The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Instrumentation
2.3. Preparation of Nanosensor
2.4. Preparation of Nanobiosensor
3. Results and Discussion
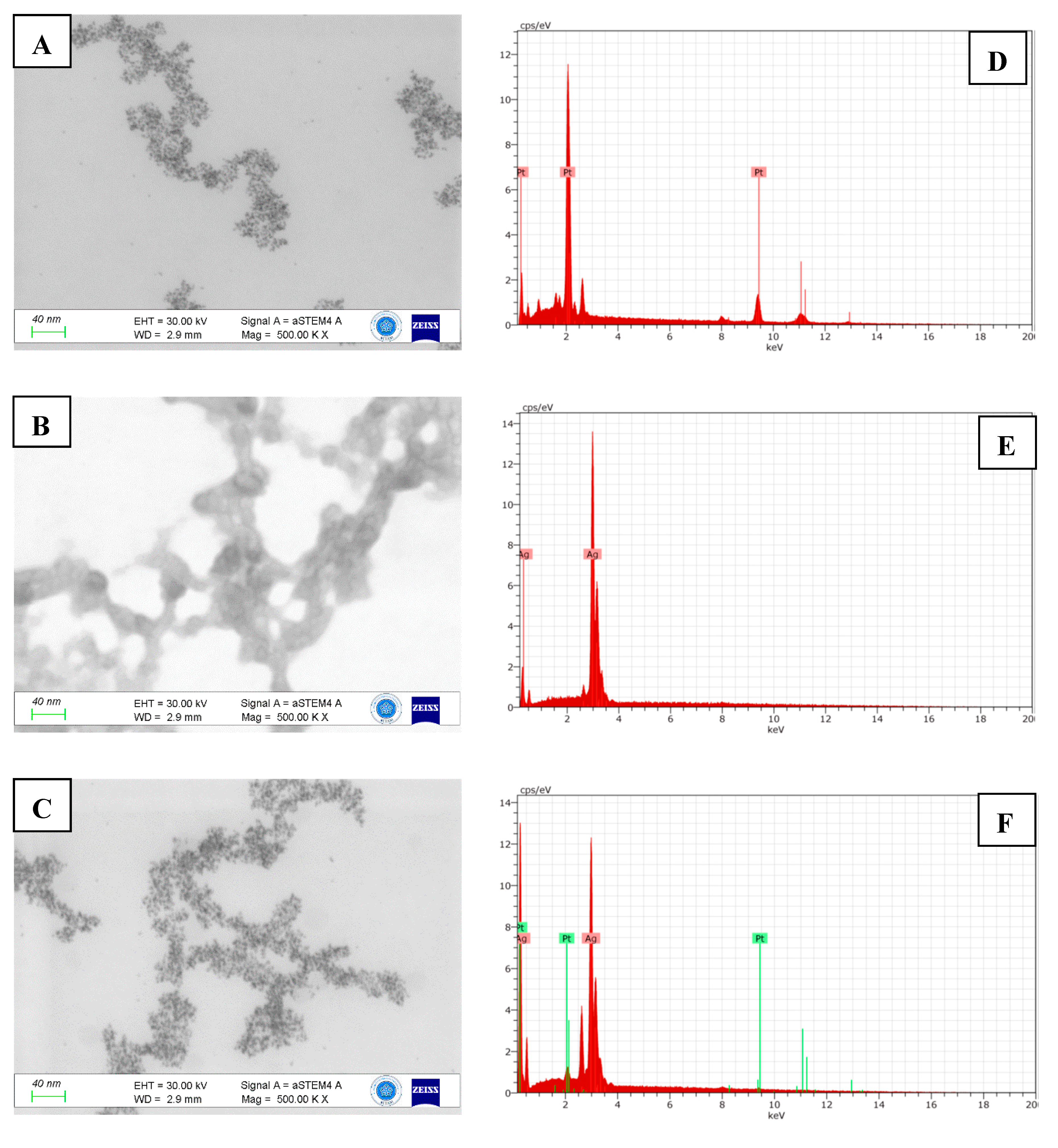
3.1. Characterization of Nanomaterials
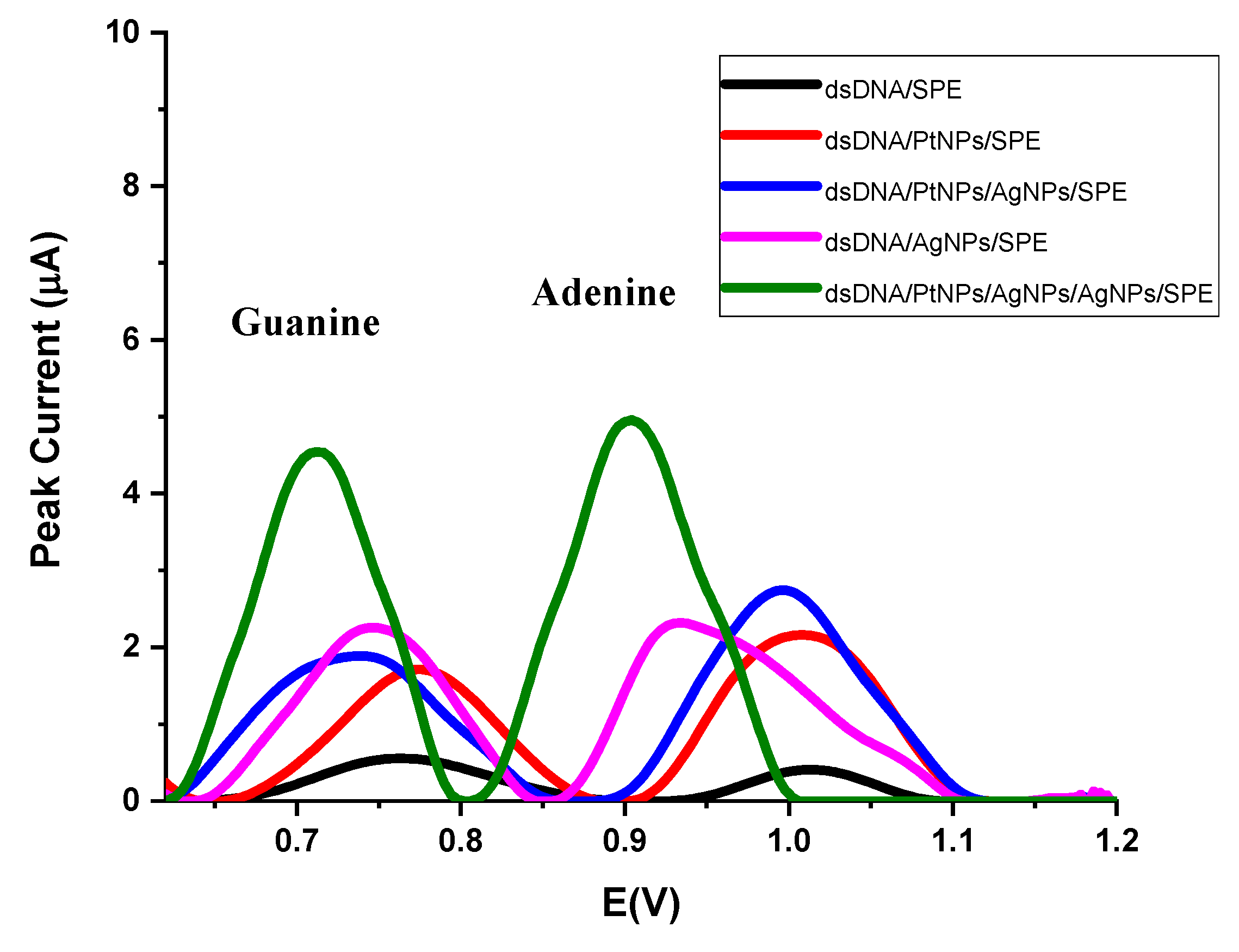
3.2. Optimization of Modified Electrodes
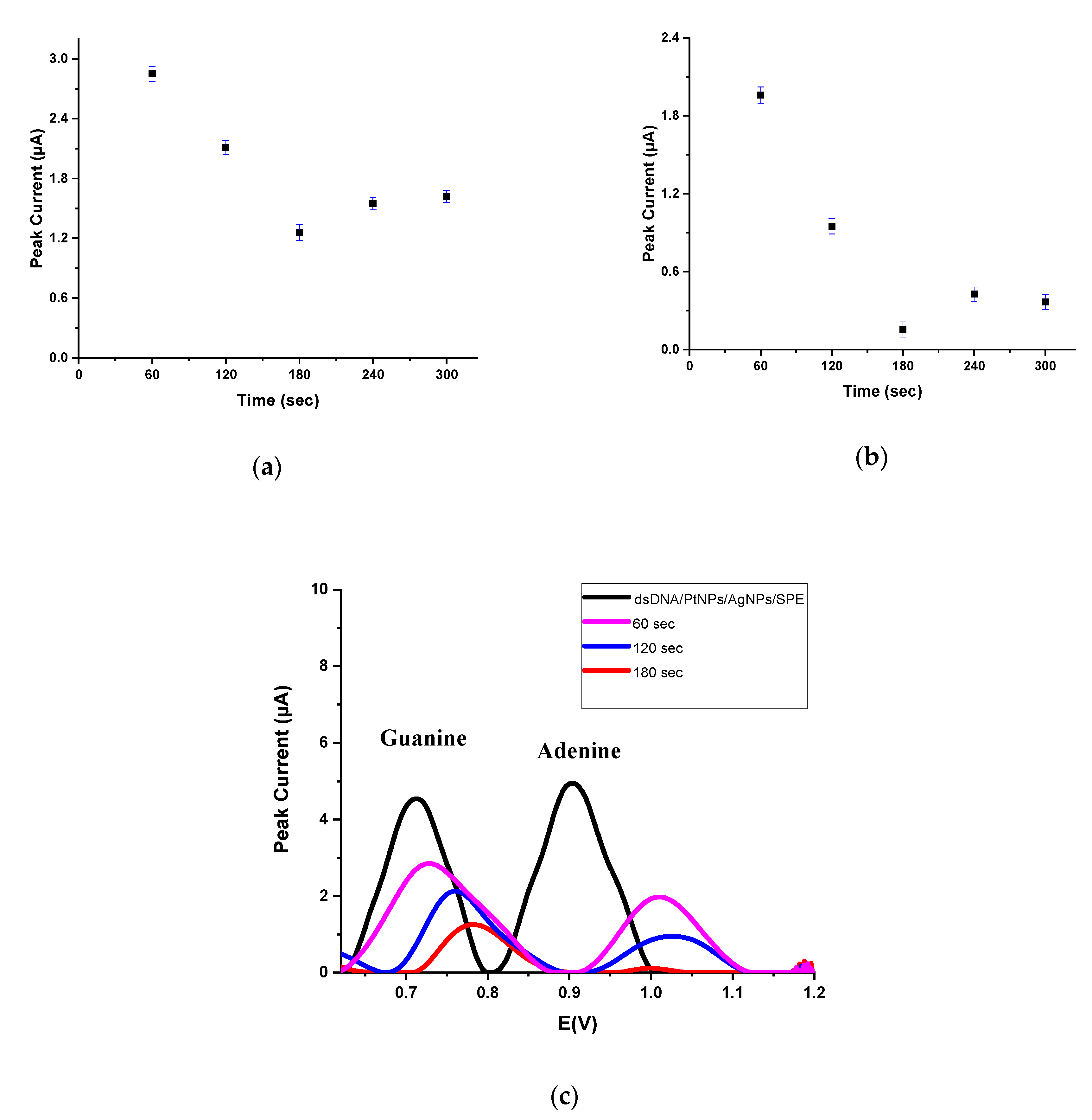
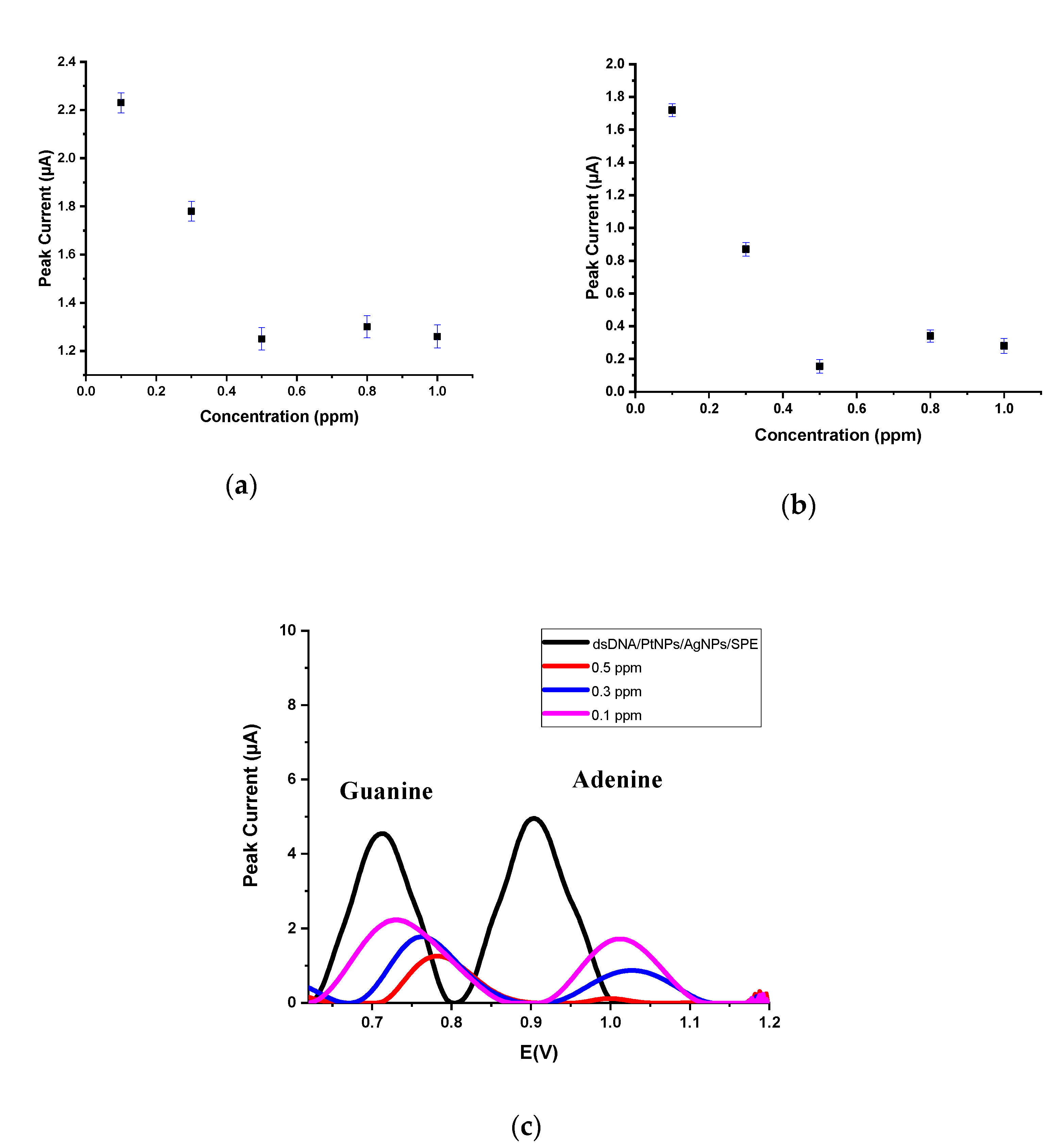
3.3. Application of Nanobiosensor for Drugs–DNA Interaction
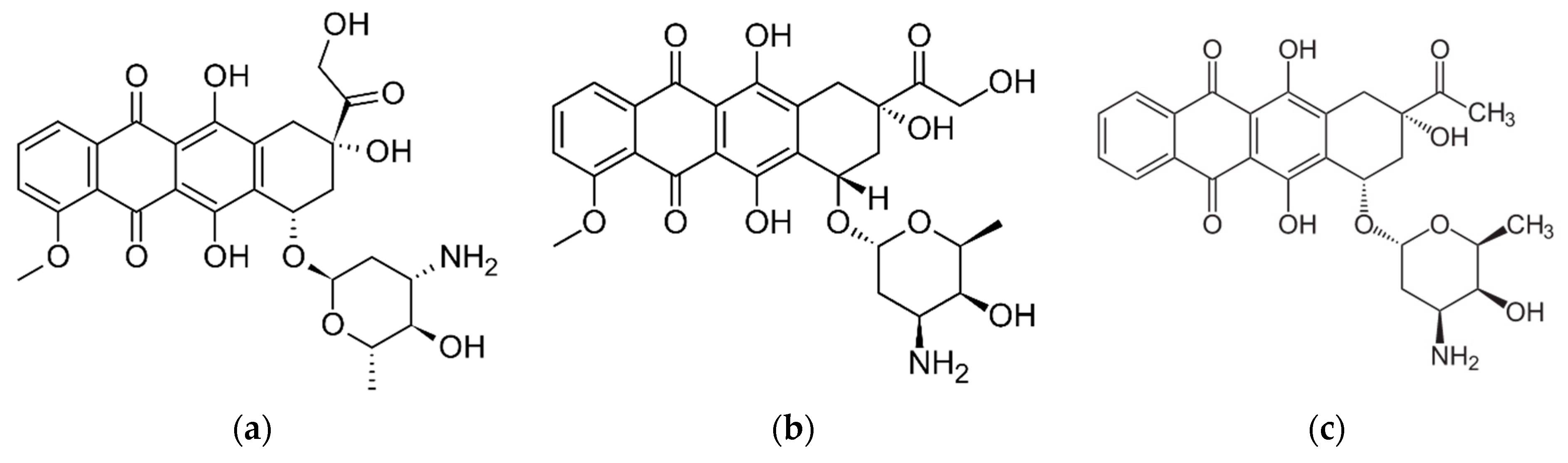
3.3.1. The Interaction between dsDNA and EPI
3.3.2. The Interaction between dsDNA and IDA
3.3.3. The Interaction between dsDNA and DOX
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palaska, P.; Aritzoglou, E.; Girousi, S. Sensitive detection of cyclophosphamide using DNA-modified carbon paste, pencil graphite and hanging mercury drop electrodes. Talanta 2007, 72, 1199–1206. [Google Scholar] [CrossRef]
- Palchaudhuri, R.; Hergenrother, P.J. DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Curr. Opin. Biotechnol. 2007, 18, 497–503. [Google Scholar] [CrossRef]
- Li, Y.Q.; Guo, Y.J.; Li, X.F.; Pan, J.H. Electrochemical studies of the interaction of Basic Brown G with DNA and determination of DNA. Talanta 2007, 71, 123–128. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Honda, K.; Rao, T.N.; Fujishima, A.; Einaga, Y. Simultaneous detection of purine and pyrimidine at highly boron-doped diamond electrodes by using liquid chromatography. Talanta 2007, 71, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L. Structural