Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration
Abstract
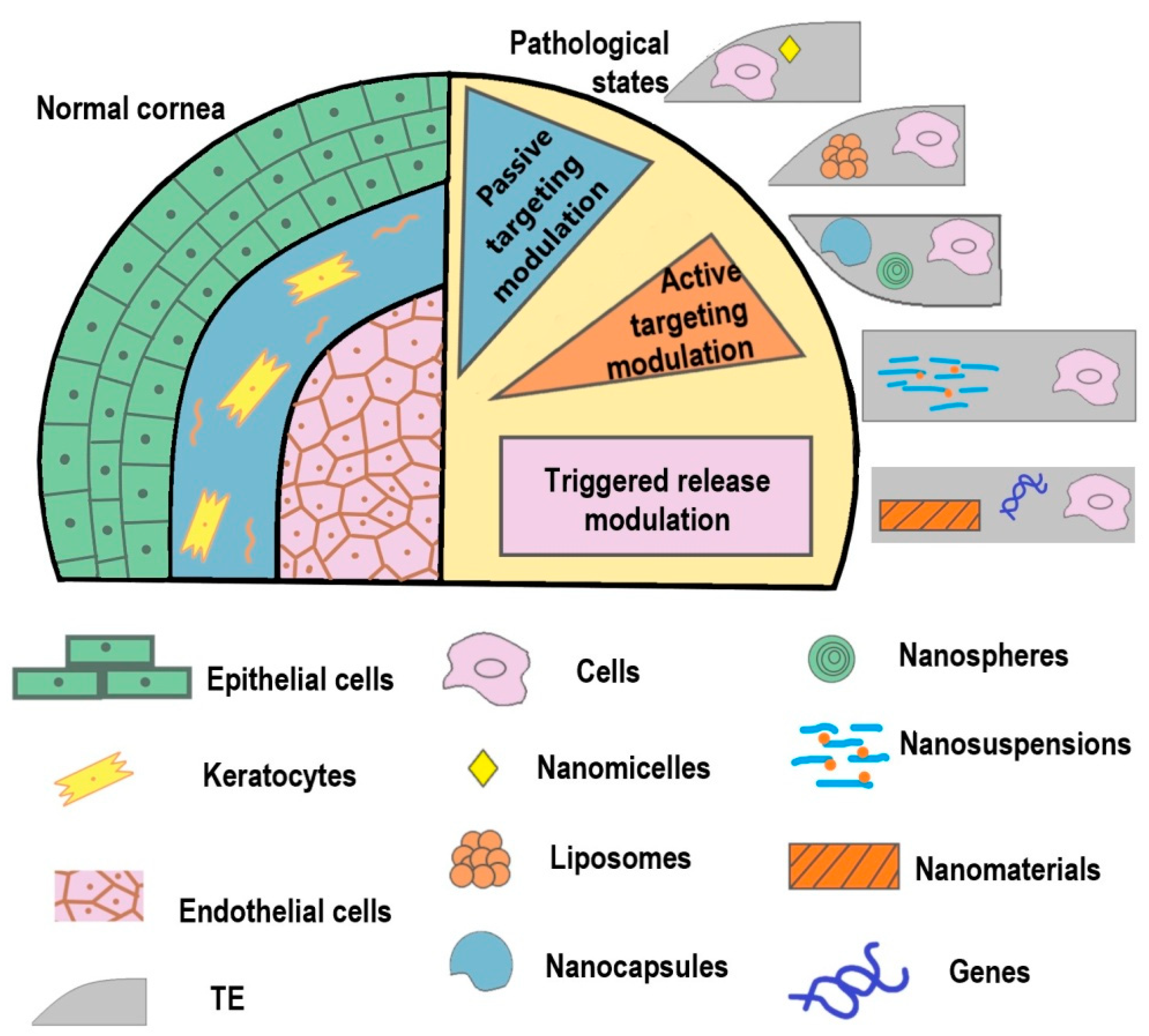
:1. Introduction
2. Corneal Tissue Engineering
2.1. Different Approaches for Cornea Replacement
2.2. Cornea TE Grafts
2.2.1. Hydrogel-Based Grafts
2.2.2. Membrane- and Film-Based Grafts
2.3. Modulation of Cell Behavior by Cornea TE Grafts
3. Molecular Pathways and Interactions between Host Tissues and a Graft
3.1. Processes Involved in Cornea Healing
3.1.1. ECM Reorganization and Re-Epithelization
3.1.2. Soluble Factors
3.1.3. Oxidative Stress
3.2. Modulation of Cornea Regeneration by Biomaterials
3.2.1. Re-Epithelization
3.2.2. ECM Analogs
3.2.3. Mechanical Properties
3.2.4. Surface Properties and Topography
3.2.5. Anti-Oxidative Properties
3.2.6. Immune Cells
4. Nanotechnology in Corneal Tissue Engineering
4.1. Nanostructured Matrices
4.2. Nanocarriers for Intracorneal Drug Delivery
5. Outlook and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ABCG2 | ATP-binding cassette super-family G member 2 |
| AM | Amniotic membrane |
| BMP4 | Bone morphogenetic protein 4 |
| CECs | Corneal epithelial cells |
| CenCs | Corneal endothelial cells |
| DC | Decellularized cornea |
| ECM | Extracellular matrix |
| ER | Endoplasmic reticulum |
| FAK | Focal adhesion kinase |
| hCECs | Human CECs |
| LESCs | Limbal epithelial stem cells |
| LSCs | Limbal stem cells |
| MMP | Matrix metallopeptidases |
| MSCs | Mesenchymal stem cells |
| NM | Nanomaterials |
| NPs | Nanoparticles |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| PMMA | Poly (methyl methacrylate) |
| PLGA | Poly lacto-glycolic acid |
| RF | Riboflavin |
| RhoA | Ras homolog family member A |
| sFit-1 | Soluble VEGF receptor 1 |
| TE | Tissue-engineered |
| Tregs | T regulatory cells |
| VEGF-A | Vascular endothelial growth factor A |
| YAP | Yes-associated protein |
| α-SMA | Smooth muscle alpha-actin (alpha smooth muscle actin) |
References
- Morishige, N.; Shin-Gyou-Uchi, R.; Azumi, H.; Ohta, H.; Morita, Y.; Yamada, N.; Kimura, K.; Takahara, A.; Sonoda, K.H. Quantitative analysis of collagen lamellae in the normal and keratoconic human cornea by second harmonic generation imaging microscopy, Investig. Ophthalmol. Vis. Sci. 2014, 55, 8377–8385. [Google Scholar] [CrossRef] [Green Version]
- Mobaraki, M.; Abbasi, R.; Vandchali, S.O.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal repair and regeneration: Current concepts and future directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, G.; Sasamoto, Y.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Limbal Stem Cells: Identity, Developmental Origin and Therapeutic Potential, Wiley Interdiscip. Rev. Dev. Biol. 2018, 7. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamrah, Y.Q.P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J. Clin. Cell. Immunol. 2013. [Google Scholar] [CrossRef]
