High-Adhesive Flexible Electrodes and Their Manufacture: A Review
Abstract
:1. Introduction
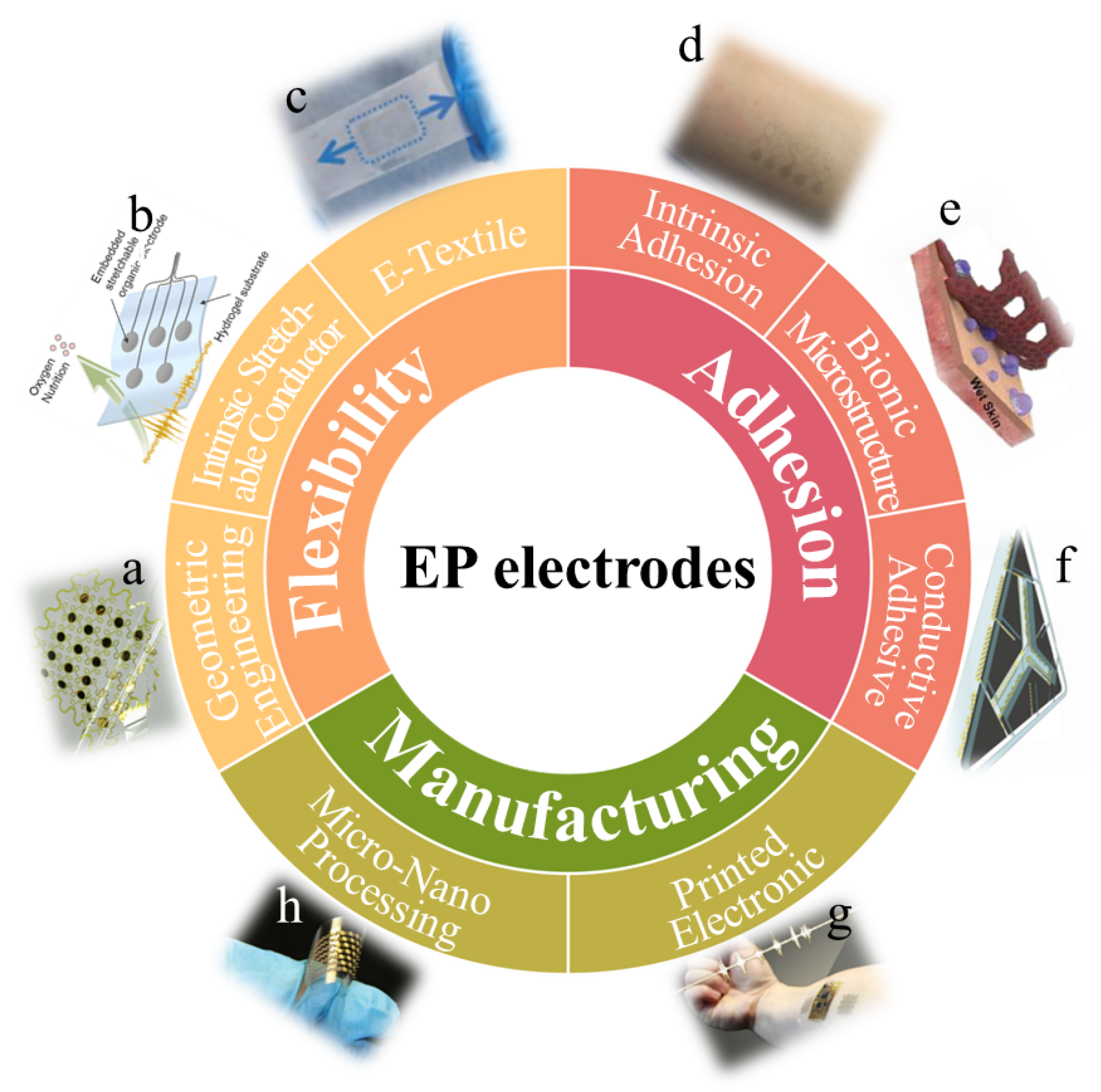
2. Fabrication Strategies for Flexible and Stretchable EP Electrodes
2.1. Geometric Engineering Fabrication
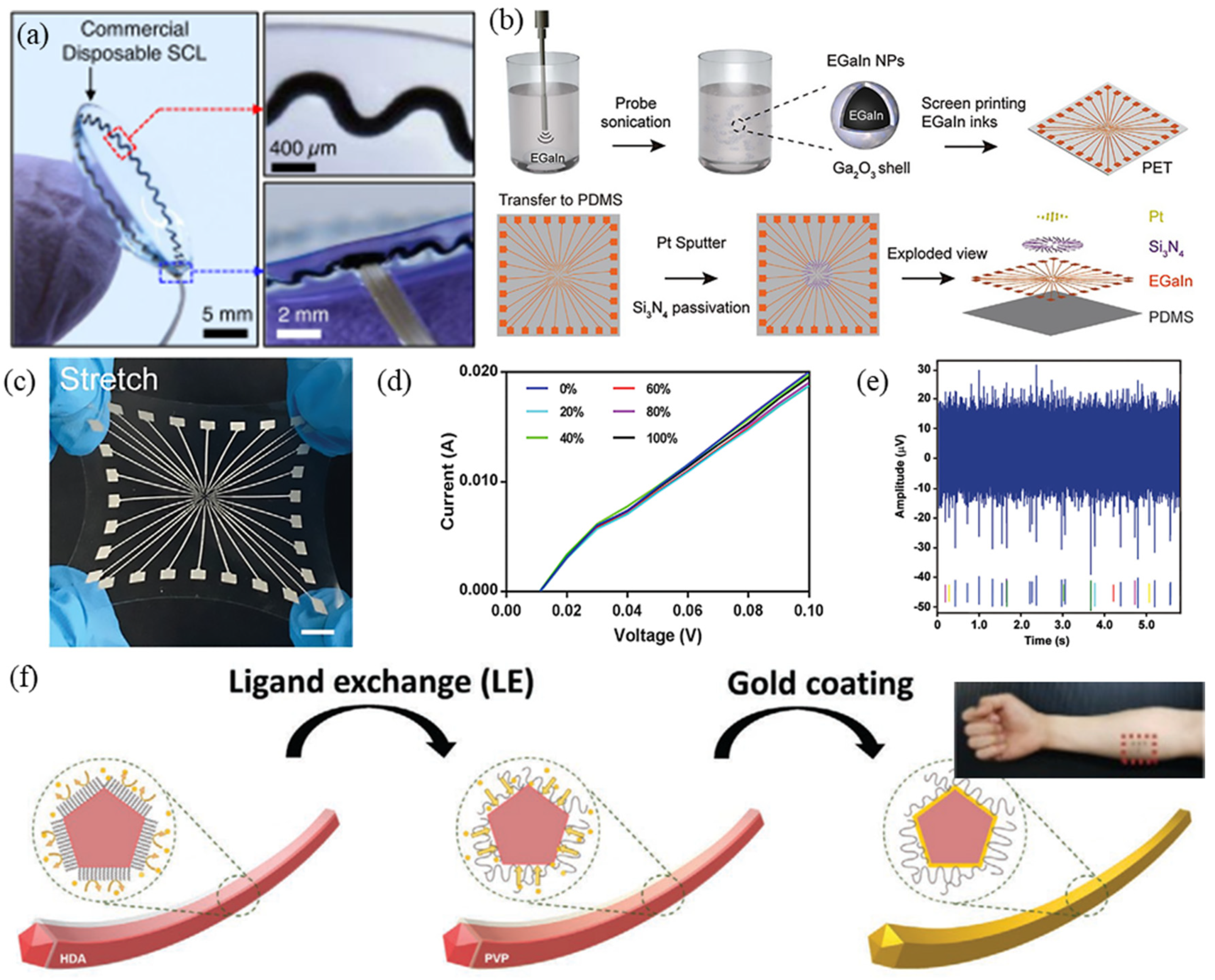
2.2. Intrinsic Stretchable Conductor Fabrication
2.2.1. Flexible EP Electrodes Based on Conductive Polymers
2.2.2. Flexible EP Electrodes Based on LM
2.2.3. Flexible EP Electrodes Based on Conductive Hydrogel
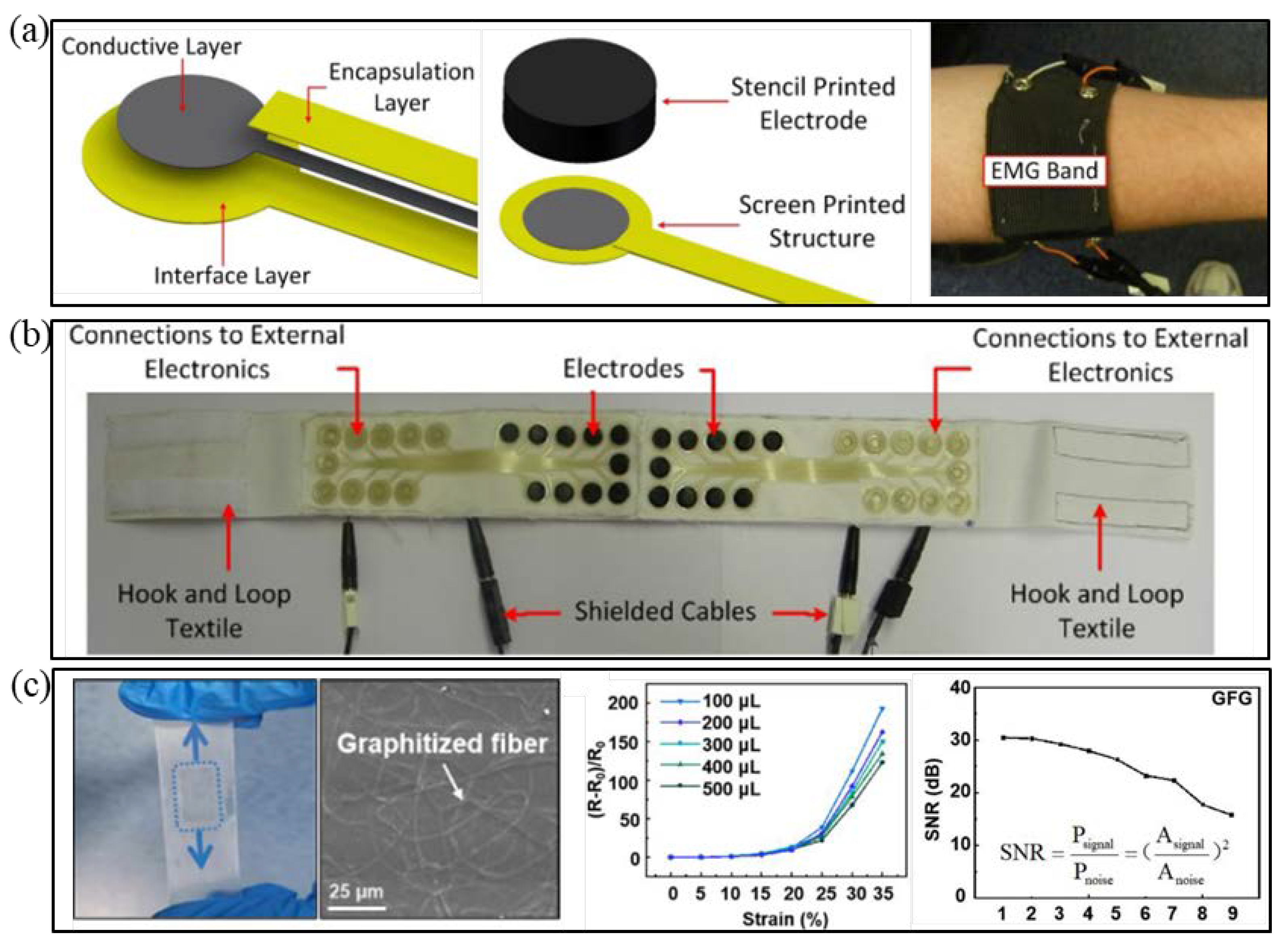
2.3. E-Textile Fabrication
3. Fabrication Strategies for High-Adhesive EP Electrodes
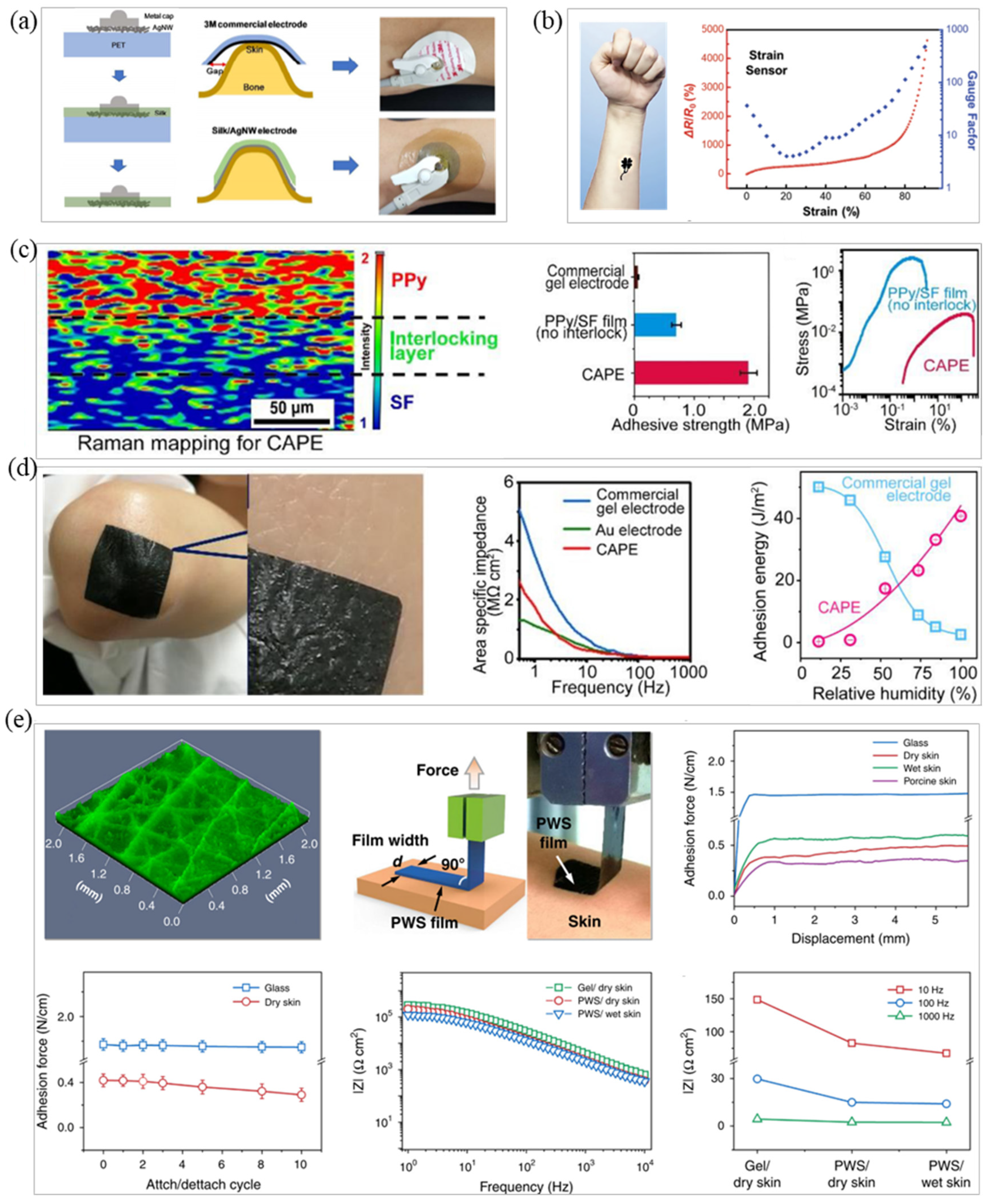
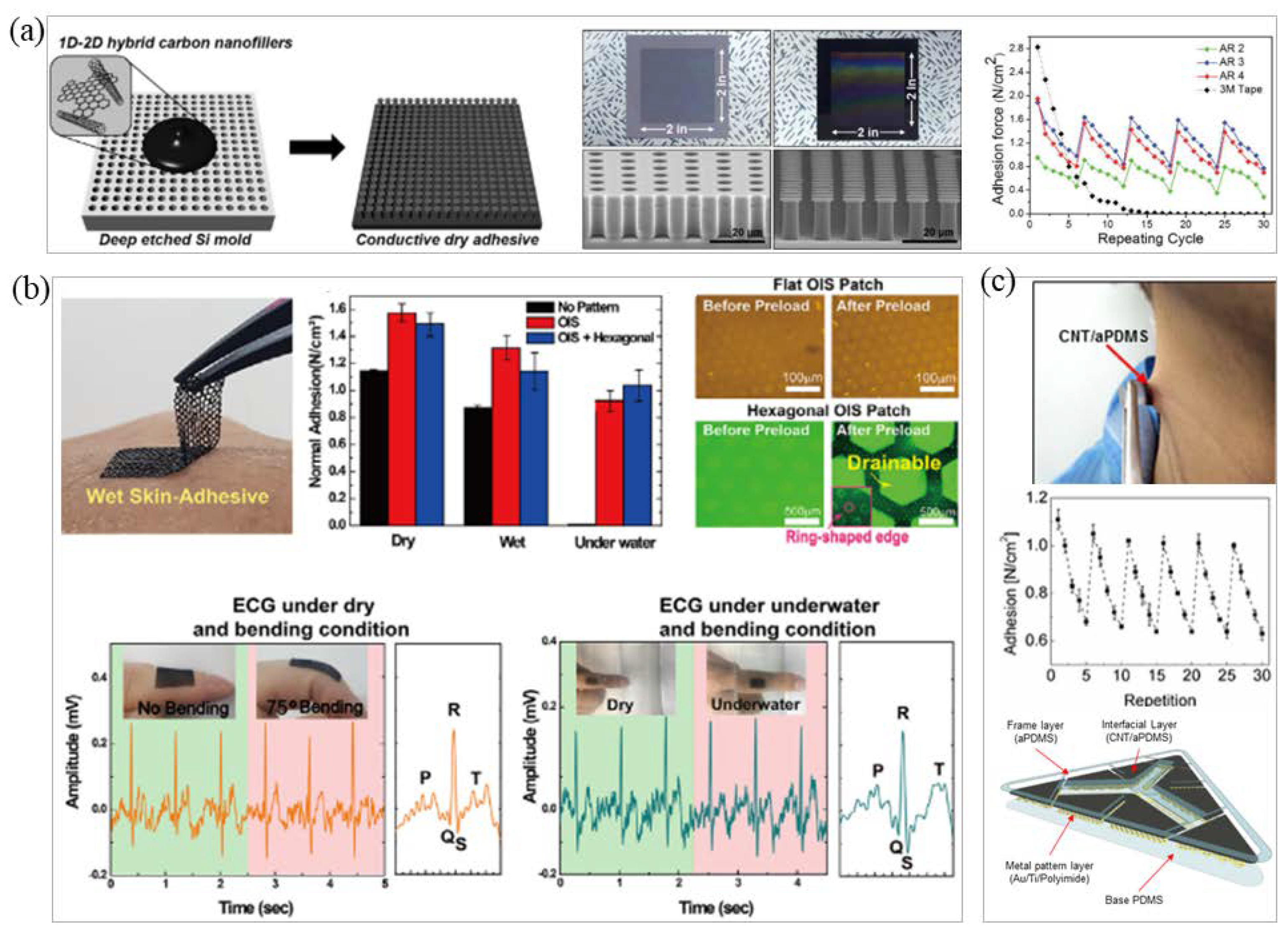
3.1. Intrinsic Adhesive Material Fabrication
3.2. Bionic Microstructure Fabrication
3.3. Conductive Adhesive Fabrication
4. Processing Technology for High-Adhesion Flexible EP Electrodes
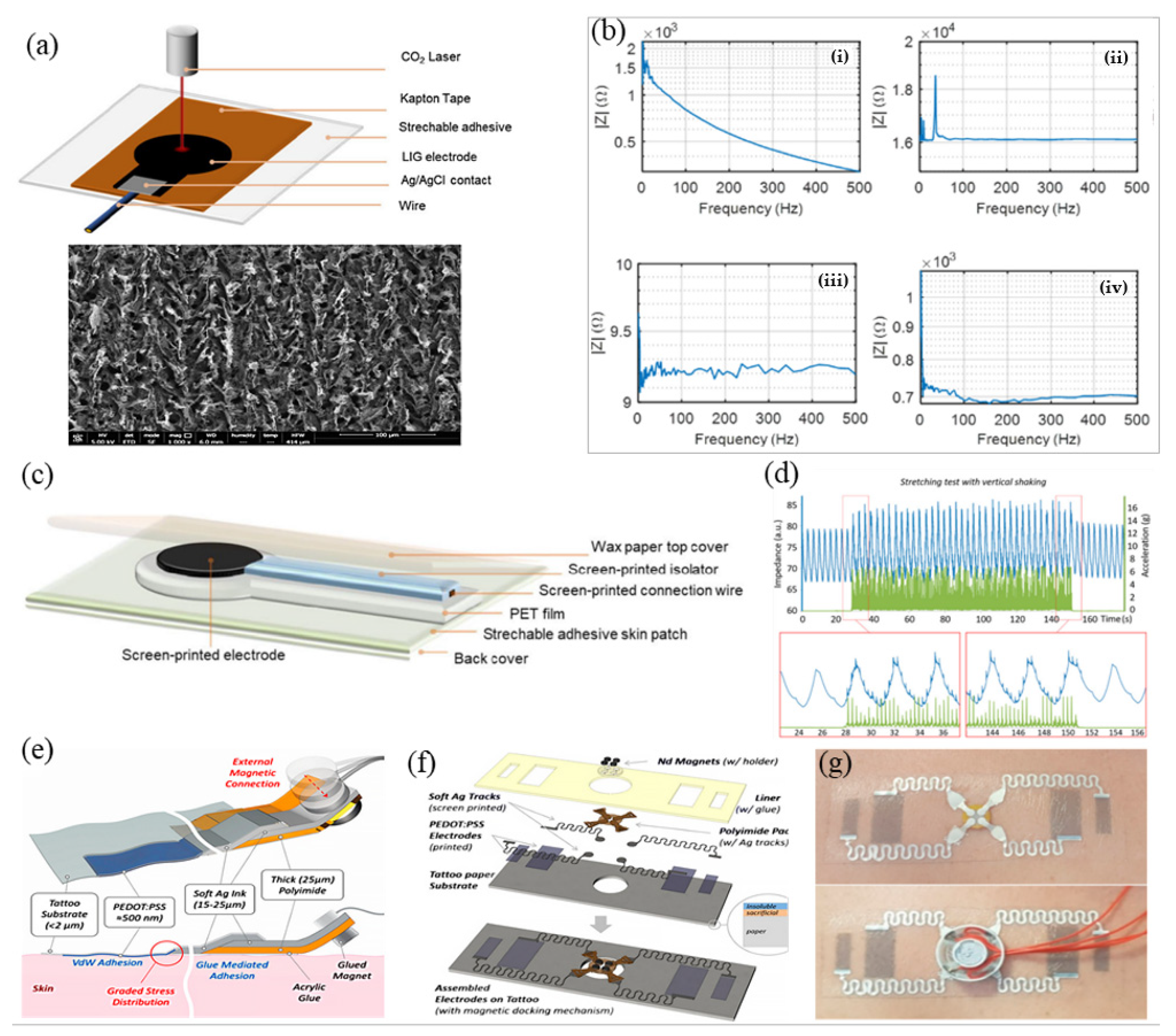
4.1. Manufacture of Micro-Nano Processing Technology
4.2. Manufacture of Printed Electronic Technology
4.2.1. Manufacturing Based on 2D PEs
4.2.2. Manufacturing Based on 3D PEs
5. Conclusions
Funding
Conflicts of Interest
References
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. The development of screen printed conductive networks on textiles for biopotential monitoring applications. Sens. Actuators A Phys. 2014, 206, 35–41. [Google Scholar] [CrossRef]
- Sugiarto, T.; Hsu, C.-L.; Sun, C.-T.; Hsu, W.-C.; Ye, S.-H.; Lu, K.-T. Surface EMG vs. High-Density EMG: Tradeoff Between Performance and Usability for Head Orientation Prediction in VR Application. IEEE Access 2021, 9, 45418–45427. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Hwang, J.-Y.; Chung, J.; Jang, T.-M.; Seo, D.G.; Gao, Y.; Lee, J.; Park, H.; Lee, S.; et al. 3D Printed, Customizable, and Multifunctional Smart Electronic Eyeglasses for Wearable Healthcare Systems and Human–Machine Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 21424–21432. [Google Scholar] [CrossRef]
- Won, Y.; Lee, J.J.; Shin, J.; Lee, M.; Kim, S.; Gandla, S. Biocompatible, Transparent, and High-Areal-Coverage Kirigami PE-DOT:PSS Electrodes for Electrooculography-Derived Human-Machine Interactions. ACS Sens. 2021, 6, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human–Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2020, 31, 2008936. [Google Scholar] [CrossRef]
- Toral, V.; Castillo, E.; Albretch, A.; Romero, F.J.; Garcia, A.; Rodriguez, N.; Lugli, P.; Morales, D.P.; Rivadeneyra, A. Cost-Effective Printed Electrodes Based on Emerging Materials Applied to Biosignal Acquisition. IEEE Access 2020, 8, 127789–127800. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Goldman, D.E. Potential, Impedance, and Rectification in Membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Lu, Q.; Zheng, L.; Wang, X.; Huang, W. Flexible Sensing Technology for Bioelectricity. Mater. Rep. 2020, 34, 1095–1106. [Google Scholar]
- Liu, J.; Liu, M.; Bai, Y.; Zhang, J.; Liu, H.; Zhu, W. Recent Progress in Flexible Wearable Sensors for Vital Sign Monitoring. Sensors 2020, 20, 4009. [Google Scholar] [CrossRef]
- Lu, F.; Wang, C.; Zhao, R.; Du, L.; Fang, Z.; Guo, X.; Zhao, Z. Review of Stratum Corneum Impedance Measurement in Non-Invasive Penetration Application. Biosensors 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piervirgili, G.; Petracca, F.; Merletti, R. A new method to assess skin treatments for lowering the impedance and noise of indi-vidual gelled Ag–AgCl electrodes. Physiol. Meas. 2014, 35, 2101–2118. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Qin, Z.; Xu, H.; Shu, X.; Gu, G.; Zhu, X. Soft ionic-hydrogel electrodes for electroencephalography signal recording. Sci. China Ser. E Technol. Sci. 2021, 64, 273–282. [Google Scholar] [CrossRef]
- Qin, Q.; Li, J.; Yao, S.; Liu, C.; Huang, H.; Zhu, Y. Electrocardiogram of a Silver Nanowire Based Dry Electrode: Quantitative Comparison With the Standard Ag/AgCl Gel Electrode. IEEE Access 2019, 7, 20789–20800. [Google Scholar] [CrossRef]
- Harati, A.; Jahanshahi, A. A reliable stretchable dry electrode for monitoring of EEG signals. Sens. Actuators A Phys. 2021, 326, 112727. [Google Scholar] [CrossRef]