considerations in the interaction of DNA and acridines. J. Mol. Biol. 1961, 3, IN13–IN14. [Google Scholar] [CrossRef]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Fojta, M. Electrochemical Sensors for DNA Interactions and Damage. Electroanalysis 2002, 14, 1449–1463. [Google Scholar] [CrossRef]
- Castañeda, M.T.; Alegret, S.; Merkoçi, A. Electrochemical Sensing of DNA Using Gold Nanoparticles. Electroanalysis 2007, 19, 743–753. [Google Scholar] [CrossRef]
- Zhang, S.; Wright, G.; Yang, Y. Materials and techniques for electrochemical biosensor design and construction. Biosens. Bioelectron. 2000, 15, 273–282. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Nilsen, T.W.; Getts, R.C. Dendritic Nucleic Acid Probes for DNA Biosensors. J. Am. Chem. Soc. 1998, 120, 8281–8282. [Google Scholar] [CrossRef]
- Parab, H.J.; Jung, C.; Lee, J.-H.; Park, H.G. A gold nanorod-based optical DNA biosensor for the diagnosis of pathogens. Biosens. Bioelectron. 2010, 26, 667–673. [Google Scholar] [CrossRef]
- Fan, H.; Ju, P.; Ai, S. Controllable synthesis of CdSe nanostructures with tunable morphology and their application in DNA biosensor of Avian Influenza Virus. Sens. Actuators B Chem. 2010, 149, 98–104. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Y.; Qi, H.; Gao, Q.; Zhang, C. Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator. Biosens. Bioelectron. 