Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation for Micromachining
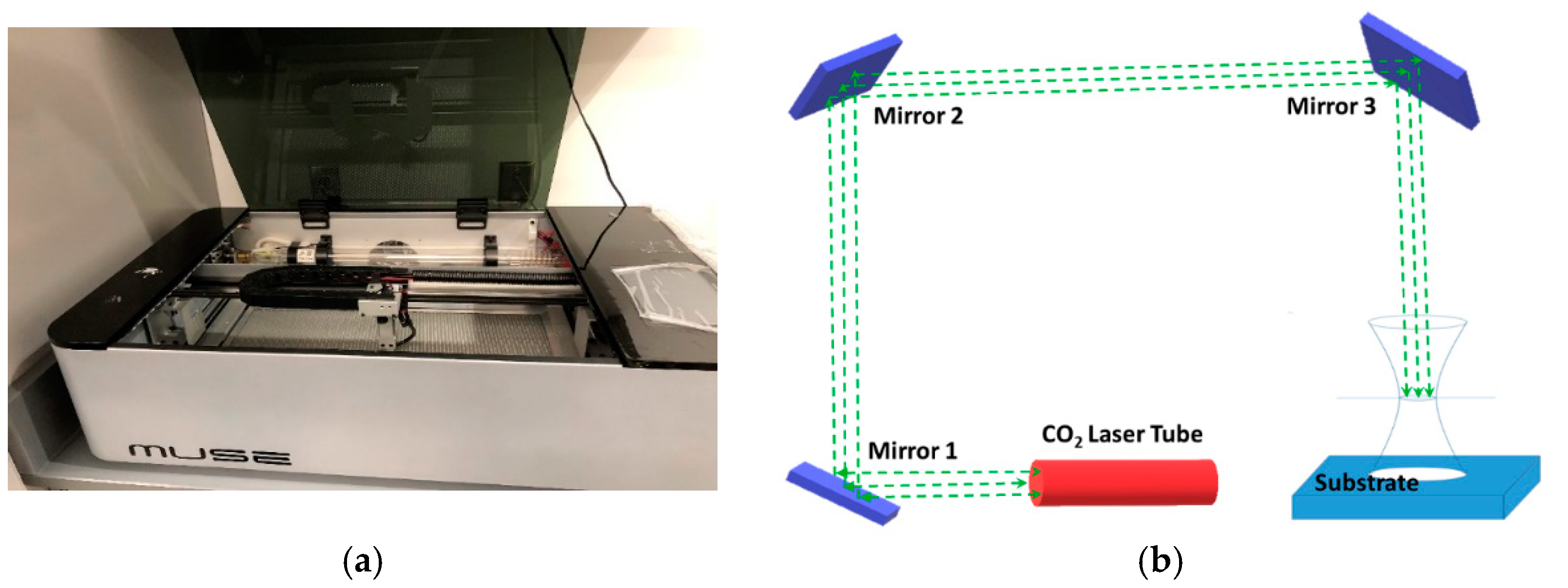
2.2. Experimental Setup
2.3. Characterization Protocols
2.4. Sample Preparation for Cell Adhesion Tests
2.5. Statistical Analysis