- Janz, G.J.; Ives, D.J.G. Silver, silver chloride electrodes. Ann. N. Y. Acad. Sci. 1968, 148, 210–221. [Google Scholar] [CrossRef]
- Spiegler, K. On the measurement of streaming potentials with silver-silver chloride electrodes. Desalination 1974, 15, 135–140. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef]
- Fonseca, C.; Cunha, J.P.S.; Martins, R.E.; Ferreira, V.M.; De Sa, J.P.M.; Barbosa, M.A.; Da Silva, A.M. A Novel Dry Active Electrode for EEG Recording. IEEE Trans. Biomed. Eng. 2006, 54, 162–165. [Google Scholar] [CrossRef]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.; Tai, L.-C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhu, Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Niu, X.; Gao, X.; Liu, Y.; Liu, H. Surface Bioelectric Dry Electrodes: A Review. Measurement 2021, 183, 109774. [Google Scholar] [CrossRef]
- Kim, N.; Lim, T.; Song, K.; Yang, S.; Lee, J. Stretchable Multichannel Electromyography Sensor Array Covering Large Area for Controlling Home Electronics with Distinguishable Signals from Multiple Muscles. ACS Appl. Mater. Interfaces 2016, 8, 21070–21076. [Google Scholar] [CrossRef]
- Oribe, S.; Yoshida, S.; Kusama, S.; Osawa, S.-I.; Nakagawa, A.; Iwasaki, M.; Tominaga, T.; Nishizawa, M. Hydrogel-Based Organic Subdural Electrode with High Conformability to Brain Surface. Sci. Rep. 2019, 9, 13379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Yu, T.; Zhang, W.; Zhao, Z.; Zhang, Y.; Ye, G.; Zhao, Y.; Du, X.; Liu, X.; Yang, L.; et al. A Bioinspired, Durable, and Nondisposable Transparent Graphene Skin Electrode for Electrophysiological Signal Detection. ACS Mater. Lett. 2020, 2, 999–1007. [Google Scholar] [CrossRef]
- Ameri, S.K.; Hongwoo, J.; Jang, H.; Tao, L.; Wang, Y.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N. Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641. [Google Scholar] [CrossRef]
- Min, H.; Jang, S.; Kim, D.W.; Kim, J.; Baik, S.; Chun, S.; Pang, C. Highly Air/Water-Permeable Hierarchical Mesh Architectures for Stretchable Underwater Electronic Skin Patches. ACS Appl. Mater. Interfaces 2020, 12, 14425–14432. [Google Scholar] [CrossRef]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.-H. Self-adhesive epidermal carbon nanotube electronics for tether-free long-term continuous recording of biosignals. Sci. Rep. 2014, 4, srep06074. [Google Scholar] [CrossRef]
- Kwon, Y.-T.; Kim, H.; Mahmood, M.; Kim, Y.-S.; Demolder, C.; Yeo, W.-H. Printed, Wireless, Soft Bioelectronics and Deep Learning Algorithm for Smart Human–Machine Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 49398–49406. [Google Scholar] [CrossRef]
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of Flexible Microneedle Array Electrodes for Wearable Bio-Signal Recording. Sensors 2018, 18, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Kang, S.-Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A.; et al. An elastic second skin. Nat. Mater. 2016, 15, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, Devices, and Systems of On-Skin Electrodes for Elec-trophysiological Monitoring and Human-Machine Interfaces. Adv. Sci. (Weinh.) 2021, 8, 2001938. [Google Scholar]
- Marques, D.G.; Lopes, P.A.; de Almeida, A.T.; Majidi, C.; Tavakoli, M. Reliable interfaces for EGaIn multi-layer stretchable circuits and microelectronics. Lab A Chip 2019, 19, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, e1904765. [Google Scholar] [CrossRef] [Green Version]
- Mackanic, D.G.; Chang, T.-H.; Huang, Z.; Cui, Y.; Bao, Z. Stretchable electrochemical energy storage devices. Chem. Soc. Rev. 2020, 49, 4466–4495. [Google Scholar] [CrossRef]
- Shahandashti, P.F.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly conformable stretchable dry electrodes based on inexpensive flex substrate for long-term biopotential (EMG/ECG) monitoring. Sens. Actuators A Phys. 2019, 295, 678–686. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.J.; Zhang, H.; Park, W.; Meyer, D.; Kim, M.K.; Kim, B.; Park, H.; Xu, B.; Kollbaum, P.; et al. All-printed stretchable corneal sensor on soft contact lenses for noninvasive and painless ocular electrodiagnosis. Nat. Commun. 2021, 12, 1544. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Bai, Y.; Liu, S.; Wang, L.; Zhou, Y.; Hou, C.; Yang, Z.; Wu, H.; Ma, J.; et al. Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale. Sci. Adv. 2020, 6, eabd0996. [Google Scholar] [CrossRef]