2010, 25, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathiraja, C.; Kamatchiammal, S.; Adaikkappan, P.; Santhosh, D.; Alagar, M. Specific detection of Mycobacterium sp. genomic DNA using dual labeled gold nanoparticle-based electrochemical biosensor. Anal. Biochem. 2011, 417, 73–79. [Google Scholar] [CrossRef]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
- Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Martínez, M.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef] [Green Version]
- Stradiotto, N.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.; Radhakrishnan, S.; Sumathi, C.; Dharuman, V. Polypyrrole–polyaniline–Au (PPy–PANi–Au) nano composite films for label-free electrochemical DNA sensing. Sens. Actuators B Chem. 2012, 171–172, 216–222. [Google Scholar] [CrossRef]
- Rohrbach, F.; Karadeniz, H.; Erdem, A.; Famulok, M.; Mayer, G. Label-free impedimetric aptasensor for lysozyme detection based on carbon nanotube-modified screen-printed electrodes. Anal. Biochem. 2012, 421, 454–459. [Google Scholar] [CrossRef]
- Erdem, A.; Papakonstantinou, P.; Murphy, H. Direct DNA Hybridization at Disposable Graphite Electrodes Modified with Carbon Nanotubes. Anal. Chem. 2006, 78, 6656–6659. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical nucleic acid biosensors. Anal. Chim. Acta 2002, 469, 63–71. [Google Scholar] [CrossRef]
- Rafique, B.; Khalid, A.M.; Akhtar, K.; Jabbar, A. Interaction of anticancer drug methotrexate with DNA analyzed by electrochemical and spectroscopic methods. Biosens. Bioelectron. 2013, 44, 21–26. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Mangiferin DNA biosensor using double-stranded DNA modified pencil graphite electrode based on guanine and adenine signals. J. Electroanal. Chem. 2014, 720–721, 134–138. [Google Scholar] [CrossRef]