3. Results and Discussions
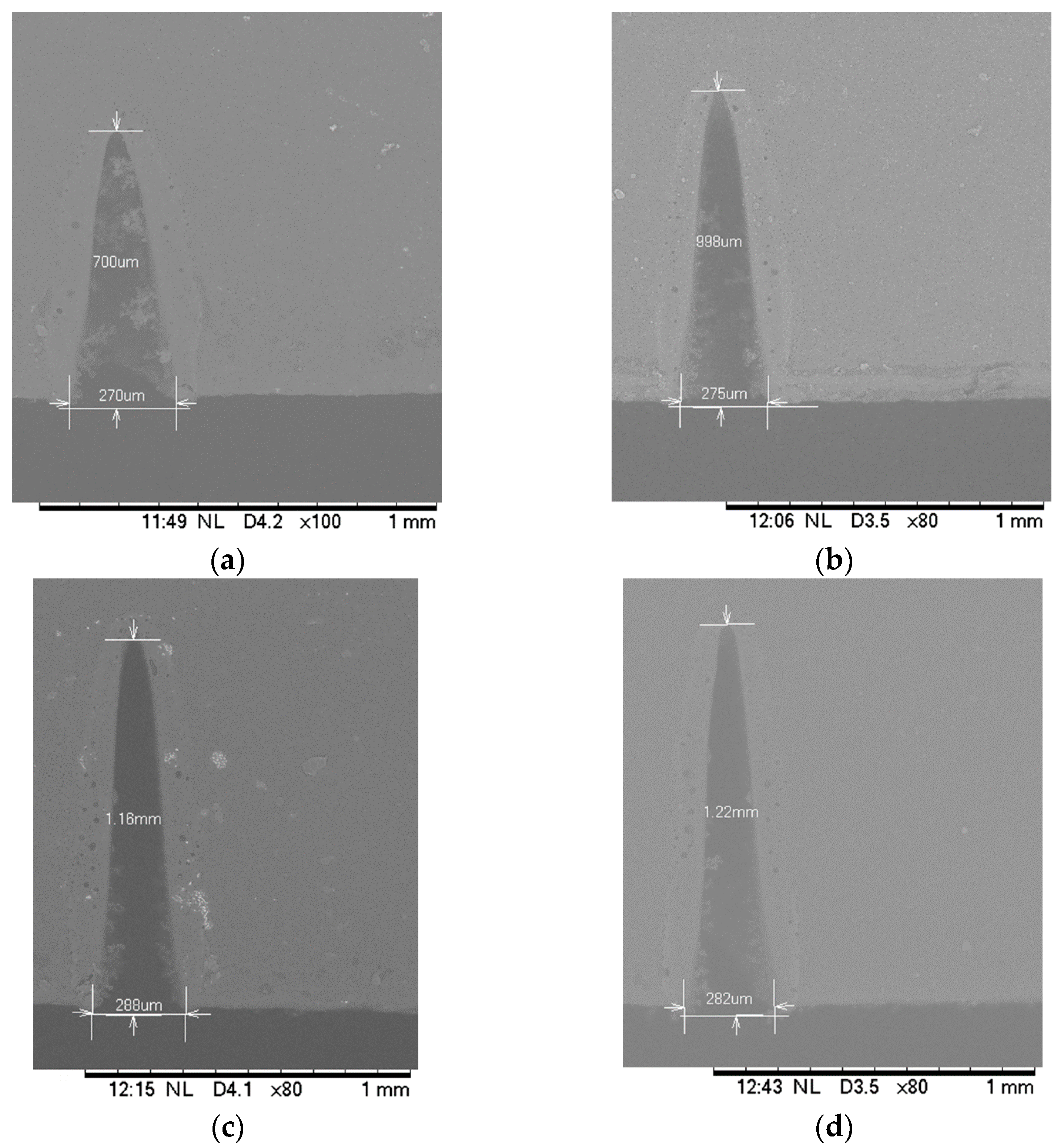
3.1. Effect of Distances between the Specimen and the Focusing Lens
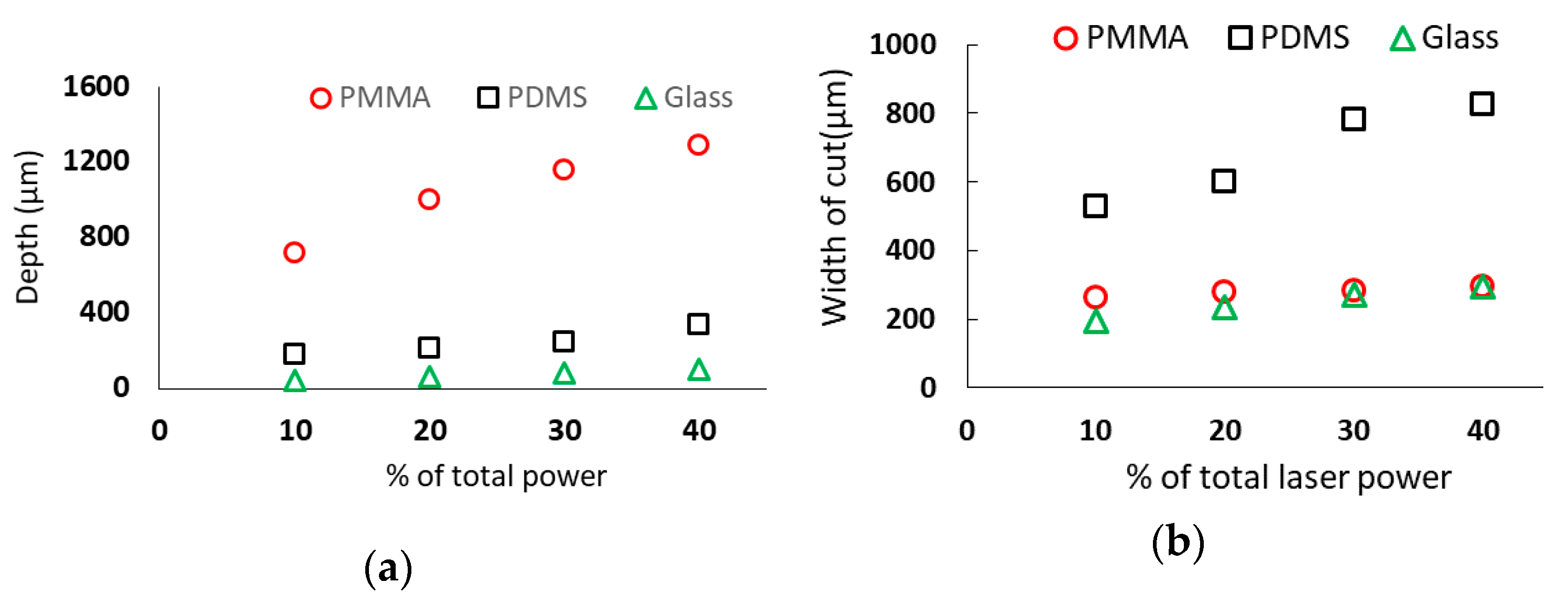
3.2. Effect of Laser Power
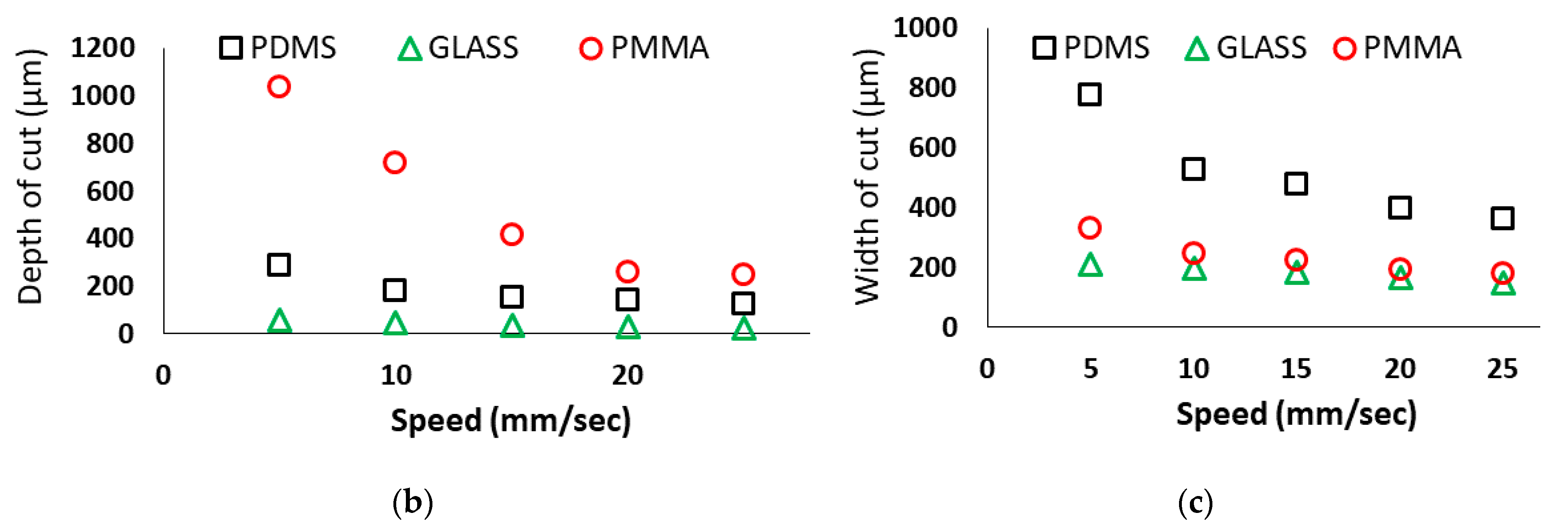