- Fan, J.A.; Yeo, W.-H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.-Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Liu, X.; Cheng, S.; Tang, L.; Chen, M.; Zhong, L.; Chen, Z.; Liu, S.; Jiang, X. Highly Stretchable Metal–Polymer Conductor Electrode Array for Electrophysiology. Adv. Heal. Mater. 2021, 10, 2000641. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bang, J.; Won, P.; Kim, Y.; Jung, J.; Lee, J.; Kwon, J.; Lee, H.; Hong, S.; Jeon, N.L.; et al. Biocompatible Cost-Effective Electrophysiological Monitoring with Oxidation-Free Cu–Au Core–Shell Nanowire. Adv. Mater. Technol. 2020, 5, 2000661. [Google Scholar] [CrossRef]
- Koncar, V. Smart textiles for monitoring and measurement applications. In Smart Textiles for In Situ Monitoring of Composites; Elsevier BV: Amsterdam, The Netherlands, 2019; 151p. [Google Scholar]
- Upadhyay, J.; Das, T.M.; Borah, R. Electrochemical performance study of polyaniline and polypyrrole based flexible electrodes. Int. J. Polym. Anal. Charact. 2021, 26, 354–363. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Fiáth, R.; Hofer, K.T.; Csikós, V.; Horváth, D.; Nánási, T.; Tóth, K.; Pothof, F.; Böhler, C.; Asplund, M.; Ruther, P.; et al. Long-term recording performance and biocompatibility of chronically implanted cylindrically-shaped, polymer-based neural interfaces. Biomed. Tech. Eng. 2018, 63, 301–315. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z. Printed Electronics: Materials, Technologies and Applications; Higher Education Press: Beijing, China, 2016; 341p. [Google Scholar] [CrossRef]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, e1901408. [Google Scholar] [CrossRef]
- Jeerapan, I.; Ma, N. Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices. C 2019, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, Y.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. A novel method of fabricating carbon nanotubes-polydimethylsiloxane composite electrodes for electrocardiography. J. Biomater. Sci. Polym. Ed. 2015, 26, 1229–1235. [Google Scholar] [CrossRef]
- Cheng, X.; Bao, C.; Dong, W. Soft dry electroophthalmogram electrodes for human machine interaction. Biomed. Microdevices 2019, 21, 103. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zhong, M.; Zhou, Y.; Wang, Y.; Yu, T.; Xu, X.; Shen, W.; Yang, L.; Liu, N.; et al. Micro-nano hybrid-structured conductive film with ultrawide range pressure-sensitivity and bioelectrical acquirability for ubiquitous wearable applications. Appl. Mater. Today 2020, 20, 100651. [Google Scholar] [CrossRef]
- Fan, Y.J.; Yu, P.T.; Liang, F.; Li, X.; Li, H.Y.; Liu, L.; Cao, J.W.; Zhao, X.J.; Wang, Z.L.; Zhu, G. Highly conductive, stretchable, and breathable epidermal electrode based on hierarchically interactive nano-network. Nanoscale 2020, 12, 16053–16062. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Lee, S.-H.; Ko, P.; Kwon, S.; Youn, H.; Seok, J.Y.; Woo, K. Control of thermal deformation with photonic sintering of ultrathin nanowire transparent electrodes. Nanoscale 2020, 12, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-T.; Youn, H. Facile Fabrication of Highly Conductive, Ultrasmooth, and Flexible Silver Nanowire Electrode for Organic Optoelectronic Devices. ACS Appl. Mater. Interfaces 2019, 11, 42469–42478. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Woo, K.; Kim, I.; Kim, H.; Ko, P.; Kang, D.; Kwon, S.; Kim, H.; Youn, H.; Moon, J. Transparent Electronics: De-fect-Free, Highly Uniform Washable Transparent Electrodes Induced by Selective Light Irradiation (Small 21/2018). Small 2018, 14, 1870094. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yao, S.; Wang, H.; Du, Q.; Ma, Y.; Zhu, Y. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepulveda, M.S. Toxicological studies on silver nano-particles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine (Lond.) 2011, 6, 879–898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiu, W.; Gan, S.; Shan, J.; Ren, S.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Antibody-Functionalized MoS2 Nanosheets for Targeted Photothermal Therapy of Staphylococcus aureus Focal Infection. Front. Bioeng. Biotechnol. 2019, 7, 218. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Liu, Q.; Cheng, W.; Wang, X.; Pan, L.; Xu, B.; Xu, H. A Self-Healable, Highly Stretchable, and Solution Processable Conductive Polymer Composite for Ultrasensitive Strain and Pressure Sensing. Adv. Funct. Mater. 2018, 28, 1705551. [Google Scholar] [CrossRef]
