.T.; Whitesides, G.M. Integrating electronics and microfluidics on paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, S.R.; Rabbi, F.; Doğan, Z.; Yetisen, A.K.; Tasoglu, S. Machine learning-enabled multiplexed microfluidic sensors. Biomicrofluidics 2020, 14, 061506. [Google Scholar] [CrossRef] [PubMed]
- Social Revolution in Menstrual Hygiene for Women. Available online: https://www.himalayanhemp.in/hempsanitarypads (accessed on 12 January 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temirel, M.; Dabbagh, S.R.; Tasoglu, S. Hemp-Based Microfluidics. Micromachines 2021, 12, 182. https://doi.org/10.3390/mi12020182
Temirel M, Dabbagh SR, Tasoglu S. Hemp-Based Microfluidics. Micromachines. 2021; 12(2):182. https://doi.org/10.3390/mi12020182
Chicago/Turabian StyleTemirel, Mikail, Sajjad Rahmani Dabbagh, and Savas Tasoglu. 2021. "Hemp-Based Microfluidics" Micromachines 12, no. 2: 182. https://doi.org/10.3390/mi12020182
APA StyleTemirel, M., Dabbagh, S. R., & Tasoglu, S. (2021). Hemp-Based Microfluidics. Micromachines, 12(2), 182. https://doi.org/10.3390/mi12020182







