Recent Insights and Multifactorial Applications of Carbon Nanotubes
Abstract
:1. Introduction
2. Review of the Carbon Nanotube Construction
3. Commercial Applications of Carbon Nanotubes
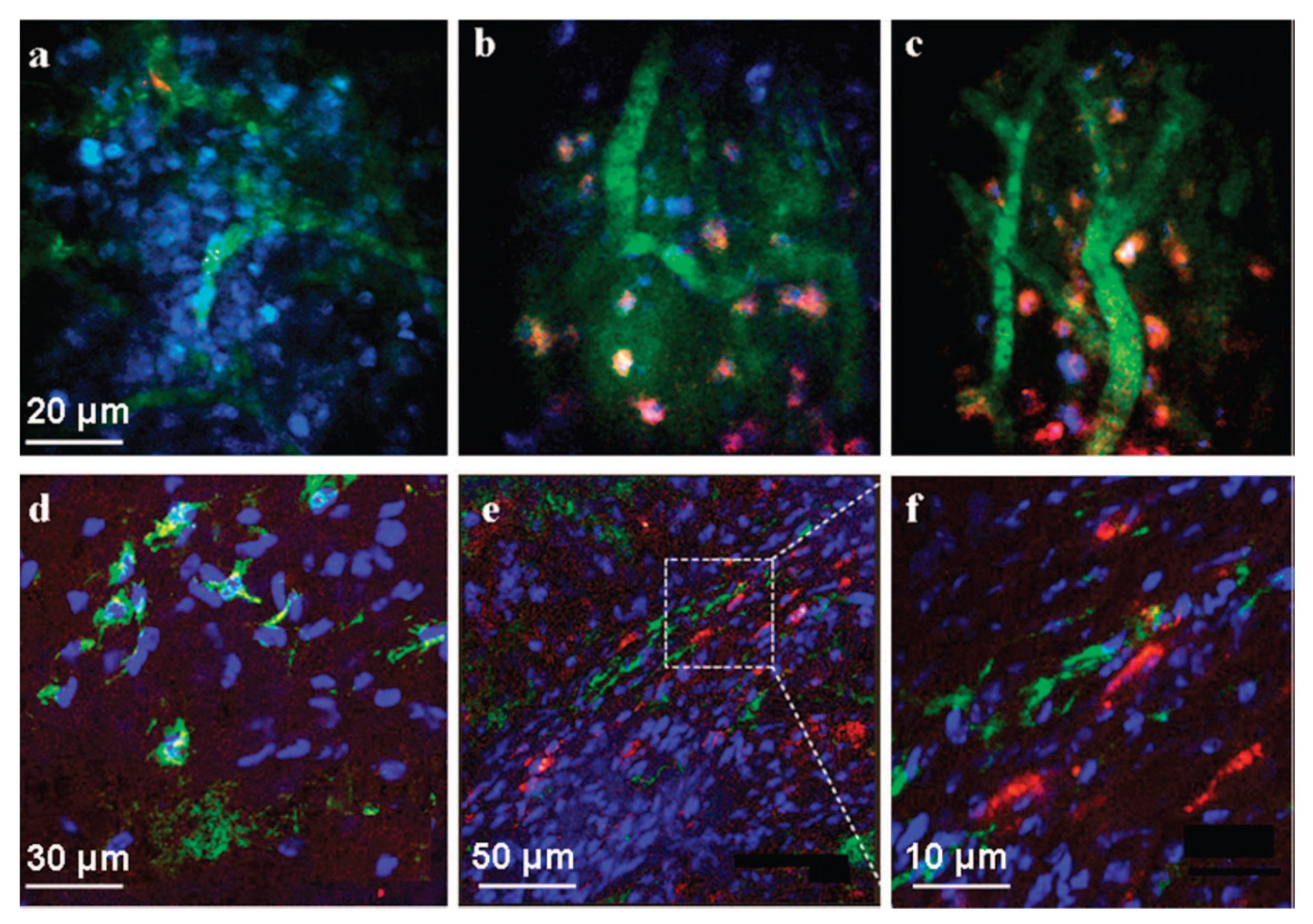
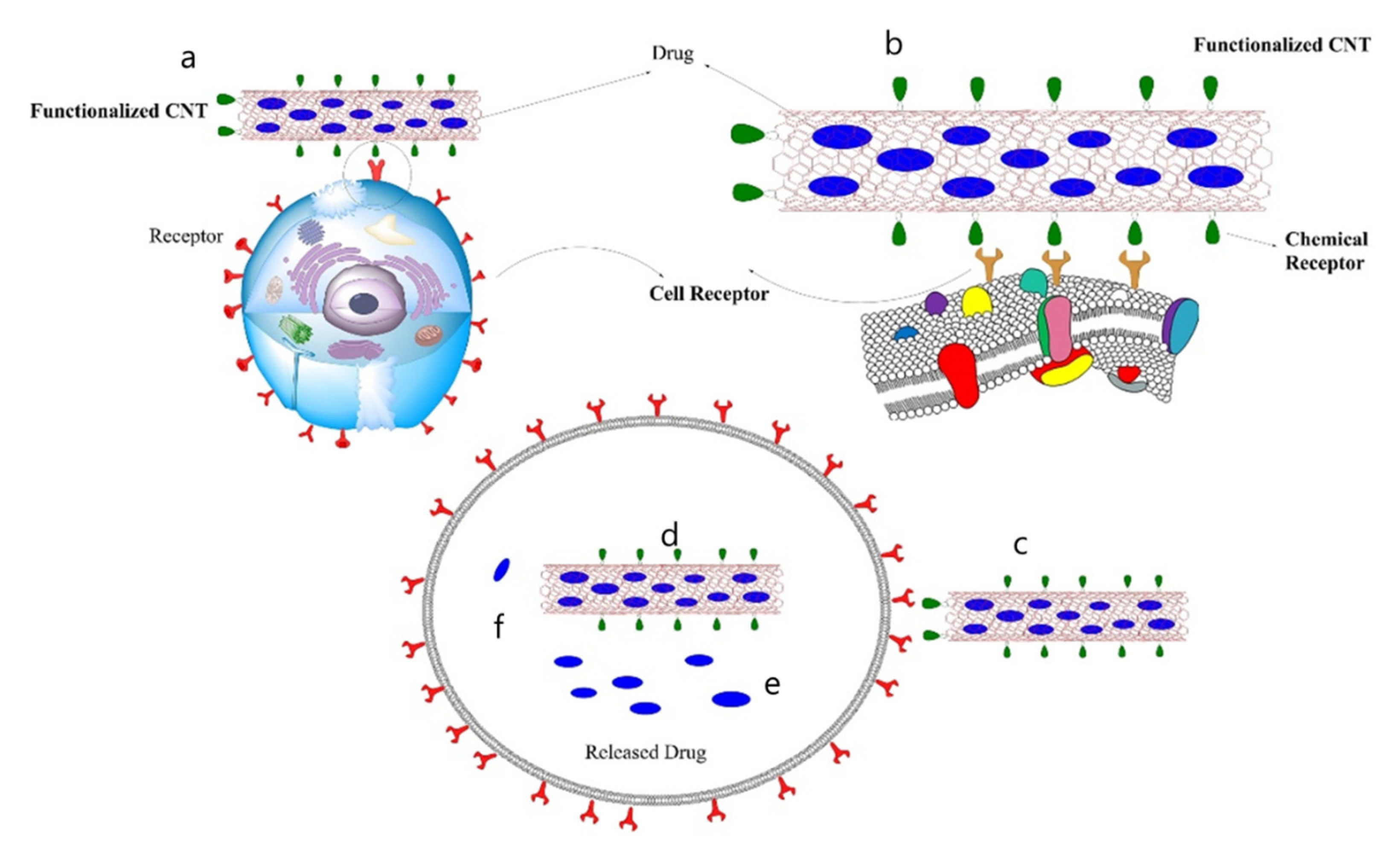
4. Uses of CNTs in Biomedicine
5. Adsorption of Carbon Nanotubes
6. Silicon Chips with Carbon Nanotubes
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elmoselhy, S.A.M. Hybrid organic/inorganic Nano-I-Beam for structural nano-mechanics. Sci. Rep. 2019, 9, 18324. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of carbon nanotubes by arc-discharge and chemical vapor deposition method with analysis of its morphology, dispersion and functionalization characteristics. Cogent. Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Takakura, A.; Beppu, K.; Nishihara, T.; Fukui, A.; Kozeki, T.; Namazu, T.; Miyauchi, Y.; Itami, K. Strength of carbon nanotubes depends on their chemical structures. Nat. Commun. 2019, 10, 3040. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Al-Marzouki, F.; Al-Ghamdi, A.A.; Abdel-Daiem, A. Different technical applications of carbon nanotubes. Nanoscale Res. Lett. 2015, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon nanotube assembly and integration for applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aqel, A.; El-Nour, K.M.M.A.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterization. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Rümmeli, M.H.; Bachmatiuk, A.; Börrnert, F.; Schäffel, F.; Ibrahim, I.; Cendrowski, K.; Simha-Martynkova, G.; Plachá, D.; Borowiak-Palen, E.; Cuniberti, G.; et al. Synthesis of carbon nanotubes with and without catalyst particles. Nanoscale Res. Lett. 2011, 6, 303. [Google Scholar] [CrossRef] [Green Version]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta 2006, 1758, 404–412. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Galano, A. Carbon nanotubes: Promising agents against free radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Miura, T.; Shiratori, T.; Weng, M.; Nagahama, T.; Shimada, T. Synthesis of carbon nanotubes by plasma-enhanced chemical vapor deposition using Fe1−xMnxO nanoparticles as catalysts: How does the catalytic activity of graphitization affect the yields and morphology? C 2019, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Journet, C.; Maser, W.; Bernier, P.; Loiseau, A.; de la Chapelle, M.L.