- Zhuo, G.; Jiang, X. Direct carboxylation of benzene catalyzed by cobalt complex. Chin. J. Org. Chem. 2003, 23, 54–56. [Google Scholar]
- Saunier, V.; Flahaut, E.; Blatche, M.C.; Bergaud, C.; Maziz, A. Carbon nanofiber-PEDOT composite films as novel microelec-trode for neural interfaces and biosensing. Biosens. Bioelectron. 2020, 165, 112413. [Google Scholar] [CrossRef]
- Lee, S.; Ozlu, B.; Eom, T.; Martin, D.C.; Shim, B.S. Electrically conducting polymers for bio-interfacing electronics: From neural and cardiac interfaces to bone and artificial tissue biomaterials. Biosens. Bioelectron. 2020, 170, 112620. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Ke, K.; Manas-Zloczower, I. Interface Design Strategy for the Fabrication of Highly Stretchable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 36483–36492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tao, J.; Li, S.; Khashab, N.M. Compositing polyetherimide with polyfluorene wrapped carbon nanotubes for en-hanced interfacial interaction and conductivity. ACS Appl. Mater. Interfaces 2014, 6, 9013–9022. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, S.; Rao, W.; Liu, J. Transformable soft liquid metal micro/nanomaterials. Mater. Sci. Eng. R Rep. 2019, 138, 1–35. [Google Scholar] [CrossRef]
- Kramer, R.K.; Majidi, C.; Wood, R.J. Masked Deposition of Gallium-Indium Alloys for Liquid-Embedded Elastomer Con-ductors. Adv. Funct. Mater. 2013, 23, 5292–5296. [Google Scholar] [CrossRef]
- Lu, T.; Finkenauer, L.; Wissman, J.; Majidi, C. Rapid Prototyping for Soft-Matter Electronics. Adv. Funct. Mater. 2014, 24, 3351–3356. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Wang, Y.; Zhang, A. Progress of Polymer/Gallium-based Liquid Metal Composites. Polym. Mater. Sci. Eng. 2021, 37, 327–334. [Google Scholar]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, e1706846. [Google Scholar] [CrossRef]
- Lear, T.R.; Hyun, S.-H.; Boley, J.W.; White, E.L.; Thompson, D.H.; Kramer, R.K. Liquid metal particle popping: Macroscale to nanoscale. Extrem. Mech. Lett. 2017, 13, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication, and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. (Weinh.) 2020, 7, 2000192. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Peng, B.; Weng, X.; Jiang, H. Fabrication of a novel liquid metal microelectrode in microfluidic chip. Mod. Phys. Lett. B 2021, 35, 2140005. [Google Scholar] [CrossRef]
- Ren, L.; Sun, S.; Casillas-Garcia, G.; Nancarrow, M.; Peleckis, G.; Turdy, M.; Du, K.; Xu, X.; Li, W.; Jiang, L.; et al. A Liquid-Metal-Based Magnetoactive Slurry for Stimuli-Responsive Mechanically Adaptive Electrodes. Adv. Mater. 2018, 30, e1802595. [Google Scholar] [CrossRef]
- Li, F.; Qin, Q.; Zhou, Y.; Wu, Y.; Xue, W.; Gao, S.; Shang, J.; Liu, Y.; Li, R.-W. Recyclable Liquid Metal-Based Circuit on Paper. Adv. Mater. Technol. 2018, 3, 1800131. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Wang, M.; Zhang, Y.; Liu, L.; Yang, W. An Electrically and Mechanically Autonomic Self-healing Hybrid Hydrogel with Tough and Thermoplastic Properties. ACS Appl. Mater. Interfaces 2017, 9, 11134–11143. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tsai, Y.-L.; Hsu, S.-H. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 5040062. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, M.; Wang, K.; Fang, L.; Zhou, J.; Fang, J.; Ren, F.; Lu, X. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018, 30, 5561–5572. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Liu, Y.; Rodrigo, M.; Loftus, P.D.; Aparicio-Valenzuela, J.; Zheng, J.; Pong, T.; Cyr, K.J.; Babakhanian, M.; et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl. Acad. Sci. USA 2020, 117, 14769–14778. [Google Scholar] [CrossRef]