3.3. Effect of Laser Scanning Speed
3.4. Effect of Number of Passes
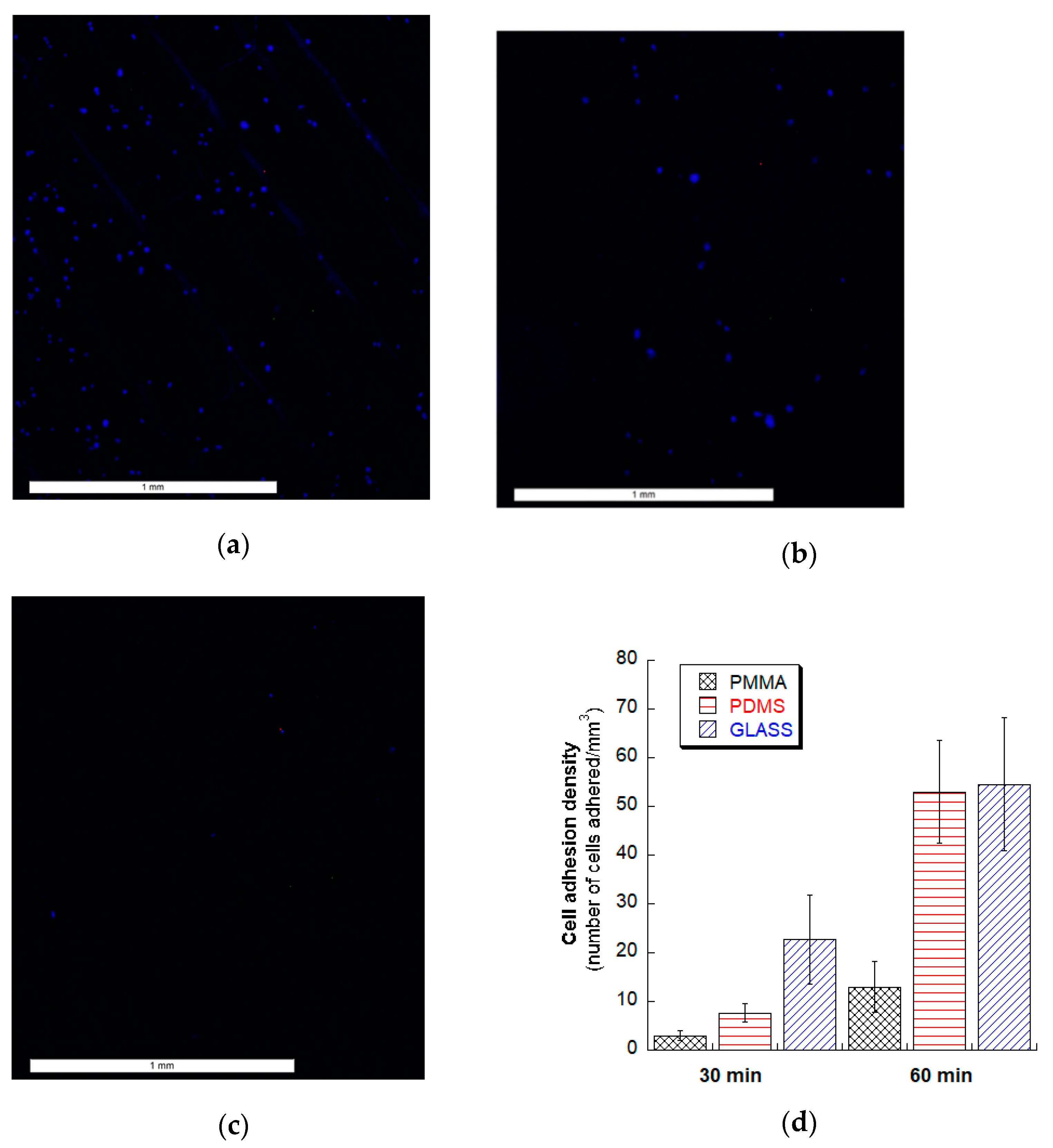
3.5. Fibroblasts Cell Adhesion Test
4. Conclusions
- Depth and width of a microchannel increase with increasing the laser power in all three substrates. However, the rate of increase in width in PMMA and glass substrate is less pronounced than the increase in depth. For a given laser power, PMMA materials provided the deepest channel and PDMS substrates provided the widest channel.