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J.E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Moise, C.; Rachmani, L.; Mihai, G.; Lazar, O.; Enăchescu, M.; Naveh, N. Pulsed laser deposition of SWCNTs on carbon fibres: Effect of deposition temperature. Polymers 2021, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Liu, P. Modification Strategies for Carbon Nanotubes as a Drug Delivery System. Ind. Eng. Chem. Res. 2013, 52, 13517–13527. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in construction materials and other applications, and implications of nanoparticle use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, S.K.; Rudžionis, Ž.; Rajapriya, R. The effect of carbon nanotubes on the flowability, mechanical, microstructural and durability properties of cementitious composite: An overview. Sustainability 2020, 12, 8362. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Laukaitis, A.; Sinica, M.; Balevicius, S.; Levitas, B. Investigation of electromagnetic wave absorber based on carbon fiber reinforced aerated concrete using time-domain method. Acta Phys. Polonica A 2008, 113, 1047–1050. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ham, S.R.; Kim, J.H.; Yoo, T.H.; Kim, S.R.; Lee, Y.T.; Hwang, D.K.; Angadi, B.; Seo, W.S.; Ju, B.K.; et al. Highly dispersible buckled nanospring carbon nanotubes for polymer nano composites. Sci. Rep. 2018, 8, 4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon nanotubes: A summary of beneficial and dangerous aspects of an increasingly popular group of nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere. 2018, 195, 351–364. [Google Scholar] [CrossRef]
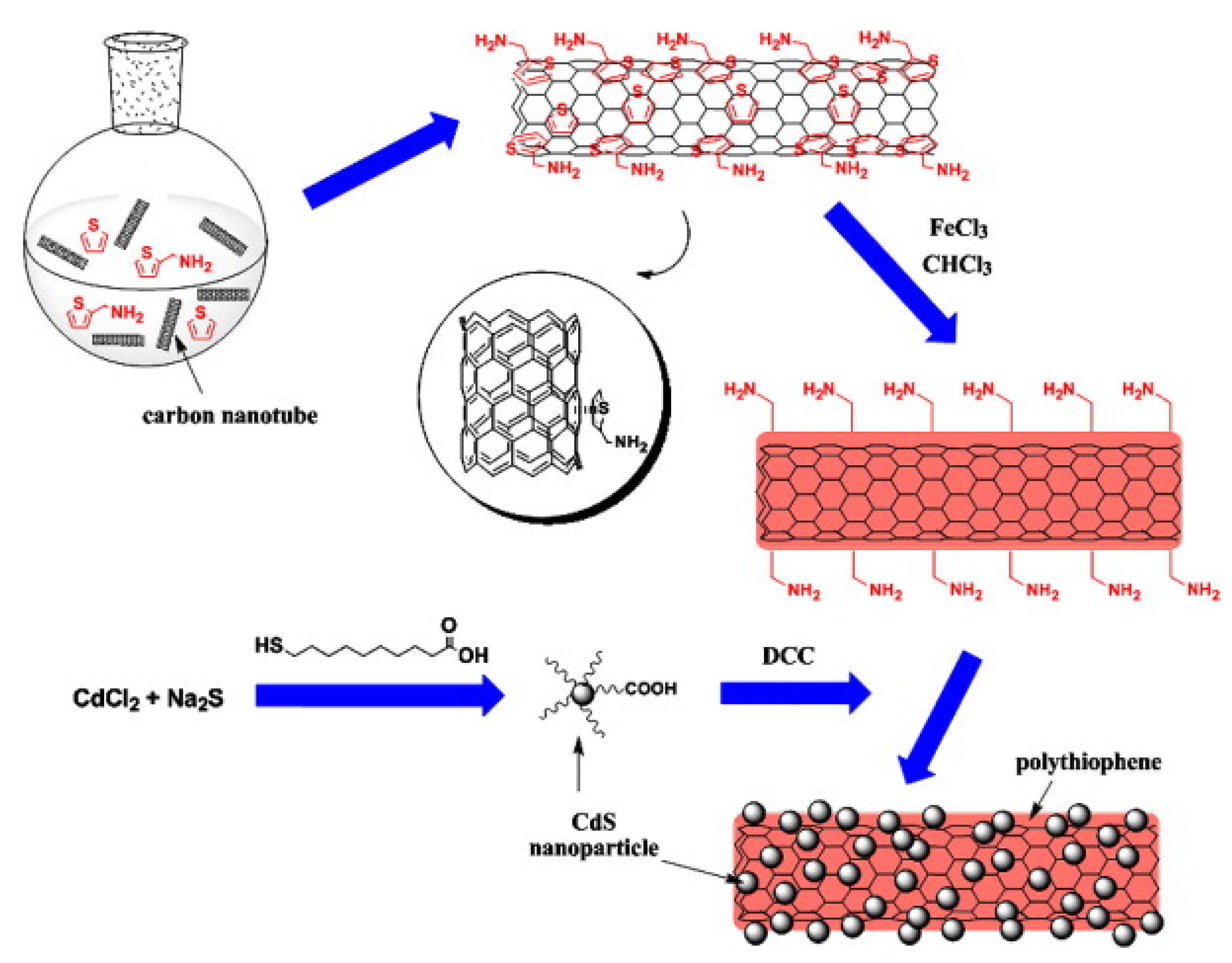
- Feng, M.; Sun, R.; Zhan, H.; Chen, Y. Decoration of carbon nanotubes with CdS nanoparticles by polythiophene interlinking for optical limiting enhancement. Carbon 2010, 48, 1177–1185. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Qian, L.; Xie, Y.; Zhang, S.; Zhang, J. Band engineering of carbon nanotubes for device applications. Matter 2020, 3, 664–695. [Google Scholar] [CrossRef]
- Cwirzen, A.; Habermehl-Cwirzen, K.; Penttala, V. Surface decoration of carbon nanotubes and mechanical properties of cement/carbon nanotube composites. Adv. Cement Res. 2008, 20, 65–73. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Pitroda, J.; Jethwa, B. A Critical review on carbon nanotubes. Int. J. Constr. Res. Civil Eng. 2016, 2, 36–42. [Google Scholar]