- Sun, C.; Hou, C.; Zhang, H.; Li, Y.; Zhang, Q.; Wang, H. Ultra-stretchable, self-adhesive, transparent, and ionic conductive organohydrogel for flexible sensor. APL Mater. 2021, 9, 011101. [Google Scholar] [CrossRef]
- He, P.; Wu, J.; Pan, X.; Chen, L.; Liu, K.; Gao, H.; Wu, H.; Cao, S.; Huang, L.; Ni, Y. Anti-freezing and moisturizing conductive hydrogels for strain sensing and moist-electric generation applications. J. Mater. Chem. A 2020, 8, 3109–3118. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Fleury, A.; Sugar, M.; Chau, T. E-textiles in Clinical Rehabilitation: A Scoping Review. Electronics 2015, 4, 173–203. [Google Scholar] [CrossRef] [Green Version]
- Ismar, E.; Bahadir, S.K.; Kalaoglu, F.; Koncar, V. Futuristic Clothes: Electronic Textiles and Wearable Technologies. Glob. Chall. 2020, 4, 1900092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoppa, M.; Chiolerio, A. Testing and evaluation of wearable electronic textiles and assessment thereof. In Performance Testing of Textiles; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 65–101. [Google Scholar]
- Huang, Y.; Hu, H.; Zhu, M.; Meng, W.; Liu, C.; Pei, Z.; Hao, C.; Wang, Z.; Zhi, C. From Industrially Weavable and Knittable Highly Conductive Yarns to Large Wearable Energy Storage Textiles. ACS Nano 2015, 9, 4766–4775. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, F.; Li, Y.; Lu, H.; Zhang, N.; Ma, M. Bioinspired ultra-stretchable and anti-freezing conductive hydrogel fibers with ordered and reversible polymer chain alignment. Nat. Commun. 2018, 9, 3579. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhu, J.; Ye, M.; Li, Y.; Ma, J.; Liu, J. Super wear-resistant and conductive cotton fabrics based on sliver nanowires. J. Ind. Text. 2021, 18, 152808372098200. [Google Scholar] [CrossRef]
- Babu, K.F.; Subramanian, S.S.; Kulandainathan, M.A. Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr. Polym. 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.V.; Seshadri, D.T.; Nate, M.M.; Gore, A.V. Development of conductive cotton fabrics for heating devices. J. Appl. Polym. Sci. 2006, 102, 4690–4695. [Google Scholar] [CrossRef]
- Babu, K.F.; Senthilkumar, R.; Noel, M.; Kulandainathan, M.A. Polypyrrole microstructure deposited by chemical and electrochemical methods on cotton fabrics. Synth. Met. 2009, 159, 1353–1358. [Google Scholar] [CrossRef]
- Knittel, D.; Schollmeyer, E. Electrically high-conductive textiles. Synth. Met. 2009, 159, 1433–1437. [Google Scholar] [CrossRef]
- Homayounfar, S.Z.; Rostaminia, S.; Kiaghadi, A.; Chen, X.; Alexander, E.T.; Ganesan, D.; Andrew, T.L. Multimodal Smart Eyewear for Longitudinal Eye Movement Tracking. Matter 2020, 3, 1275–1293. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, S.; Shu, L.; Tao, X.-M. Low-dimensional carbon based sensors and sensing network for wearable health and environmental monitoring. Carbon 2017, 121, 353–367. [Google Scholar] [CrossRef]
- Lam, C.L.; Rajdi, N.N.Z.M.; Wicaksono, D.H.B. MWCNT/Cotton-based flexible electrode for electrocardiography. In SENSORS, 2013 IEEE; IEEE: Piscataway, NJ, USA, 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Eskandarian, L.; Lam, E.; Rupnow, C.; Meghrazi, M.A.; Naguib, H.E. Robust and Multifunctional Conductive Yarns for Biomedical Textile Computing. ACS Appl. Electron. Mater. 2020, 2, 1554–1566. [Google Scholar] [CrossRef]
- Li, Q.; Ding, C.; Yuan, W.; Xie, R.; Zhou, X.; Zhao, Y.; Yu, M.; Yang, Z.; Sun, J.; Tian, Q.; et al. Highly Stretchable and Permeable Conductors Based on Shrinkable Electrospun Fiber Mats. Adv. Fiber Mater. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Taccola, S.; Poliziani, A.; Santonocito, D.; Mondini, A.; Denk, C.; Ide, A.N.; Oberparleiter, M.; Greco, F.; Mattoli, V. Toward the Use of Temporary Tattoo Electrodes for Impedancemetric Respiration Monitoring and Other Electrophysiological Recordings on Skin. Sensors 2021, 21, 1197. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-R.; Kil, H.-J.; Kim, Y.-C.; Park, J.-W. Highly Conformable, Transparent Electrodes for Epidermal Electronics. Nano Lett. 2018, 18, 4531–4540. [Google Scholar] [CrossRef]