- Congur, G.; Erdem, A.; Mese, F. Electrochemical investigation of the interaction between topotecan and DNA at disposable graphite electrodes. Bioelectrochemistry 2015, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Katrahalli, U.; Seetharamappa, J. Electrochemical studies and spectroscopic investigations on the interaction of an anticancer drug with DNA and their analytical applications. J. Electroanal. Chem. 2009, 636, 93–100. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H.; Torkzadeh-Mahani, M. Electrochemical determination of the anticancer drug taxol at a ds-DNA modified pencil-graphite electrode and its application as a label-free electrochemical biosensor. Talanta 2015, 134, 60–64. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Sparapani, S.; Neidle, S.; Oliveira-Brett, A.M. Triazole–acridine conjugates: Redox mechanisms and in situ electrochemical evaluation of interaction with double-stranded DNA. Bioelectrochemistry 2013, 89, 50–56. [Google Scholar] [CrossRef]
- Dogan-Topal, B.; Bozal-Palabiyik, B.; Ozkan, S.A.; Uslu, B. Investigation of anticancer drug lapatinib and its interaction with dsDNA by electrochemical and spectroscopic techniques. Sens. Actuators B Chem. 2014, 194, 185–194. [Google Scholar] [CrossRef]
- Radi, A.-E.; Alnaggar, A.; Nassef, H.M. Electrochemical and Spectral studies on the Interaction of the Antiparasitic Drug Nitazoxanide with DNA. Electrochimica Acta 2014, 129, 259–265. [Google Scholar] [CrossRef]