- Yakovlev, G.; Pervushin, G.; Maeva, I.; Keriene, J.; Pudov, I.; Shaybadullina, A.; Buryanov, A.; Korzhenko, A.; Senkov, S. Modification of construction materials with multiwalled carbon nanotubes. Procedia Eng. 2013, 57, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ouyang, D.; Xu, W. Mechanical properties and durability of ultra-high strength concrete incorporating multi-walled carbon nanotubes. Materials 2016, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Gamal, H.A.; El-Feky, M.S.; Alharbi, Y.R.; Abadel, A.A.; Kohail, M. Enhancement of the concrete durability with hybrid nano materials. Sustainability 2021, 13, 1373. [Google Scholar] [CrossRef]
- Siddique, R.; Mehta, A. Effect of carbon nanotubes on properties of cement mortars. Constr. Build. Mater. 2014, 50, 116–129. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Wu, K.; Niu, Y.; Zhang, Y.; Yong, Z.; Li, Q. Continuous growth of carbon nanotube films: From controllable synthesis to real applications. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106359. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Bu, F.; Zhou, W.; Xu, Y.; Du, Y.; Guan, C.; Huang, W. Recent developments of advanced micro-supercapacitors: Design, fabrication and applications. Npj Flex. Electron. 2020, 4, 31. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Y.; Tang, X.; Pan, Y.; Hu, S. Fully packaged carbon nanotube supercapacitors by direct ink writing on flexible substrates. ACS Appl. Mater. Interfaces 2017, 9, 28433–28440. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.M.; Timmermans, M.Y.; Tian, Y.; Nasibulin, A.G.; Kauppinen, E.I.; Kishimoto, S.; Mizutani, T.; Ohno, Y. Flexible high-performance carbon nanotube integrated circuits. Nat. Nanotechnol. 2011, 6, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Komatsu, T.; Arai, K.; Yamazaki, T.; Kijima, M.; Shimizu, H.; Takasawa, Y.; Nakamura, J. Reduction of Pt usage in fuel cell electrocatalysts with carbon nanotube electrodes. Chem. Commun. 2004, 7, 840–841. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, A.; Artero, V.; Jousselme, B.; Tran, P.D.; Guillet, N.; Métayé, R.; Fihri, A.; Palacin, S.; Fontecave, M. From hydrogenases to noble metal–free catalytic nanomaterials for H2 production and uptake. Science 2009, 326, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabor, N.M.; Zhong, Z.; Bosnick, K.; Park, J.; McEuen, P.L. Extremely efficient multiple electron-hole pair generation in carbon nanotube photodiodes. Science 2009, 325, 1367–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Yamauchi, Y.; Honda, S.; Maki, H. An electrically driven, ultrahigh-speed, on-chip light emitter based on carbon nanotubes. Nano Lett. 2014, 14, 3277–3283. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Choi, J.-W. Inkjet printing of carbon nanotubes. Nanomaterials 2013, 3, 453–468. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Nobusa, Y.; Gocho, S.; Kudou, H.; Yanagi, K.; Kataura, H.; Takenobu, T. Inkjet printing of aligned single-walled carbon-nanotube thin films. Appl. Phys. Lett. 2013, 102, 143107. [Google Scholar] [CrossRef]
- Wang, C.; Chien, J.C.; Takei, K.; Takahashi, T.; Nah, J.; Niknejad, A.M.; Javey, A. Extremely bendable, high-performance integrated circuits using semiconducting carbon nanotube networks for digital, analog, and radio-frequency applications. Nano Lett. 2012, 12, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.H.; Takei, K.; Wang, C.; Ju, Y.; Kim, J.; Yu, Z.; Takahashi, T.; Cho, G.; Javey, A. Fully printed, high performance carbon nanotube thin-film transistors on flexible substrates. Nano Lett. 2013, 13, 3864–3869. [Google Scholar] [CrossRef] [PubMed]
- Sajed, F.; Rutherglen, C. All-printed and transparent single walled carbon nanotube thin film transistor devices. Appl. Phys. Lett. 2013, 103, 143303. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mat. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Tsentalovich, D.E.; Headrick, R.J.; Mirri, F.; Hao, J.; Behabtu, N.; Young, C.C.; Pasquali, M. Influence of Carbon Nanotube Characteristics on Macroscopic Fiber Properties. ACS Appl. Mater. Interfaces 2017, 9, 36189–36198. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew Chem. Int. Ed. Engl. 2009, 48, 7668–7672. [Google Scholar] [CrossRef]
- Lay, C.L.; Liu, H.Q.; Tan, H.R.; Liu, Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol)(PEG)-graft carbon nanotubes for potent cancer therapeutics. Nanotechnology 2010, 21, 065101. [Google Scholar] [CrossRef]