- Zahed, A.; Das, P.S.; Maharjan, P.; Barman, S.C.; Sharifuzzaman, M.; Yoon, S.H.; Park, J.Y. Flexible and robust dry electrodes based on electroconductive polymer spray-coated 3D porous graphene for long-term electrocardiogram signal monitoring system. Carbon 2020, 165, 26–36. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xu, Z.; Yang, Y.; Xu, Q.; Li, X.; Cheng, X.; Huang, Y.; Zhang, F.; Zhao, J.; Li, S.; et al. Transparent, stretchable and degradable protein electronic skin for biomechanical energy scavenging and wireless sensing. Biosens. Bioelectron. 2020, 169, 112567. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ji, S.; Chaturvedi, I.; Xia, H.; Wang, T.; Chen, G.; Pan, L.; Wan, C.; Qi, D.; Ong, Y.-S.; et al. Adhesive Biocomposite Electrodes on Sweaty Skin for Long-Term Continuous Electrophysiological Monitoring. ACS Mater. Lett. 2020, 2, 478–484. [Google Scholar] [CrossRef]
- Chen, G.; Matsuhisa, N.; Liu, Z.; Qi, D.; Cai, P.; Jiang, Y.; Wan, C.; Cui, Y.; Leow, W.R.; Liu, Z.; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater. 2018, 30, e1800129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Yao, B.; He, H.; Zhang, L.; Yue, S.; Wang, Z.; Ouyang, J. Self-Adhesive, Stretchable, Biocompatible, and Conductive Nonvolatile Eutectogels as Wearable Conformal Strain and Pressure Sensors and Biopotential Electrodes for Precise Health Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 20735–20745. [Google Scholar] [CrossRef]
- Agueda, J.R.H.S.; Chen, Q.; Maalihan, R.D.; Ren, J.; da Silva, Í.G.M.; Dugos, N.P.; Caldona, E.B.; Advincula, R.C. 3D printing of biomedically relevant polymer materials and biocompatibility. MRS Commun. 2021, 11, 197–212. [Google Scholar] [CrossRef]
- Jo, M.; Min, K.; Roy, B.; Kim, S.; Lee, S.; Park, J.-Y.; Kim, S. Protein-Based Electronic Skin Akin to Biological Tissues. ACS Nano 2018, 12, 5637–5645. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.X.; Du, X.H.; Yang, H. Highly Adhesive and Stretchable Polymers for the Interface of Cyber-human Inter-action. Chem. J. Chin. Univ.-Chin. 2021, 42, 1093–1113. [Google Scholar]
- Xu, Z.; Chen, L.; Lu, L.; Du, R.; Ma, W.; Cai, Y.; An, X.; Wu, H.; Luo, Q.; Xu, Q.; et al. A Highly-Adhesive and Self-Healing Elastomer for Bio-Interfacial Electrode. Adv. Funct. Mater. 2020, 31, 2006432. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Lee, Y.; Yim, S.G.; Lee, G.W.; Kim, S.; Kim, H.S.; Hwang, D.Y.; An, B.S.; Lee, J.H.; Seo, S.; Yang, S.Y. Self-Adherent Biodegradable Gelatin-Based Hydrogel Electrodes for Electrocardiography Monitoring. Sensors 2020, 20, 5737. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Yap, L.W.; Zhu, B.; Cheng, W. Multiscale Soft–Hard Interface Design for Flexible Hybrid Electronics. Adv. Mater. 2019, 32, e1902278. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D–2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, T.; Mondal, H.; Mondal, S.; Das, D.; Biri, S. Fabrication of a low-cost strap for holding precordial electrodes on the hirsute chest. J. Fam. Med. Prim. Care 2020, 9, 2359–2363. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Song, C.; Jin, S.W.; Lee, H.; Keum, K.; Lee, Y.H.; Lee, G.; Jeong, Y.R.; Ha, J.S. High performance flexible mi-cro-supercapacitor for powering a vertically integrated skin-attachable strain sensor on a bio-inspired adhesive. Nano Energy 2021, 83, 105837. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Wang, J.; Wang, T.; Jiang, Y.; Hu, J.; Liu, Z.; Chen, X.; Yu, J. Bioinspired, Microstructured Silk Fibroin Adhesives for Flexible Skin Sensors. ACS Appl. Mater. Interfaces 2020, 12, 5601–5609. [Google Scholar] [CrossRef]
- Jung, J.; Shin, S.; Kim, Y.T. Dry electrode made from carbon nanotubes for continuous recording of bio-signals. Microelectron. Eng. 2019, 203-204, 25–30. [Google Scholar] [CrossRef]
- Inoue, A.; Yuk, H.; Lu, B.; Zhao, X. Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv. 2020, 6, eaay5394. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nat. Cell Biol. 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, L.; Li, B.; Zhao, K.; Wang, Z.; Zhou, Y.; Ma, C.; Li, J.; Zhang, H.; Herrmann, A.; et al. Genetically Engineered Polypeptide Adhesive Coacervates for Surgical Applications. Angew. Chem. Int. Ed. Engl. 2021, 22, 3375. [Google Scholar]