- Erdem, A.; Congur, G. Impedimetric detection of in situ interaction between anti-cancer drug bleomycin and DNA. Int. J. Biol. Macromol. 2013, 61, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yunus, G.; Srivastava, S.; Kuddus, M.; Gupta, V. Drug–DNA interaction: A theoretical study on the binding of thionine with DNAs of varying base composition. Curr. Appl. Phys. 2013, 13, 322–326. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Impedimetric DNA-biosensor for the study of anti-cancer action of mitomycin C: Comparison between acid and electroreductive activation. Biosens. Bioelectron. 2014, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Zhao, W.; Chiuman, W.; Lam, J.C.F.; McManus, S.A.; Chen, W.; Cui, Y.; Pelton, R.; Brook, M.A.; Li, Y. DNA Aptamer Folding on Gold Nanoparticles: From Colloid Chemistry to Biosensors. J. Am. Chem. Soc. 2008, 130, 3610–3618. [Google Scholar] [CrossRef]
- Daniel, M.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- He, F.-G.; Yin, J.-Y.; Sharma, G.; Kumar, A.; Stadler, F.J.; Du, B. Facile Fabrication of Hierarchical rGO/PANI@PtNi Nanocomposite via Microwave-Assisted Treatment for Non-Enzymatic Detection of Hydrogen Peroxide. Nanomaterials 2019, 9, 1109. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Huai, S.; Jiao, J.; Xu, Q.; Wu, H.; Zhang, H. Fullerene and platinum composite-based electrochemical sensor for the selective determination of catechol and hydroquinone. J. Electroanal. Chem. 2020, 878, 114726. [Google Scholar] [CrossRef]