- Bhirde, A.A.; Patel, V.; Gavard, J.; Zhang, G.; Sousa, A.A.; Masedunskas, A.; Leapman, R.D.; Weigert, R.; Gutkind, J.S.; Rusling, J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 2009, 3, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Gannon, C.J.; Cherukuri, P.; Yakobson, B.I.; Cognet, L.; Kanzius, J.S.; Kittrell, C.; Weisman, R.B.; Pasquali, M.; Schmidt, H.K.; Smalley, R.E.; et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007, 110, 2654–2665. [Google Scholar] [CrossRef]
- Torti, S.V.; Byrne, F.; Whelanetal, O. Thermalablation therapeutics based on CNx multiwalled nanotubes. Int. J. Nanomed. 2007, 2, 707–714. [Google Scholar]
- Chakravarty, P.; Marches, R.; Zimmerman, N.S.; Swafford, A.D.; Bajaj, P.; Musselman, I.H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2008, 105, 8697–8702. [Google Scholar] [CrossRef] [Green Version]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Cancian, G.; Tozzi, G.; Hussain, A.A.B.; De Mori, A.; Roldo, M. Carbon nanotubes play an important role in the spatial arrangement of calcium deposits in hydrogels for bone regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhao, H.; Huang, C.; Du, Y. Mechanically and electrically enhanced CNT–collagen hydrogels as potential scaffolds for engineered cardiac constructs. ACS Biomater. Sci. Eng. 2017, 3, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.G.; Im, O.; Li, J.; Keidar, M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int. J. Nanomed. 2012, 7, 2087–2099. [Google Scholar] [CrossRef] [Green Version]
- Kaboudin, B.; Saghatchi, F.; Kazemi, F.; Akbari-Birgani, S. A novel magnetic carbon nanotubes functionalized with pyridine groups: Synthesis, characterization and their application as an efficient carrier for plasmid DNA and aptamer. Chem. Sel. 2018, 3, 6743–6749. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Feng, L.; Dong, S.; Hao, J.; Yu, Q. Carbon nanotubes modified by a paramagnetic cationic surfactant for migration of DNA and proteins. Colloids Surf. Physicochem. Eng. Asp. 2018, 559, 201–208. [Google Scholar] [CrossRef]
- Folkmann, J.K.; Risom, L.; Jacobsen, N.R.; Wallin, H.; Loft, S.; Møller, P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ. Health Perspect. 2009, 117, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Fraczek, A.; Menaszek, E.; Paluszkiewicz, C.; Blazewicz, M. Comparative in vivo biocompatibility study of single- and multi-wall carbon nanotubes. Acta Biomater. 2008, 4, 1593–1602. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of carbon nanotubes in bone tissue regeneration and engineering: Superiority, concerns, current advancements, and prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wu, R.; Zhao, L.; Wu, M.; Yang, L.; Zou, H. P-glycoprotein antibody functionalized carbon nanotube over- comes the multidrug resistance of human leukemia cells. ACS Nano 2010, 4, 1399–1408. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Yang, Y.; Sun, L.; Han, D.; Li, H.; Wang, C. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine 2010, 6, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, W.; Jie, F.; Zhao, Z.; Zhou, K.; Liu, H. The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater. Sci Rep. 2021, 11, 16878. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental remediation applications of carbon nanotubes and graphene oxide: Adsorption and catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Wang, S.; Wei, J.; Zhang, X.; Xu, C.; Luan, Z.; Wu, D.; Wei, B. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Z.; Cao, J.; Yu, F. Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 2020, 242, 125188. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Binti Jaafar, W.Z.; AlOmar, M.K.; Fayaed, S.S.; Binti Mohd, N.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Grace, T.; Yu, L.; Gibson, C.; Tune, D.; Alturaif, H.; Al Othman, Z.; Shapter, J. Investigating the effect of carbon nanotube diameter and wall number in carbon nanotube/silicon heterojunction solar cells. Nanomaterials 2016, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.; Tahir, N.; Rubab, A.; Beller, M.; Sharif, M. Facile synthesis of iron-titanate nanocomposite as a sustainable material for selective amination of substitued nitro-arenes. Catalysts 2020, 11, 871. [Google Scholar] [CrossRef]
- Zhou, L.; Forman, H.J.; Ge, Y.; Lunec, J. Multi-walled carbon nanotubes: A cytotoxicity study in relation to functionalization, dose and dispersion. Toxicol. Vitro 2017, 42, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Johnen, S.; Meißner, F.; Krug, M.; Baltz, T.; Endler, I.; Mokwa, W.; Peter, W. Properties of retinal precursor cells grown on vertically aligned multiwalled carbon nanotubes generated for the modification of retinal implant-embedded microelectrode arrays. J. Ophthalmol. 2016, 2016, 2371021. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Sheng, T.; Hu, Y.; Baig, S.A.; Lv, X.; Xu, X. Adsorption–dechlorination of 2,4-dichlorophenol using two specified MWCNTs-stabilized Pd/Fe nanocomposites. Chem. Eng. J. 2013, 219, 162–173. [Google Scholar] [CrossRef]