- For a given laser parameter, the depth of cut becomes maximum and the width of the cut becomes minimum when substrates are placed at the focal distance of the system in all three substrate materials.
- Both the depth and width of the microchannel decrease with the increased laser scanning speed in all three substrates.
- With the increase of the number of passes, the channel profile becomes more circular in glass and PDMS substrate while in PMMA it introduces a sharper corner at the bottom of the channel.
- The cut width is more sensitive for PDMS while the cut depth is more sensitive in PMMA for different combinations of laser system parameters. On the other hand, glass is more delicate for laser machining because it breaks more easily and produces more broken edges at the machined microchannels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Srivastava, V.C. Microfluidic-based photocatalytic microreactor for environmental application: A review of fabrication substrates and techniques, and operating parameters. Photochem. Photobiological Sci. 2016, 15, 714–730. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D printing microfluidics and applications in chemistry and biology: A review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Venkatesan, S.; Jerald, J.; Asokan, P.; Prabakaran, R. A Comprehensive Review on Microfluidics Technology and its Applications. In Recent Advances in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–245. [Google Scholar]
- Li, S.; Xu, Z.; Mazzeo, A.D.; Burns, D.J.; Fu, G.; Dirckx, M.; Shilpiekandula, V.; Chen, X.; Nayak, N.C.; Wong, E.; et al. Review of production of microfluidic devices: Material, manufacturing and metrology. In MEMS, MOEMS, and Micromachining III; International Society for Optics and Photonics: Bellingham, WA, USA, 2008; Volume 6993, p. 69930F. [Google Scholar]
- Hossan, M.R.; Dutta, D.; Islam, N.; Dutta, P. Electric field driven pumping in microfluidic device. Electrophoresis 2018, 39, 702–731. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.i.; Tachikawa, K.; Manz, A. Microfluidics: Applications for analytical purposes in chemistry and biochemistry. Electrophoresis 2008, 29, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimfam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Lawes, R. Manufacturing costs for microsystems/MEMS using high aspect ratio microfabrication techniques. Microsyst. Technol. 2007, 13, 85–95. [Google Scholar] [CrossRef]