- Ko, E.; Tran, V.-K.; Son, S.E.; Hur, W.; Choi, H.; Seong, G.H. Characterization of Au@PtNP/GO nanozyme and its application to electrochemical microfluidic devices for quantification of hydrogen peroxide. Sens. Actuators B Chem. 2019, 294, 166–176. [Google Scholar] [CrossRef]
- Stankovića, V.; Djurdjic, S.; Ognjanović, M.; Mutićb, J.; Kalcherd, K.; M.Stankovićbc, D. A novel nonenzymatic hydrogen peroxide amperometric sensor based on AgNp@GNR nanocomposites modified screen-printed carbon electrode. J. Electroanal. Chem. 2020, 876, 2–7. [Google Scholar] [CrossRef]
- Charak, S.; Jangir, D.K.; Tyagi, G.; Mehrotra, R. Interaction studies of Epirubicin with DNA using spectroscopic techniques. J. Mol. Struct. 2011, 1000, 150–154. [Google Scholar] [CrossRef]
- Erdem, A.; Ozsoz, M. Interaction of the anticancer drug epirubicin with DNA. Anal. Chim. Acta 2001, 437, 107–114. [Google Scholar] [CrossRef]
- Jawad, B.; Poudel, L.; Podgornik, R.; Steinmetz, N.F.; Ching, W.-Y. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys. Chem. Chem. Phys. 2019, 21, 3877–3893. [Google Scholar] [CrossRef] [PubMed]
- Ozluer, C.; Kara, H.E.S. In vitro DNA binding studies of anticancer drug idarubicin using spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2014, 138, 36–42. [Google Scholar] [CrossRef]