- Dou, J.; Tang, L.; Mou, L.; Zhang, R.; Jiang, X. Stretchable conductive adhesives for connection of electronics in wearable devices based on metal-polymer conductors and carbon nanotubes. Compos. Sci. Technol. 2020, 197, 108237. [Google Scholar] [CrossRef]
- Shustak, S.; Inzelberg, L.; Steinberg, S.; Rand, D.; Pur, M.D.; Hillel, I.; Katzav, S.; Fahoum, F.; De Vos, M.; Mirelman, A.; et al. Home monitoring of sleep with a temporary-tattoo EEG, EOG and EMG electrode array: A feasibility study. J. Neural Eng. 2018, 16, 026024. [Google Scholar] [CrossRef]
- Espera, A.H.; Dizon, J.R.C.; Chen, Q.; Advincula, R.C. 3D-printing and advanced manufacturing for electronics. Prog. Addit. Manuf. 2019, 4, 245–267. [Google Scholar] [CrossRef]
- Da Luz, L.L.; Milani, R.; Felix, J.F.; Ribeiro, I.R.B.; Talhavini, M.; Neto, B.A.D.; Chojnacki, J.; Rodrigues, M.O.; Júnior, S. Inkjet Printing of Lanthanide–Organic Frameworks for Anti-Counterfeiting Applications. ACS Appl. Mater. Interfaces 2015, 7, 27115–27123. [Google Scholar] [CrossRef] [PubMed]
- Chou, N.; Yoo, S.; Kim, S. A Largely Deformable Surface Type Neural Electrode Array Based on PDMS. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, G.; Jeong, J.-W.; Li, X.; Scott, D.K.; Jang, K.-I.; Chung, H.-J.; Rogers, J.A.; Rieger, J. Epidermal electronics for electromyography: An application to swallowing therapy. Med. Eng. Phys. 2016, 38, 807–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, G.J.; Uddin, M.J.; Shim, J.S. Biomimetic Cilia-Patterned Rubber Electrode Using Ultra Conductive Polydimethylsiloxane. Adv. Funct. Mater. 2018, 28, 1804351. [Google Scholar] [CrossRef]
- Jung, J.M.; Cha, D.Y.; Kim, D.S.; Yang, H.J.; Choi, K.S.; Choi, J.M.; Chang, S.P. Development of PDMS-based flexible dry type SEMG electrodes by micromachining technologies. Appl. Phys. A 2014, 116, 1395–1401. [Google Scholar] [CrossRef]
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Zanow, F.; Haueisen, J. In Novel flexible dry PU/TiN-multipin electrodes: First application in EEG measurements. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 55–58. [Google Scholar]
- Yun, I.; Jeung, J.; Lim, H.; Kang, J.; Lee, S.; Park, S.; Seong, S.; Park, S.; Cho, K.; Chung, Y. Stable Bioelectric Signal Acquisition Using an Enlarged Surface-Area Flexible Skin Electrode. ACS Appl. Electron. Mater. 2021, 3, 1842–1851. [Google Scholar] [CrossRef]
- Kunnari, E.; Valkama, J.; Keskinen, M.; Mansikkamäki, P. Environmental evaluation of new technology: Printed electronics case study. J. Clean. Prod. 2009, 17, 791–799. [Google Scholar] [CrossRef]
- Cao, X.; Wu, F.; Lau, C.; Liu, Y.; Liu, Q.; Zhou, C. Top-Contact Self-Aligned Printing for High-Performance Carbon Nanotube Thin-Film Transistors with Sub-Micron Channel Length. ACS Nano 2017, 11, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Lim, H.-R.; Park, S.-W.; Kang, S.-O.; Choi, J.J.; Herbert, R.; Jang, Y.C.; et al. All-printed nanomembrane wireless bioelectronics using a biocompatible solderable graphene for multimodal human-machine interfaces. Nat. Commun. 2020, 11, 3450. [Google Scholar] [CrossRef]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R.; et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Chen, L.; Zhang, Y.; He, Z.; Zhang, W.; Zhao, J.; Lu, D.; He, J.; Zhu, H.; et al. Flexible and Stretchable Dry Active Electrodes With PDMS and Silver Flakes for Bio-Potentials Sensing Systems. IEEE Sens. J. 2021, 21, 12255–12268. [Google Scholar] [CrossRef]
- Guo, R.; Tang, J.; Dong, S.; Lin, J.; Wang, H.; Liu, J.; Rao, W. One-Step Liquid Metal Transfer Printing: Toward Fabrication of Flexible Electronics on Wide Range of Substrates. Adv. Mater. Technol. 2018, 3, 1800265. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, S.; Zhang, L.; Mi, H.; Mou, L.; Yang, S.; Huang, Z.; Shi, X.; Jiang, X. Printable Metal-Polymer Conductors for Highly Stretchable Bio-Devices. iScience 2018, 4, 302–311. [Google Scholar] [CrossRef]
- Zeng, X.; Dong, Y.; Wang, X. Flexible Electrode by Hydrographic Printing for Surface Electromyography Monitoring. Materials 2020, 13, 2339. [Google Scholar] [CrossRef] [PubMed]