- Matthyssen, S.; van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2018, 69, 31–41. [Google Scholar] [CrossRef]
- Susaimanickam, P.J.; Maddileti, S.; Pulimamidi, V.K.; Boyinpally, S.R.; Naik, R.R.; Naik, M.N.; Reddy, G.B.; Sangwan, V.S.; Mariappan, I. Generating minicorneal organoids from human induced pluripotent stem cells. Development 2017, 144, 2338–2351. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering Approaches for Corneal Regenerative Medicine. Tissue Eng. Regen. Med. 2020. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing, Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar]
- Zarei-Ghanavati, M.; Liu, C. Aspects of corneal transplant immunology. J. Ophthalmic Vis. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72. [Google Scholar] [CrossRef]
- Fuchsluger, T.A.; Jurkunas, U.; Kazlauskas, A.; Dana, R. Corneal endothelial cells are protected from apoptosis by gene therapy. Hum. Gene Ther. 2011, 22, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente-Pascual, M.; Albano, A.; Solinís, M.; Serpe, L.; Rodríguez-Gascón, A.; Foglietta, F.; Muntoni, E.; Torrecilla, J.; del Pozo-Rodríguez, A.; Battaglia, L. Gene delivery in the cornea: In vitro & ex vivo evaluation of solid lipid nanoparticle-based vectors. Nanomedicine 2018, 13, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Raub, C.B.; Orwin, E.J.; Haskell, R. Immunogold labeling to enhance contrast in optical coherence microscopy of tissue engineered corneal constructs. In Annual International Conference IEEE Engineering Medicine Biology; IEEE: Piscataway, NJ, USA, 2004; pp. 1210–1213. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-J.; Buznyk, O.; Kuffova, L.; Rajendran, V.; Forrester, J.V.; Phopase, J.; Islam, M.M.; Skog, M.; Ahlqvist, J.; Griffith, M. Cathelicidin LL-37 and HSV-1 Corneal Infection: Peptide Versus Gene Therapy. Transl. Vis. Sci. Technol. 2014, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomchalao, P.; Nimtrakul, P.; Pham, D.T.; Tiyaboonchai, W. Development of amphotericin B-loaded fibroin nanoparticles: A novel approach for topical ocular application. J. Mater. Sci. 2020, 55, 5268–5279. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell Loaded GelMA:HEMA IPN hydrogels for corneal stroma engineering. J. Mater. Sci. Mater. Med. 2019, 31, 1–15. [Google Scholar] [CrossRef]
- Shavkuta, B.S.; Gerasimov, M.Y.; Minaev, N.V.; Kuznetsova, D.S.; Dudenkova, V.V.; Mushkova, I.A.; Malyugin, B.E.; Kotova, S.L.; Timashev, P.S.; Kostenev, S.V.; et al. Highly effective 525 nm femtosecond laser crosslinking of collagen and strengthening of a human donor cornea. Laser Phys. Lett. 2018, 15, 015602. [Google Scholar] [CrossRef]
