Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Spheroid Formation
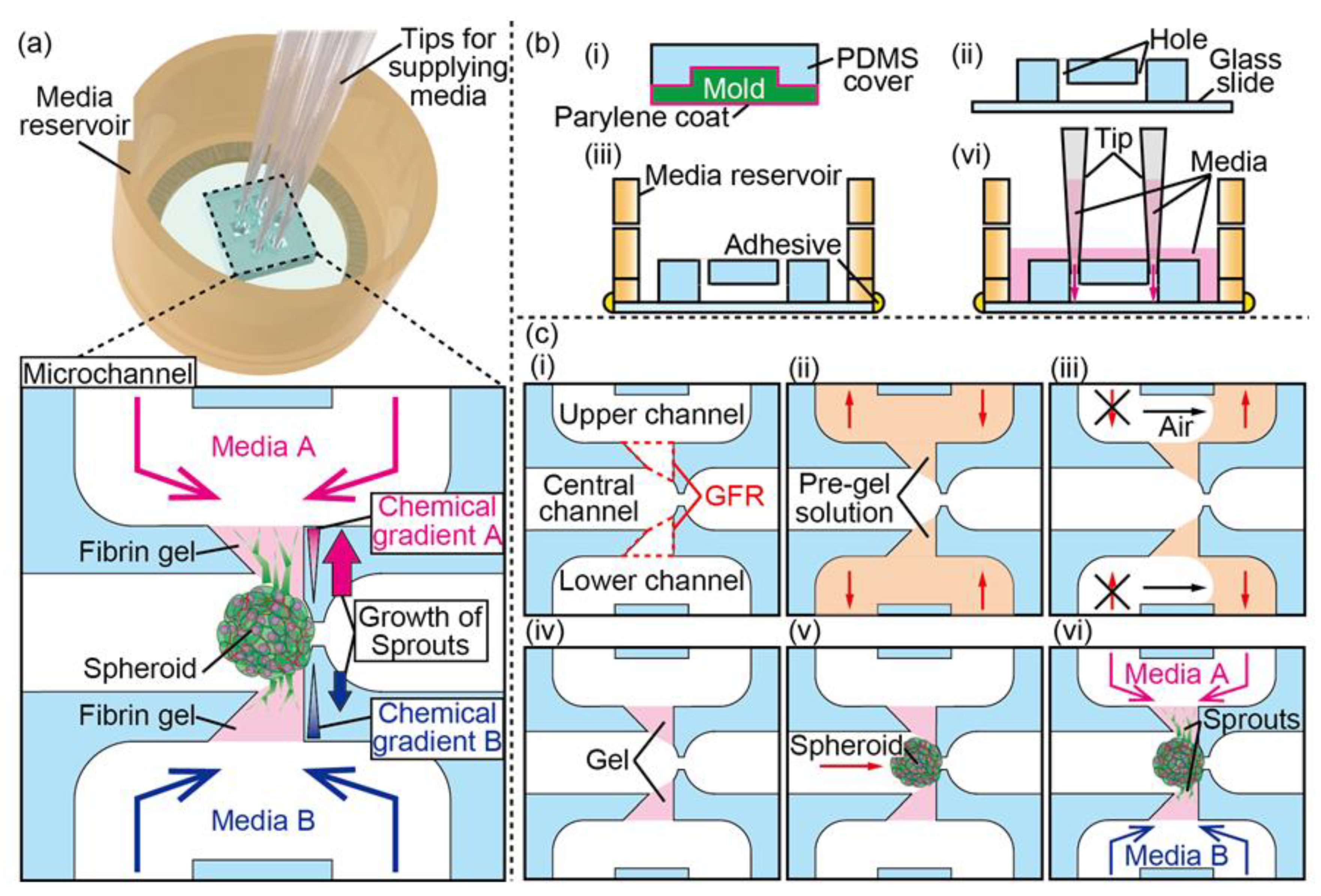
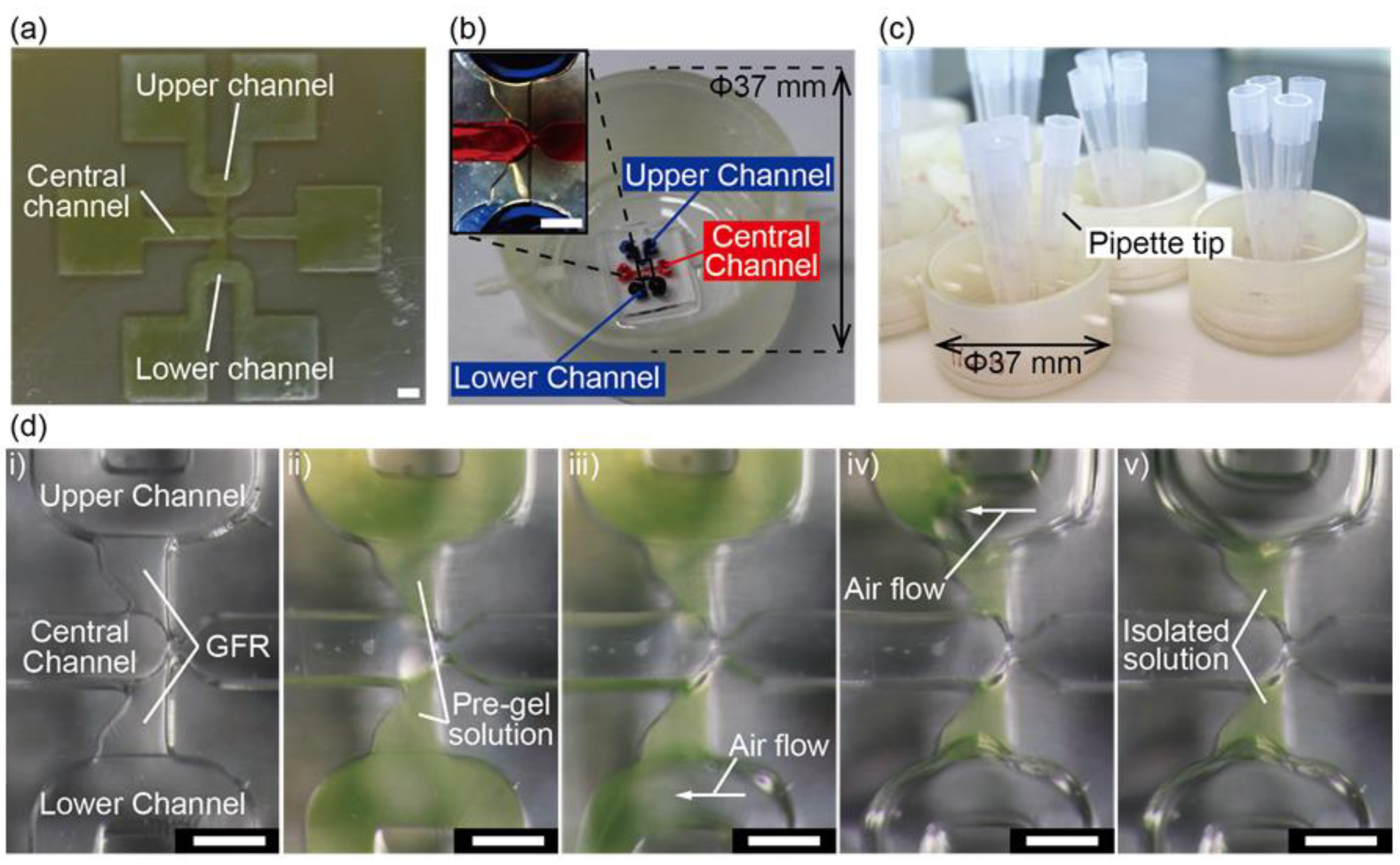
2.3. Device Fabrication
2.4. Gel Formation
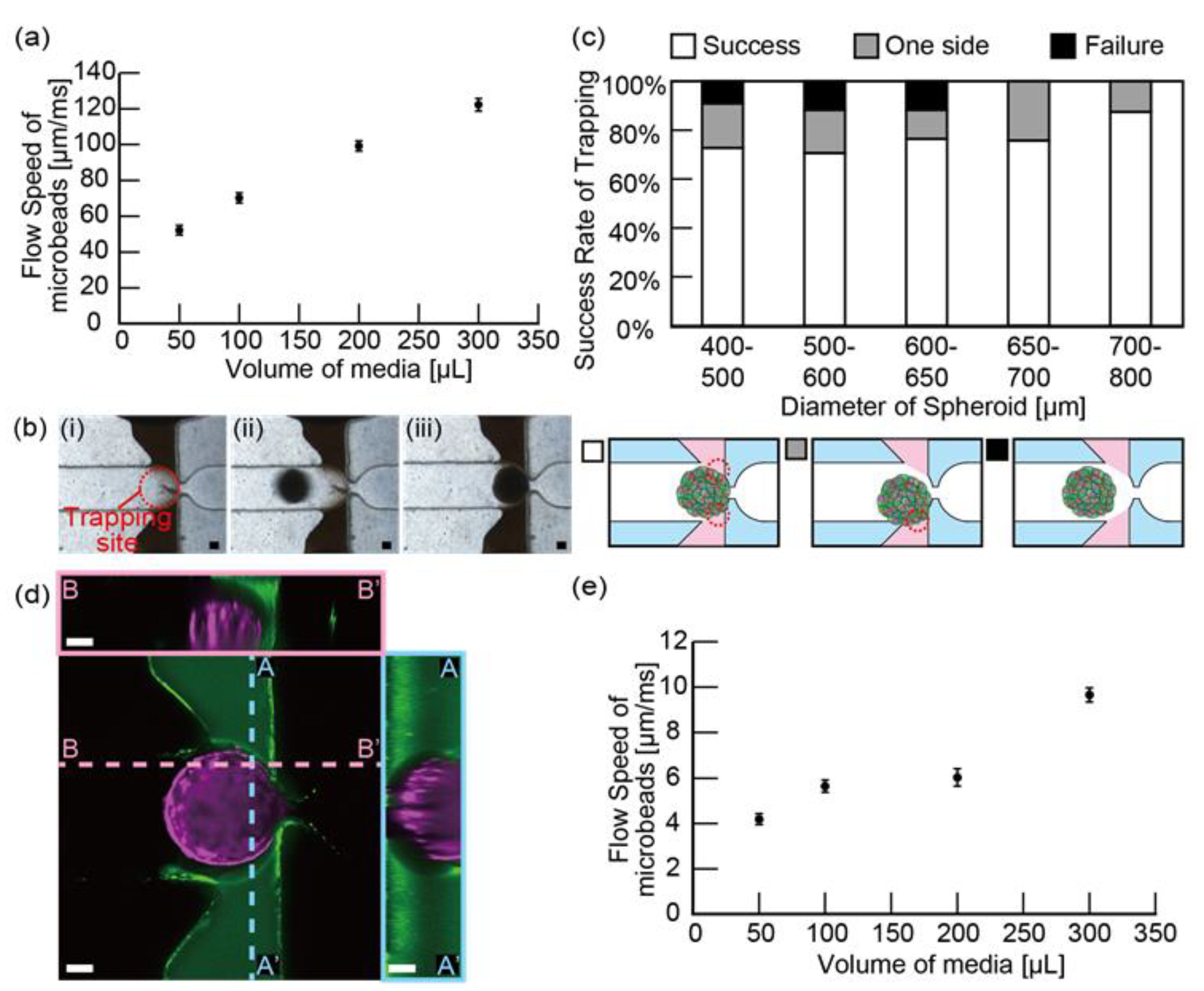
2.5. Spheroid Trapping
2.6. Spheroid Culture under Biochemical Gradient
2.7. Analysis of the Angiogenic Sprouting
3. Results and Discussion
3.1. Device Design and Fabrication
3.2. Spheroid Trapping
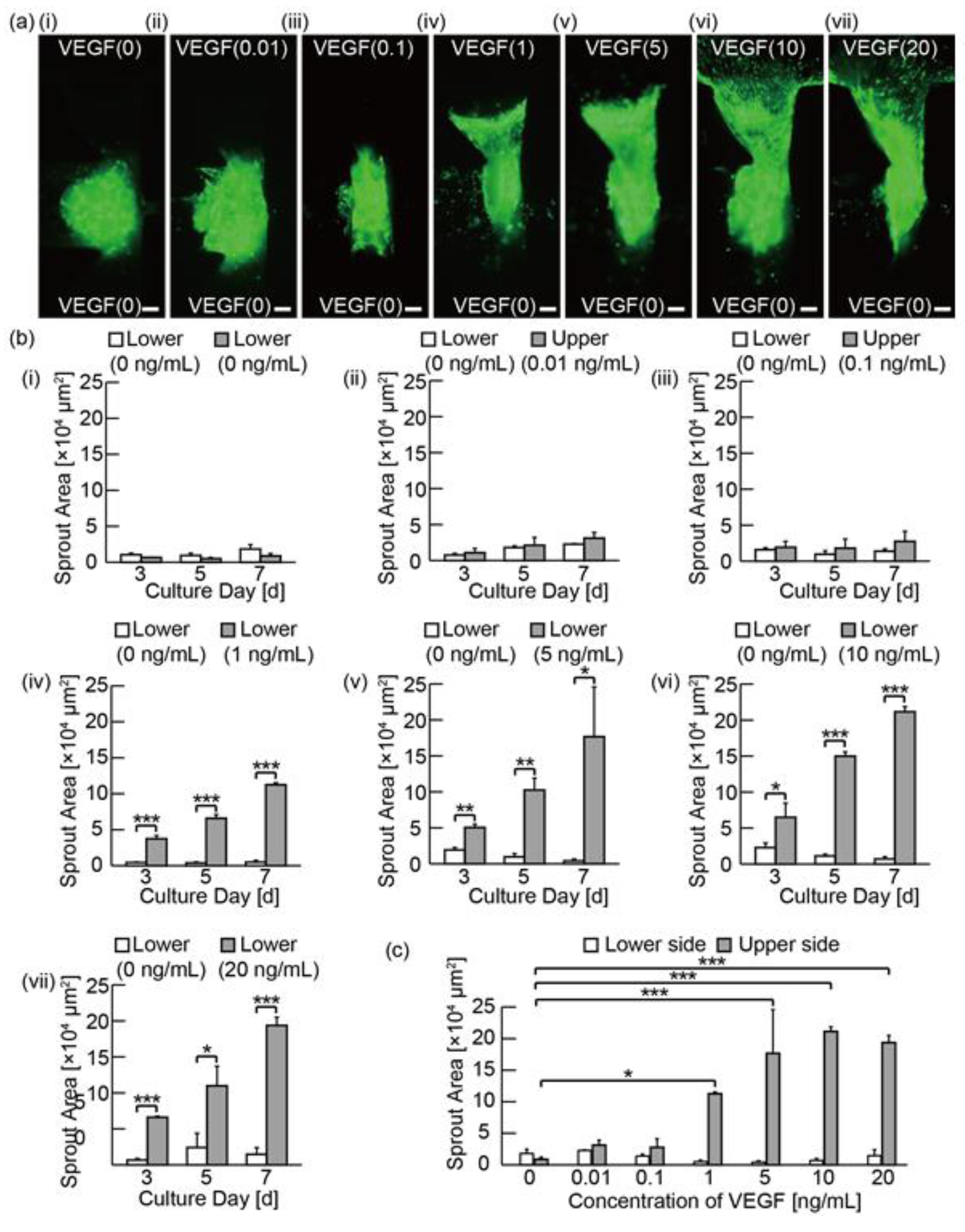
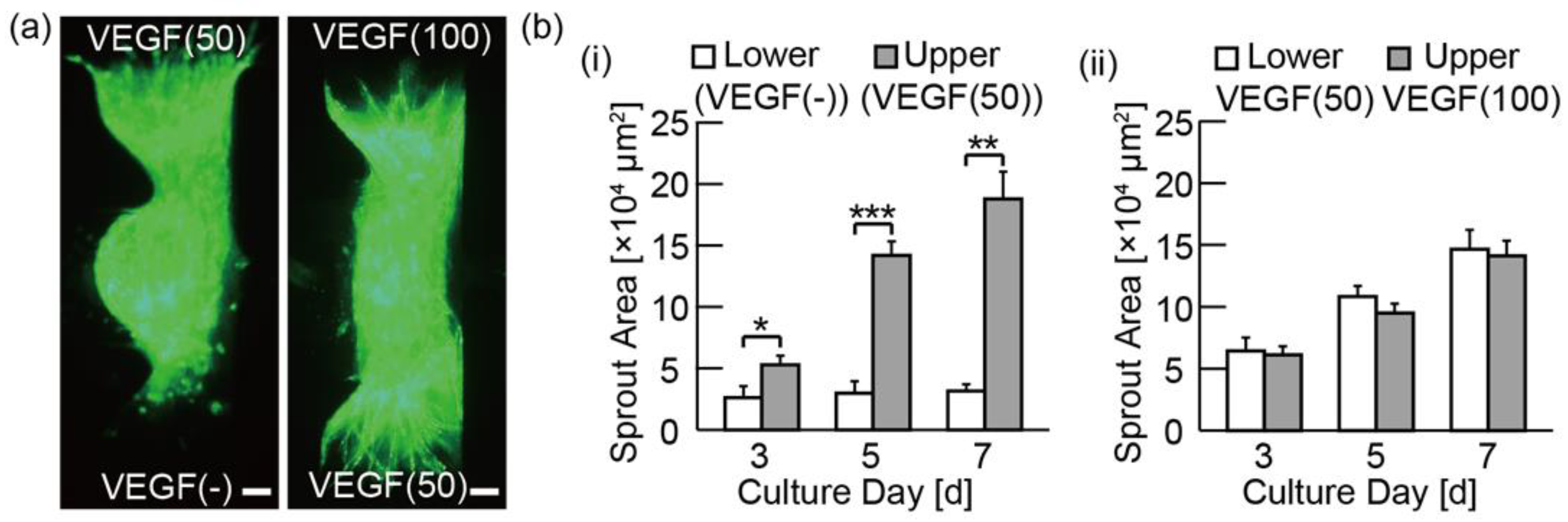
3.3. Analysis of the Angiogenic Sprouting in Media with Various Concentrations of VEGF
3.4. Analysis of Angiogenic Sprouts in Media with Various Kind of Biochemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, K.; Krebs, L.T.; Tweedie, E.; Conley, B.; Mancini, M.; Arthur, H.M.; Liaw, L.; Gridley, T.; Vary, C.P.H. Endoglin is required in Pax3-derived cells for embryonic blood vessel formation. Dev. Biol. 2016, 409, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.; Gardiner, T.A.; van Haperen, R.; de