- Zhou, J.; Lou, Z.; Yang, K.; Xu, J.; Xu, X. Electrocatalytic dechlorination of 2,4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: Adsorption and dechlorination activity. Appl. Cat. B Environ. 2019, 244, 215–224. [Google Scholar] [CrossRef]
- Si, S.; Gao, T.; Wang, J.; Liu, Q.; Zhou, G. Mussel inspired polymerized P(TA-TETA) for facile functionalization of carbon nanotube. Appl. Surf. Sci. 2018, 433, 94–100. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected heavy metals removal from electroplating wastewater by purified and polyhydroxylbutyrate functionalized carbon nanotubes adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Yun, D.J.; Theilmann, P.; Bandaru, P. Superior electrical and mechanical characteristics observed through the incorporation of coiled carbon nanotubes, in comparison to non-coiled forms, in polymers. Polymer 2013, 54, 1318–1322. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ionsby thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef]
- Duc Vu Quyen, N.; Khieu, D.Q.; Tuyen, T.N.; Tin, D.X.; Hoang Diem, B.T. Carbon nanotubes: Synthesis via chemical vapour deposition without hydrogen, surface modification, and application. J. Chem. 2019, 2019, 4260153. [Google Scholar] [CrossRef] [Green Version]
- Venkata Ramana, D.K.; Harikishore Kumar Reddy, D.; Naresh Kumar, B.; Seshaiah, K.; Purna Chandra Rao, G.; Lu, C. Adsorption of Pb(II) from aqueous solutions by chemically modified zeolite supported carbon nanotubes: Equilibrium, kinetic, and thermodynamic studies. Sep. Sci. Technol. 2013, 48, 403–412. [Google Scholar] [CrossRef]
- Chen, P.H.; Hsu, C.F.; Tsai, D.D.-W.; Lu, Y.M.; Huang, W.-J. Adsorption of mercury from water by modified multiwalled carbon nanotubes: Adsorption behaviour and interference resistance by coexisting anions. Environ. Technol. 2014, 35, 1935–1944. [Google Scholar] [CrossRef]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269. [Google Scholar] [CrossRef]
- Lee, B.Y.; Seo, S.M.; Lee, D.J.; Lee, M.; Lee, J.; Cheon, J.H.; Cho, E.; Lee, H.; Chung, I.Y.; Park, Y.J.; et al. Biosensor system-on-a-chip including CMOS-based signal processing circuits and 64 carbon nanotube-based sensors for the detection of a neurotransmitter. Lab Chip 2010, 10, 894–898. [Google Scholar] [CrossRef]