- Jo, D.H.; Lee, T.G.; Kim, J.H. Nanotechnology and nanotoxicology in retinopathy. Int. J. Mol. Sci. 2011, 12, 8288–8301. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Wu, X. Development and Characterization of a Full-Thickness Acellular Porcine Cornea Matrix for Tissue Engineering. Artif. Organs. 2011, 35, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, Y.; Li, N.; Huang, M.; Duan, H.; Ge, J.; Xiang, P.; Wang, Z. The use of phospholipase A2 to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 2009, 30, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; Carrasquilla, S.D.; Feinberg, A.W. Natural Biomaterials for Corneal Tissue Engineering, Repair, and Regeneration. Adv. Healthc. Mater. 2018, 7, 1701434. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, J.; Roy, S.; Murab, S.; Ravani, R.; Kaur, K.; Devi, S.; Singh, D.; Sharma, S.; Mohanty, S.; Dinda, A.K.; et al. Modulation of Macrophage Phenotype, Maturation, and Graft Integration through Chondroitin Sulfate Cross-Linking to Decellularized Cornea. ACS Biomater. Sci. Eng. 2019, 5, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Sidney, L.; Dunphy, S.; Rose, J.; Hopkinson, A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. J. Funct. Biomater. 2013, 4, 114–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; de Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalcione, C.; Ortiz-Vaquerizas, D.; Said, D.G.; Dua, H.S. Fibrin glue as agent for sealing corneal and conjunctival wound leaks. Eye 2018, 32, 463–466. [Google Scholar] [CrossRef]
- Jacob, S.; Dhawan, P.; Tsatsos, M.; Agarwal, A.; Narasimhan, S.; Kumar, A. Fibrin Glue–Assisted Closure of Macroperforation in Predescemetic Deep Anterior Lamellar Keratoplasty With a Donor Obtained From Small Incision Lenticule Extraction. Cornea 2019, 38, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Crawford, G.J. The development and results of an artificial cornea: AlphaCorTM. Biomater. Regen. Med. Ophthalmol. 2016, 443–462. [Google Scholar] [CrossRef]
- Nouri, M.; Terada, H.; Alfonso, E.C.; Foster, C.S.; Durand, M.L.; Dohlman, C.H. Endophthalmitis after keratoprosthesis: Incidence, bacterial causes, and risk factors. Arch. Ophthalmol. 2001, 119, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Rico-Sánchez, L.; Garzón, I.; González-Andrades, M.; Ruíz-García, A.; Punzano, M.; Lizana-Moreno, A.; Muñoz-Ávila, J.I.; Sánchez-Quevedo, M.d.; Martínez-Atienza, J.; Lopez-Navas, L.; et al. Successful development and clinical translation of a novel anterior lamellar artificial cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.M.; Faraj, L.A.; McIntosh, O.D.; Dhillon, V.K.; Dua, H.S. Fibrin glue inhibits migration of ocular surface epithelial cells. Eye 2016, 30, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, Y.; Bai, Y.; Quan, D.; Wang, Z.; Xiong, L.; Shao, Z.; Sun, W.; Mi, S. A core-skirt designed artificial cornea with orthogonal microfiber grid scaffold. Exp. Eye Res. 2020, 195, 108037. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.K.; Chen, H.C.; Ma, K.S.K.; Lai, J.Y.; Yang, U.; Yeh, L.K.; Hsueh, Y.J.; Chu, W.K.; Lai, C.H.; Chen, J.K. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016, 31, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, D.; Hsu, C.C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef]
- Haj, E.; Sidney, L.E.; Patient, J.; White, L.J.; Dua, H.S.; El Haj, A.J.; Hopkinson, A.; Rose, F.R. In vitro evaluation of electrospun blends of gelatin and PCL for application as a partial thickness corneal graft. J. Biomed. Mater. Res. Part A. 2018, 107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Young, T.H.; Wang, T.J. Investigating the effect of chitosan/ polycaprolactone blends in differentiation of corneal endothelial cells and extracellular matrix compositions. Exp. Eye Res. 2019, 185, 107679. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Sim, B.R.; Jeon, Y.S.; Kim, H.S.; Shin, E.Y.; Carlomagno, C.; Khang, G. Characterization of surface modified glycerol/silk fibroin film for application to corneal endothelial cell regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Takezawa, T. Fabrication of a corneal model composed of corneal epithelial and endothelial cells via a collagen vitrigel membrane functioned as an acellular stroma and its application to the corneal permeability test of chemicals. Drug Metab. Dispos. 2018, 46, 1684–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, J.; Yokoo, S.; Oshikata-Miyazaki, A.; Amano, S.; Takezawa, T.; Yamagami, S. Transplantation of Human Corneal Endothelial Cells Cultured on Bio-Engineered Collagen Vitrigel in a Rabbit Model of Corneal Endothelial Dysfunction. Curr. Eye Res. 2017, 42, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef] [Green Version]
- Hatami-Marbini, H.; Jayaram, S.M. UVA/riboflavin collagen crosslinking stiffening effects on anterior and posterior corneal flaps. Exp. Eye Res. 2018, 176, 53–58. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, X.; Liu, S.; Chen, M.; Liu, B.; Ge, J.; Jia, Y.G.; Ren, L. Collagen−hydroxypropyl methylcellulose membranes for corneal regeneration. ACS Omega 2018, 3, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- In situ-forming bio-orthogonally crosslinked collagen-hyaluronate co-polymeric hydrogel to treat deep corneal stromal defects: In vivo biological response. IOVS ARVO J. 2020, 61, 1208.