- Wlodarczyk, K.L.; Carter, R.M.; Jahanbakhsh, A.; Lopes, A.A.; Mackenzie, M.D.; Maier, R.R.J.; Hand, D.P.; Maroto-Valer, M.M. Rapid laser manufacturing of microfluidic devices from glass substrates. Micromachines 2018, 9, 409. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Alam, M.N.H.Z.; Kouzani, A.; Gibson, I. Fabrication of microfluidic devices: Improvement of surface quality of CO2 laser machined poly (methylmethacrylate) polymer. J. Micromechanics Microengineering 2016, 27, 015021. [Google Scholar] [CrossRef]
- Malek, C.G.K. Laser processing for bio-microfluidics applications (part II). Anal. Bioanal. Chem. 2006, 385, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Dahotre, N.B.; Harimkar, S. Laser Fabrication and Machining of Materials; Springer Science & Business Media: Boston, MA, USA, 2008. [Google Scholar]
- Srinivasan, R. Ablation of polymers and biological tissue by ultraviolet lasers. Science 1986, 234, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Campbell, K.; Groisman, A.; Kleinfeld, D.; Schaffer, C.B. Femtosecond laser-drilled capillary integrated into a microfluidic device. Appl. Phys. Lett. 2005, 86, 201106. [Google Scholar] [CrossRef]
- Imran, M.; Rahman, R.A.; Ahmad, M.; Akhtar, M.N.; Usman, A.; Sattar, A. Fabrication of microchannels on PMMA using a low power CO2 laser. Laser Phys. 2016, 26, 96101. [Google Scholar] [CrossRef]
- Snakenborg, D.; Klank, H.; Kutter, J.P. Microstructure fabrication with a CO2 laser system. J. Micromechanics Microengineering 2003, 14, 182. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Zhou, M. Rapid fabrication of a four-layer PMMA-based microfluidic chip using CO2-laser micromachining and thermal bonding. J. Micromechanics Microengineering 2016, 26, 107001. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Guler, M.T.; Aydogdu, B.; Bilican, I.; Elbuken, C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromechanics Microengineering 2016, 26, 35008. [Google Scholar] [CrossRef]
- Klank, H.; Kutter, J.P.; Gescheke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef]
- Cheng, J.-Y.; Wei, C.-W.; Hsu, K.-H.; Young, T.-H.J.S. Direct-write laser micromachining and universal surface modification of PMMA for device development. Sens. Actuators B 2004, 99, 186–196. [Google Scholar] [CrossRef]