Crom, R.; McDonald, D.M. eNOS overexpression exacerbates vascular closure in the obliterative phase of OIR and increases angiogenic drive in the subsequent proliferative stage. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6833–6850. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.T.; Stapleton, S.C.; Yang, M.T.; Cha, S.S.; Choi, C.K.; Galie, P.A.; Chen, C.S. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 6712–6717. [Google Scholar] [CrossRef]
- Korff, T.; Kimmina, S.; Martiny-Baron, G.; Augustin, H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001, 15, 447–457. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Cartier, A.; Leigh, T.; Liu, C.H.; Hla, T. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3157–3166. [Google Scholar] [CrossRef]
- Jung, B.; Obinata, H.; Galvani, S.; Mendelson, K.; Ding, B.S.; Skoura, A.; Kinzel, B.; Brinkmann, V.; Rafii, S.; Evans, T.; et al. Flow-Regulated Endothelial S1P Receptor-1 Signaling Sustains Vascular Development. Dev. Cell 2012, 23, 600–610. [Google Scholar] [CrossRef]
- Gaengel, K.; Niaudet, C.; Hagikura, K.; Siemsen, B.L.; Muhl, L.; Hofmann, J.J.; Ebarasi, L.; Nyström, S.; Rymo, S.; Chen, L.L.; et al. The Sphingosine-1-Phosphate Receptor S1PR1 Restricts Sprouting Angiogenesis by Regulating the Interplay between VE-Cadherin and VEGFR2. Dev. Cell 2012, 23, 587–599. [Google Scholar] [CrossRef]
- Wen, H.C.; Huo, Y.N.; Chou, C.M.; Lee, W. Sen PMA inhibits endothelial cell migration through activating the PKC-δ/Syk/NF-κB-mediated up-regulation of Thy-1. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.W.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach-Teschl, C.; Lopez, J.A.; et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Canciani, B.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Mangiavini, L. Effect of Chemically Induced Hypoxia on Osteogenic and Angiogenic Differentiation of Bone Marrow Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in Direct Coculture. Cells 2020, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kasuya, J.; Jeon, J.; Chung, S.; Kamm, R.D. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15, 301–310. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Sano, E.; Mori, C.; Nashimoto, Y.; Yokokawa, R.; Kotera, H.; Torisawa, Y.S. Engineering of vascularized 3D cell constructs to model cellular interactions through a vascular network. Biomicrofluidics 2018, 12. [Google Scholar] [CrossRef]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C.W. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 2011, 22, 3791–3800. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, S.H.; Kim, I.S.; Kim, K.M.; Kwon, S.K.; Hwang, N.S. Gelatin-based micro-hydrogel carrying genetically engineered human endothelial cells for neovascularization. Acta Biomater. 2019, 95, 285–296. [Google Scholar] [CrossRef]
- Abdallah, M.; Martin, M.; El Tahchi, M.R.; Balme, S.; Faour, W.H.; Varga, B.; Cloitre, T.; Páll, O.; Cuisinier, F.J.G.; Gergely, C.; et al. Influence of Hydrolyzed Polyacrylamide Hydrogel Stiffness on Podocyte Morphology, Phenotype, and Mechanical Properties. ACS Appl. Mater. Interfaces 2019, 11, 32623–32632. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Nagarajan, S.; Martin, M.; Tamer, M.; Faour, W.H.; Bassil, M.; Cuisinier, F.J.G.; Gergely, C.; Varga, B.; Pall, O.; et al. Enhancement of Podocyte Attachment on Polyacrylamide Hydrogels with Gelatin-Based Polymers. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Fedele, C.; De Gregorio, M.; Netti, P.A.; Cavalli, S.; Attanasio, C. Azopolymer photopatterning for directional control of angiogenesis. Acta Biomater. 2017, 63, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Tanaka, R.; Takeuchi, S. Construction of 3D, Layered Skin, Microsized Tissues by Using Cell Beads for Cellular Function Analysis. Adv. Healthc. Mater. 2013, 2, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Ratel, D.; Mihoubi, S.; Beaulieu, E.; Durocher, Y.; Rivard, G.E.; Gingras, D.; Béliveau, R. VEGF increases the fibrinolytic activity of endothelial cells within fibrin matrices: Involvement of VEGFR-2, tissue type plasminogen activator and matrix metalloproteinases. Thromb. Res. 2007, 121, 203–212. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Q.; Shao, X.; Zhang, T.; Xue, C.; Shi, S.; Zhao, D.; Lin, Y. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 2017, 50, 1–10. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Nie, M.; Miura, S.; Takeuchi, S. Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients. Micromachines 2020, 11, 1049. https://doi.org/10.3390/mi11121049
Nishimura K, Nie M, Miura S, Takeuchi S. Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients. Micromachines. 2020; 11(12):1049. https://doi.org/10.3390/mi11121049
Chicago/Turabian StyleNishimura, Keigo, Minghao Nie, Shigenori Miura, and Shoji Takeuchi. 2020. "Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients" Micromachines 11, no. 12: 1049. https://doi.org/10.3390/mi11121049
APA StyleNishimura, K., Nie, M., Miura, S., & Takeuchi, S. (2020). Microfluidic Device for the Analysis of Angiogenic Sprouting under Bidirectional Biochemical Gradients. Micromachines, 11(12), 1049. https://doi.org/10.3390/mi11121049