- Fonverne, A.; Ricoul, F.; Demesmay, C.; Delattre, C.; Fournier, A.; Dijon, J.; Vinet, F. In situ synthesized carbon nanotubes as a new nanostructured stationary phase for microfabricated liquid chromatographic colum. Sens. Actuators B 2008, 129, 51510. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.; Hong, S.; Chung, T.D. Formaldehyde gas sensing chip based on single-walled carbon nanotubes and thin water layer. Chem. Commun. 2011, 47, 2892–2894. [Google Scholar] [CrossRef] [PubMed]
- Close, G.F.; Yasuda, S.; Paul, B.; Fujita, S.; Wong, H.-S.P. A 1 GHz integrated circuit with carbon nanotube interconnects and silicon transistors. Nano Lett. 2008, 8, 706–709. [Google Scholar] [CrossRef]
- Daneshvar, F.; Chen, H.; Noh, K.; Sue, H.-J. Critical challenges and advances in the carbon nanotube–metal interface for next-generation electronics. Nanoscale Adv. 2021, 3, 942–962. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Soon Huat, T. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruvengadam, M.; Rajakumar, G.; Swetha, V.; Ansari, M.A.; Alghamdi, S.; Almehmadi, M.; Halawi, M.; Kungumadevi, L.; Raja, V.; Sabura Sarbudeen, S.; et al. Recent Insights and Multifactorial Applications of Carbon Nanotubes. Micromachines 2021, 12, 1502. https://doi.org/10.3390/mi12121502
Thiruvengadam M, Rajakumar G, Swetha V, Ansari MA, Alghamdi S, Almehmadi M, Halawi M, Kungumadevi L, Raja V, Sabura Sarbudeen S, et al. Recent Insights and Multifactorial Applications of Carbon Nanotubes. Micromachines. 2021; 12(12):1502. https://doi.org/10.3390/mi12121502
Chicago/Turabian StyleThiruvengadam, Muthu, Govindasamy Rajakumar, Venkata Swetha, Mohammad Azam Ansari, Saad Alghamdi, Mazen Almehmadi, Mustafa Halawi, Lakshmanan Kungumadevi, Vaishnavi Raja, Sulthana Sabura Sarbudeen, and et al. 2021. "Recent Insights and Multifactorial Applications of Carbon Nanotubes" Micromachines 12, no. 12: 1502. https://doi.org/10.3390/mi12121502
APA StyleThiruvengadam, M., Rajakumar, G., Swetha, V., Ansari, M. A., Alghamdi, S., Almehmadi, M., Halawi, M., Kungumadevi, L., Raja, V., Sabura Sarbudeen, S., Madhavan, S., Rebezov, M., Ali Shariati, M., Sviderskiy, A., & Bogonosov, K. (2021). Recent Insights and Multifactorial Applications of Carbon Nanotubes. Micromachines, 12(12), 1502. https://doi.org/10.3390/mi12121502