- Shur, M.; Fallegger, F.; Pirondini, E.; Roux, A.; Bichat, A.; Barraud, Q.; Courtine, G.; Lacour, S.P. Soft Printable Electrode Coating for Neural Interfaces. ACS Appl. Bio Mater. 2020, 3, 4388–4397. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Saadaoui, M.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Inkjet-Printed PEDOT:PSS Electrodes on Paper for Electrocardiography. Adv. Healthc. Mater. 2017, 6, 1601167. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.M.; Sudha, S.; Tarantino, S.; Esposti, R.; Bolzoni, F.; Cavallari, P.; Cipriani, C.; Mattoli, V.; Greco, F. Ultraconformable Temporary Tattoo Electrodes for Electrophysiology. Adv. Sci. 2018, 5, 1700771. [Google Scholar] [CrossRef] [Green Version]
- Lo, L.-W.; Zhao, J.; Wan, H.; Wang, Y.; Chakrabartty, S.; Wang, C. An Inkjet-Printed PEDOT:PSS-Based Stretchable Conductor for Wearable Health Monitoring Device Applications. ACS Appl. Mater. Interfaces 2021, 13, 21693–21702. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Shi, W.; Peng, Z.; Luo, K.; Xing, S.; Li, J.; Liu, Z.; Liu, L. Self-Healing, Robust, and Stretchable Electrode by Direct Printing on Dynamic Polyurea Surface at Slightly Elevated Temperature. Adv. Funct. Mater. 2021, 31, 2102225. [Google Scholar] [CrossRef]
- Yuan, S.; Li, S.; Zhu, J.; Tang, Y. Additive manufacturing of polymeric composites from material processing to structural design. Compos. Part B Eng. 2021, 219, 108903. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, R.; Chen, K.; Wang, Y.; Niu, J.; Guo, Y.; Zhang, Y.; Yin, Z.; Xia, K.; Zhou, B.; et al. Microribbons composed of directionally self-assembled nanoflakes as highly stretchable ionic neural electrodes. Proc. Natl. Acad. Sci. USA 2020, 117, 14667–14675. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Zhan, Z.; Xie, M.; Duan, G.; Cheng, P.; Chen, Y.; Duan, H. 3D printed super-anti-freezing self-adhesive human-machine interface. Mater. Today Phys. 2021, 19, 100404. [Google Scholar] [CrossRef]


| Structures | EP Signal Type | Conductivity/Resistivity | SNR/RMS | Stretchability | Thickness | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|
| AgSEBS/disposable soft contact lens | EOG | 18.2 ± 3.8 Ω | / | 350% | 70 μm | 1500 | [38] |
| PEDOT:PSS/Ag/PI | EMG&ECG | ΔR/R = 0.1–1 Ω @64 kHz (under 10% strain) | SNR ≈ 20.7 dB vs. SNR ≈ 22.4 dB (Conv. 1) | 110% | ≈1.7 μm | / | [103] |
| PDMS/EGaIn NPs | ECG | ΔR/R < 7% (under 10% strain) | / | ≈100% | 100 μm | >400 | [41] |
| PDMS/AgNW | ECG | ≈35 Ω/sq | RMS ≈ 500 µV vs. RMS ≈ 450 µV (Conv.) | ≈400% | ≈2 mm | 1000 | [104] |
| PEDOT: PSS/WPU/ D-sorbitol | ECG&EOG&EEG | 545 S/cm | RMS ≈ 25 μV vs. RMS ≈ 28 μV (Conv.) | 130% | 20 μm | 40 | [18] |
| PEDOT:PSS/LIG | ECG | 17.4 Ω/sq@10 Hz | SNR ≈ 12.9 dB vs. SNR ≈ 13.3 dB (Conv.) | / | 100 μm | / | [105] |
| PEDOT:PSS ink | / | 155 S/cm | / | 113% | 100 μm | / | [106] |
| GFG/SEBS | EMG&ECG&EEG | ≈150 Ω/aq | SNR ≈ 30 dB | 35% | / | 10 | [26] |
| SBS/Ag-AuNWs | ECG&EMG | 72,600 S/cm | / | ≈840% | ≈20 µm | 3000 | [107] |
| SF/Au | EMG | 7 Ω/sq | SNR ≈ 17.17 dB vs. SNR ≈ 17.95 dB (Conv.) | >400% | 160 µm | 7500 | [108] |
| Materials | EP Signal Type | Adhesion | Other Performance | Ref. |
|---|---|---|---|---|
| TA-PVA-PAM organohydrogel | ECG | 80 kPa | UV-blocking > 90% | [82] |
| PDMS/dopamine/AgNWs | EMG | ~62 kPa | ≈16 kPa (underwater) | [115] |
| PU/CNT/Ag | ECG | ~1.5 N/cm2 | cycling stability ≈ 1000 | [28] |
| GET electrode | ECG&EMG&EEG | / | Transparency ≈ 85% | [27] |
| PDMS/graphene | ECG | ~1.3 N/cm2 | volume resistance (~100 Ohm·cm) | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Wang, M.; Li, Y.; Sun, Z.; Liu, Z.; He, L.; Liu, R. High-Adhesive Flexible Electrodes and Their Manufacture: A Review. Micromachines 2021, 12, 1505. https://doi.org/10.3390/mi12121505
Xiao Y, Wang M, Li Y, Sun Z, Liu Z, He L, Liu R. High-Adhesive Flexible Electrodes and Their Manufacture: A Review. Micromachines. 2021; 12(12):1505. https://doi.org/10.3390/mi12121505
Chicago/Turabian StyleXiao, Yingying, Mengzhu Wang, Ye Li, Zhicheng Sun, Zilong Liu, Liang He, and Ruping Liu. 2021. "High-Adhesive Flexible Electrodes and Their Manufacture: A Review" Micromachines 12, no. 12: 1505. https://doi.org/10.3390/mi12121505
APA StyleXiao, Y., Wang, M., Li, Y., Sun, Z., Liu, Z., He, L., & Liu, R. (2021). High-Adhesive Flexible Electrodes and Their Manufacture: A Review. Micromachines, 12(12), 1505. https://doi.org/10.3390/mi12121505