- Khodadadi, A.; Faghih-Mirzaei, E.; Karimi-Maleh, H.; Abbaspourrad, A.; Agarwal, S.; Gupta, V.K. A new epirubicin biosensor based on amplifying DNA interactions with polypyrrole and nitrogen-doped reduced graphene: Experimental and docking theoretical investigations. Sens. Actuators B Chem. 2019, 284, 568–574. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, Z.; Hoshi, T.; Kashiwagi, Y.; Anzai, J.-I.; Li, G. Electrochemical studies of danthron and the DNA–danthron interaction. Biophys. Chem. 2005, 114, 21–26. [Google Scholar] [CrossRef]
- Charak, S.; Mehrotra, R. Structural investigation of idarubicin–DNA interaction: Spectroscopic and molecular docking study. Int. J. Biol. Macromol. 2013, 60, 213–218. [Google Scholar] [CrossRef]
- Pérez-Arnaiz, C.; Busto, N.; Leal, J.M.; Garcia, B. New Insights into the Mechanism of the DNA/Doxorubicin Interaction. J. Phys. Chem. B 2014, 118, 1288–1295. [Google Scholar] [CrossRef]
- Silva, E.F.; Bazoni, R.F.; Ramos, E.B.; Rocha, M.S. DNA-doxorubicin interaction: New insights and peculiarities. Biopolymers 2017, 107, e22998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
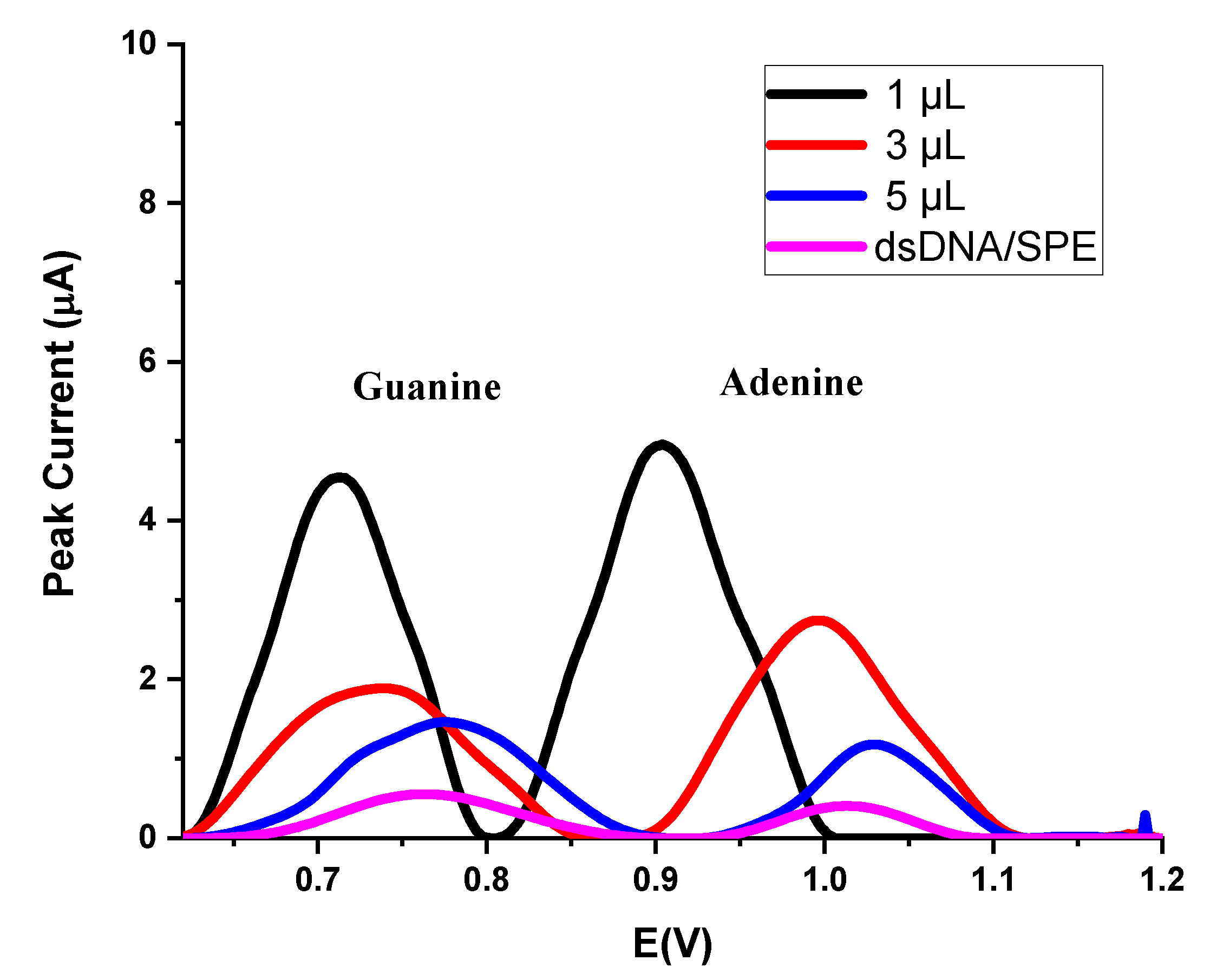
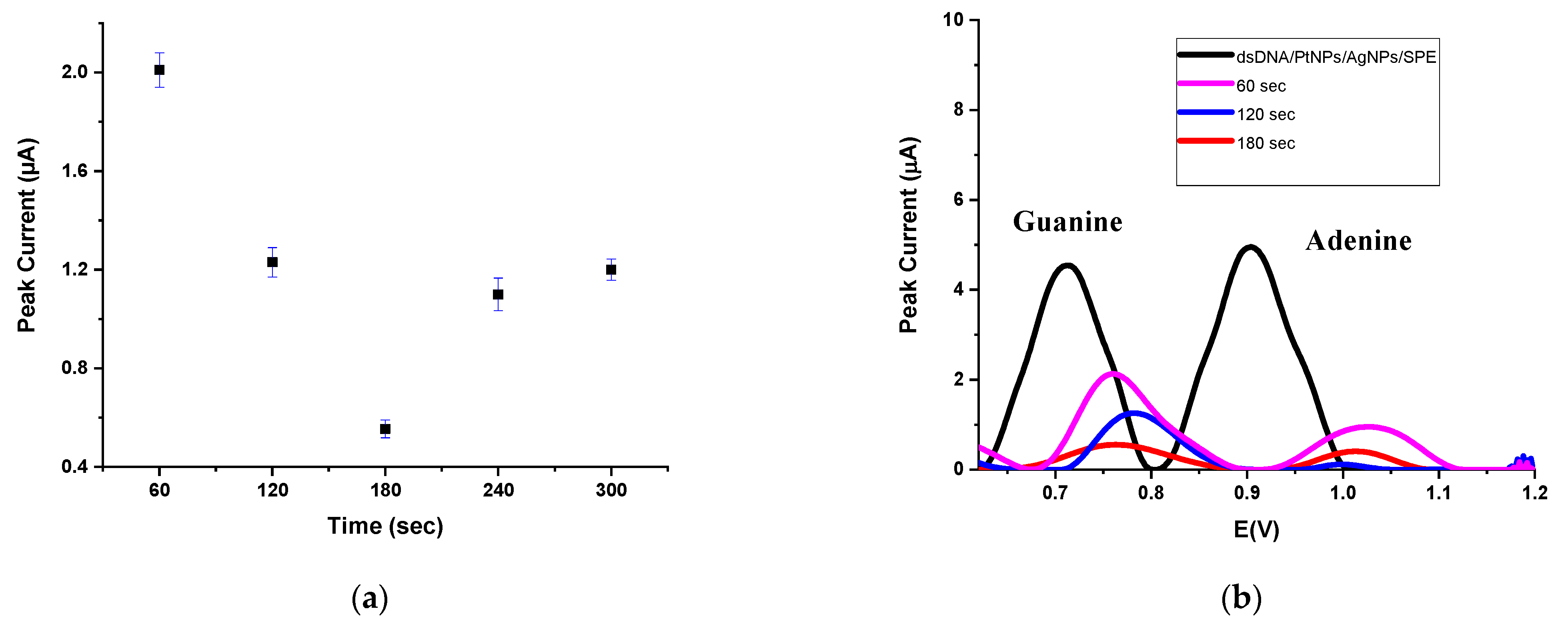
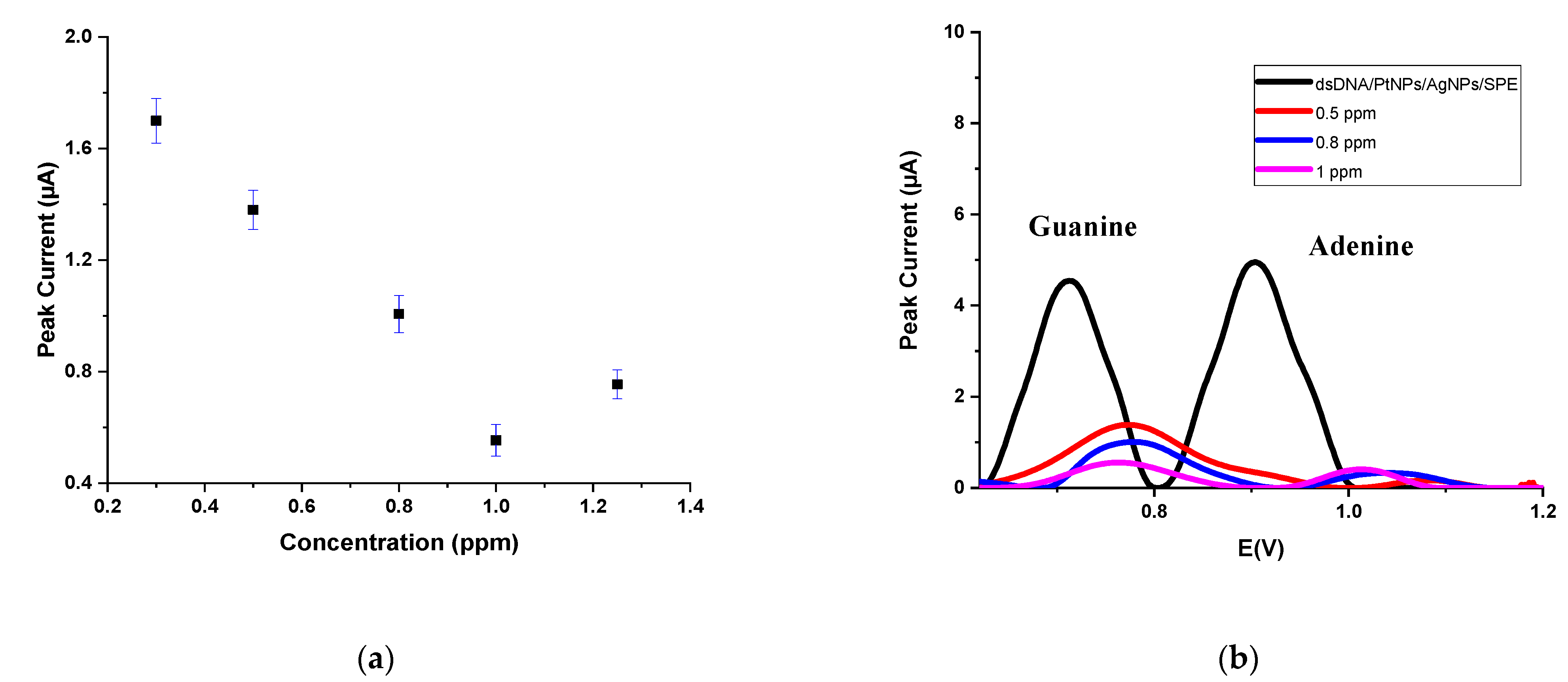

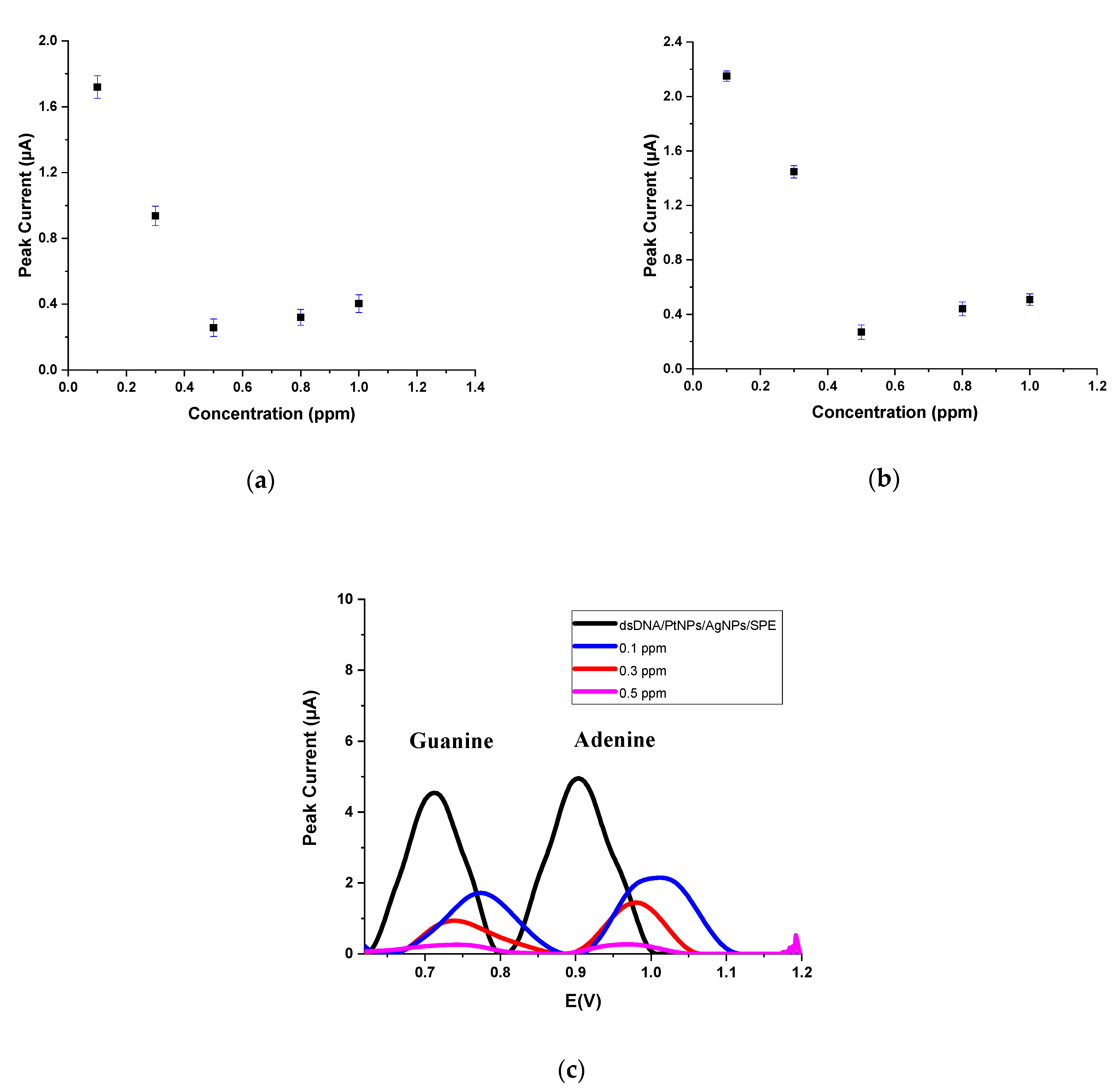
| dGuo | dAdo | |||
|---|---|---|---|---|
| Electrode | Peak Potential (V) | Peak Current (μA) | Peak Potential (V) | Peak Current (μA) |
| dsDNA/SPE | 0.764 | 0.554 | 1.014 | 0.407 |
| dsDNA/PtNPs/SPE | 0.774 | 1.712 | 1.008 | 2.161 |
| dsDNA/AgNPs/SPE | 0.738 | 1.892 | 0.996 | 2.749 |
| dsDNA/PtNPs/AgNPs (monolayer)/SPE | 0.746 | 2.254 | 0.934 | 2.317 |