- Vargas, M.J.; Nieuwoudt, M.; Yong, R.M.; Vanholsbeeck, F.; Williams, D.E.; Simpson, M.C. Excellent quality microchannels for rapid microdevice prototyping: Direct CO2 laser writing with efficient chemical postprocessing. Microfluid. Nanofluidics 2019, 23, 124. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar, S. Fabrication of rectangular cross-sectional microchannels on PMMA with a CO2 laser and underwater fabricated copper mask. Opt. Laser Technol. 2017, 94, 180–192. [Google Scholar] [CrossRef]
- Helmy, M.O.; El-Bab, A.R.F.; El-Hofy, H.A. Fabrication and characterization of polymethyl methacrylate microchannel using dry and underwater CO2 laser. Proc. Inst. Mech. Eng. N. J. Nanomater. Nanoeng. Nanosyst. 2018, 232, 23–30. [Google Scholar] [CrossRef]
- Hong, T.-F.; Ju, W.-J.; Wu, M.-C.; Tai, C.-H.; Tsai, C.; Fu, L.M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluidics 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Benton, M.; Hossan, M.R.; Konari, P.R.; Gamagedara, S. Effect of process parameters and material properties on laser micromachining of microchannels. Micromachines 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Cubaud, T.; Longtin, J.P. Ultrafast laser machining of tapered microchannels in glass and PDMS. Opt. Lasers Eng. 2012, 50, 210–214. [Google Scholar] [CrossRef]
- Fu, L.-M.; Ju, W.-J.; Yang, R.-J.; Wang, Y.-N. Rapid prototyping of glass-based microfluidic chips utilizing two-pass defocused CO2 laser beam method. Microfluid. Nanofluidics 2013, 14, 479–487. [Google Scholar] [CrossRef]
- Doan, K.T.; Kshetri, P.; Attamakulsri, N.; Newsome, D.R.; Zhou, F.; Murray, C.K.; Chen, W.R.; Xu, G.; Vaughan, M.B. The Effect of Chitosan Derivatives on the Compaction and Tension Generation of the Fibroblast-populated Collagen Matrix. Molecules 2019, 24, 2713. [Google Scholar] [CrossRef] [PubMed]
- Ravi-Kumar, S.; Lies, B.; Zhang, X.; Lyu, H.; Qin, H. Laser ablation of polymers: A review. Polym. Int. 2019, 68, 1391–1401. [Google Scholar] [CrossRef]
- Zheng, H.; Lee, T. Studies of CO2 laser peeling of glass substrates. J. Micromechanics Microengineering 2005, 15, 2093. [Google Scholar] [CrossRef]
- Radhakrishnan, T.S. Thermal degradation of poly (dimethylsilylene) and poly (tetramethyldisilylene-co-styrene). J. Appl. Polym. Sci. 2006, 99, 2679–2686. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Holle, A.W.; Chao, S.-H.; Holl, M.R.; Houkal, J.M.; Meldrum, D.R. Characterization of Program Controlled CO2 Laser-Cut PDMS Channels for Lab-on-a-chip Applications. In Proceedings of the 2007 IEEE International Conference on Automation Science and Engineering, Scottsdale, AZ, USA, 22–25 September 2007; pp. 621–627. [Google Scholar]
- Prakash, S.; Kumar, S. Manufacture Fabrication of microchannels: A review. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 1273–1288. [Google Scholar] [CrossRef]
- Allcock, G.; Dyer, P.; Elliner, G.; Snelling, H. Experimental observations and analysis of CO2 laser-induced microcracking of glass. J. Appl. Phys. 1995, 78, 7295–7303. [Google Scholar] [CrossRef]
- Andersson, H.; Van Den Berg, A. Microfabrication and microfluidics for tissue engineering: State of the art and future opportunities. Lab Chip 2004, 4, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, E.; Ranella, A.; Fotakis, C.J.B. Biomimetic micro/nanostructured functional surfaces for microfluidic and tissue engineering applications. Biomicrofluidics 2011, 5, 3411. [Google Scholar] [CrossRef] [PubMed]
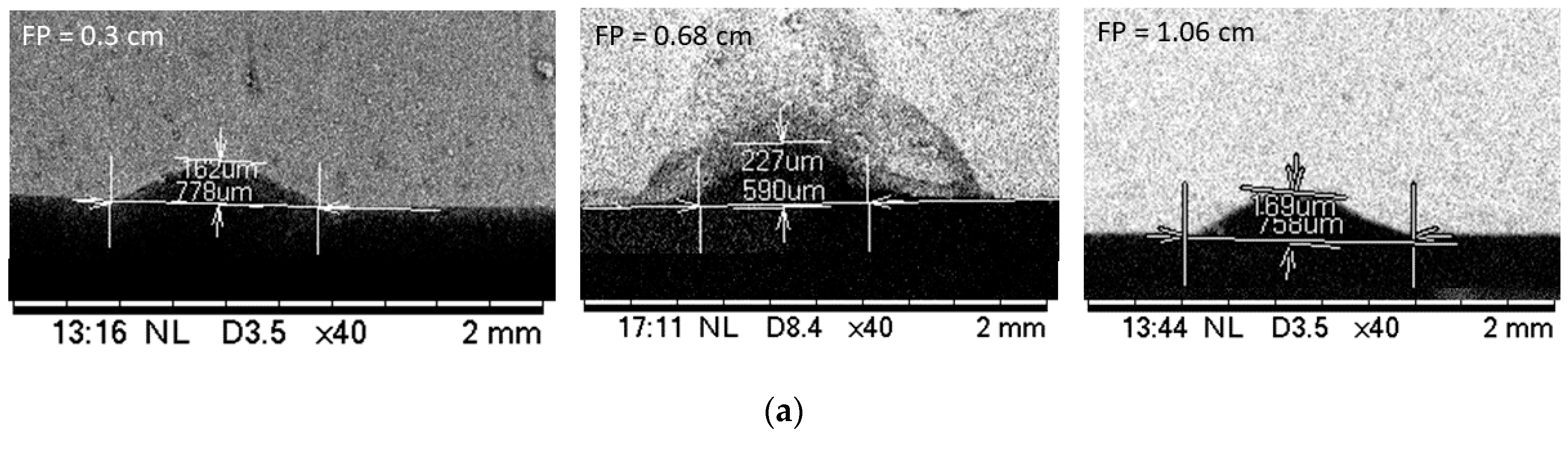
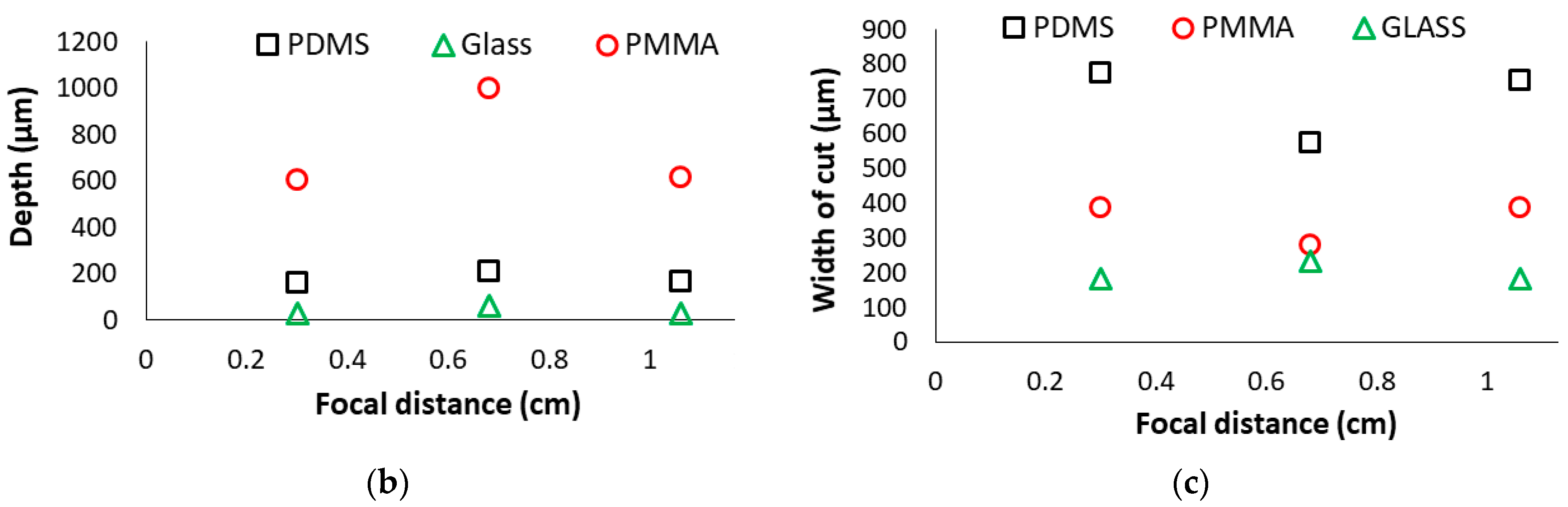


| Focal Distance | 0.3 cm | 0.68 cm | 1.06 cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| /Substrates | S1 | S2 | S3 | Avg | SE | S1 | S2 | S3 | Avg | SE | S1 | S2 | S3 | Avg | SE |
| PMMA (μm) | 616 | 603 | 599 | 606 | 5.1 | 1030 | 972 | 998 | 1000 | 16.8 | 616 | 635 | 593 | 615 | 12.1 |
| PDMS (μm) | 162 | 162 | 162 | 162 | 0 | 220 | 188 | 227 | 212 | 12 | 169 | 156 | 169 | 165 | 4.33 |
| Glass (μm) | 28.3 | 28.3 | 28.3 | 28.3 | 0 | 62.3 | 62 | 63 | 62.6 | 0.22 | 28.5 | 28 | 28 | 28.4 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konari, P.R.; Clayton, Y.-D.; Vaughan, M.B.; Khandaker, M.; Hossan, M.R. Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates. Micromachines 2021, 12, 138. https://doi.org/10.3390/mi12020138
Konari PR, Clayton Y-D, Vaughan MB, Khandaker M, Hossan MR. Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates. Micromachines. 2021; 12(2):138. https://doi.org/10.3390/mi12020138
Chicago/Turabian StyleKonari, Prashanth Reddy, Yung-Dai Clayton, Melville B. Vaughan, Morshed Khandaker, and Mohammad Robiul Hossan. 2021. "Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates" Micromachines 12, no. 2: 138. https://doi.org/10.3390/mi12020138
APA StyleKonari, P. R., Clayton, Y.-D., Vaughan, M. B., Khandaker, M., & Hossan, M. R. (2021). Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates. Micromachines, 12(2), 138. https://doi.org/10.3390/mi12020138