| dsDNA/PtNPs/AgNPs (twolayer)/SPE | 0.714 | 4.542 | 0.904 | 4.948 |
| dGuo | dAdo | |||
|---|---|---|---|---|
| Electrode | Peak Potential (V) | Peak Current (μA) | Peak Potential (V) | Peak Current (μA) |
| dsDNA/SPE | 0.764 | 0.554 | 1.014 | 0.407 |
| dsDNA/AgNPs (5 μL)/SPE | 0.738 | 1.892 | 0.996 | 2.749 |
| dsDNA/PtNPs (1 μL)/AgNPs (two-step of 3 μL)/SPE | 0.728 | 3.387 | 0.934 | 1.852 |
| dsDNA/PtNPs (1 μL)/AgNPs (two-step of 7 μL)/SPE | 0.640 | 1.936 | 0.836 | 2.730 |
| dsDNA/PtNPs (1 μL)/AgNPs (one-step of 5 μL)/SPE | 0.746 | 2.254 | 0.934 | 2.317 |
| dsDNA/PtNPs (1 μL)/AgNPs (two-step of 5 μL)/SPE | 0.714 | 4.542 | 0.904 | 4.948 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadurmus, L.; Dogan-Topal, B.; Kurbanoglu, S.; Shah, A.; Ozkan, S.A. The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode. Micromachines 2021, 12, 1337. https://doi.org/10.3390/mi12111337
Karadurmus L, Dogan-Topal B, Kurbanoglu S, Shah A, Ozkan SA. The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode. Micromachines. 2021; 12(11):1337. https://doi.org/10.3390/mi12111337
Chicago/Turabian StyleKaradurmus, Leyla, Burcu Dogan-Topal, Sevinc Kurbanoglu, Afzal Shah, and Sibel A. Ozkan. 2021. "The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode" Micromachines 12, no. 11: 1337. https://doi.org/10.3390/mi12111337
APA StyleKaradurmus, L., Dogan-Topal, B., Kurbanoglu, S., Shah, A., & Ozkan, S. A. (2021). The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode. Micromachines, 12(11), 1337. https://doi.org/10.3390/mi12111337