- Rose, J.B.; Pacelli, S.; el Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, K.; Li, T.; Zhang, W.; Dong, Y.; Lv, J.; Wang, W.; Sun, J.; Li, M.; Wang, M.; et al. An in situ hydrogel based on carboxymethyl chitosan and sodium alginate dialdehyde for corneal wound healing after alkali burn. J. Biomed. Mater. Res. Part A 2018, 107, 36589. [Google Scholar] [CrossRef] [Green Version]
- Stafiej, P.; Schubert, D.W.; Fuchsluger, T. Mechanical and Optical Properties of PCL Nanofiber Reinforced Alginate Hydrogels for Application in Corneal Wound Healing Draw resonance View project biomaterials for corneal tissue engineering and wound healing View project. Biomater. Med. Appl. 2018. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Woung, L.C.; Yen, J.C.; Tseng, P.C.; Chiou, S.H.; Sung, Y.J.; Liu, K.T.; Cheng, Y.H. Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym. 2016, 135, 308–315. [Google Scholar] [CrossRef]
- Tang, Q.; Luo, C.; Lu, B.; Fu, Q.; Yin, H.; Qin, Z.; Lyu, D.; Zhang, L.; Fang, Z.; Zhu, Y.; et al. Thermosensitive chitosan-based hydrogels releasing stromal cell derived factor-1 alpha recruit MSC for corneal epithelium regeneration. Acta Biomater. 2017, 61, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Barrio, C.; Etxebarria, J.; Hernáez-Moya, R.; del Val-Alonso, M.; Rodriguez-Astigarraga, M.; Urkaregi, A.; Freire, V.; Morales, M.-C.; Durán, J.; Vicario, M.; et al. Hyaluronic Acid Combined with Serum Rich in Growth Factors in Corneal Epithelial Defects. Int. J. Mol. Sci. 2019, 20, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Deng, Y.; Tian, B.; Wang, B.; Sun, Y.; Huang, H.; Chen, L.; Ling, S.; Yuan, J. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J. Ophthalmol. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Biazar, E.; Baradaran-Rafii, A.; Heidari-Keshel, S.; Tavakolifard, S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J. Biomater. Sci. Polym. Ed. 2015, 26, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Aghaei-Ghareh-Bolagh, B.; Guan, J.; Wang, Y.; Martin, A.D.; Dawson, R.; Mithieux, S.M.; Weiss, A.S. Optically robust, highly permeable and elastic protein films that support dual cornea cell types. Biomaterials 2019, 188, 50–62. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Sabater, A.L.; Perez, V.L. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr. Opin. Ophthalmol. 2017, 28, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mingo-Botín, D.; Balgos, M.J.T.D.; Arnalich-Montiel, F. Corneal Endothelium: Isolation and Cultivation Methods; Springer: Cham, Switzerland, 2019; pp. 425–436. [Google Scholar] [CrossRef]
- Che, X.; Wu, H.; Jia, C.; Sun, H.; Ou, S.; Wang, J.; Jeyalatha, M.V.; He, X.; Yu, J.; Zuo, C.; et al. A novel tissue-engineered corneal stromal equivalent based on amniotic membrane and keratocytes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zou, D.; Li, S.; Wang, J.; Qu, Y.; Ou, S.; Jia, C.; Li, J.; He, H.; Liu, T.; et al. An Ultra-thin Amniotic Membrane as Carrier in Corneal Epithelium Tissue-Engineering. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.; Sirivisoot, S.; Talchai, S.; Khemarangsan, V. Efficient mesenchymal stem cell conversion to corneal keratocytes by the use of specific biomaterial and Cell RevTM mSC diffKera media. Cytotherapy 2020, 22, S182. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pérez, J.; Kador, K.E.; Lynch, A.P.; Ahearne, M. Characterization of extracellular matrix modified poly(ε-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Mater. Sci. Eng. C 2020, 108, 110415. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.; Huff, T.; Zuniga, J. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J. Tissue Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008. [Google Scholar] [CrossRef] [Green Version]
- Crupi, A.; Costa, A.; Tarnok, A.; Melzer, S.; Teodori, L. Inflammation in tissue engineering: The Janus between engraftment and rejection. Eur. J. Immunol. 2015. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shiraishi, A.; Hara, Y.; Kadota, Y.; Yang, L.; Inoue, T.; Shirakata, Y.; Ohashi, Y. Stromal-epithelial interaction study: The effect of corneal epithelial cells on growth factor expression in stromal cells using organotypic culture model. Exp. Eye Res. 2015, 135, 109–117. [Google Scholar] [CrossRef]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torricelli, A.A.M.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-Stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Ma, X.; Wu, W.; Shi, M.; Ma, J.; Zhang, Y.; Zhao, E.; Yang, X. Coordinated microRNA/mRNA expression profiles reveal a putative mechanism of corneal epithelial cell transdifferentiation from skin epidermal stem cells. Int. J. Mol. Med. 2018, 41, 877–887. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Serrano, J.C.; Kamm, R.D. Cooperative Effects of Vascular Angiogenesis and Lymphangiogenesis. Regen. Eng. Transl. Med. 2018, 4, 120–132. [Google Scholar] [CrossRef]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guérin, S.L. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Suri, K.; Amouzegar, A.; Li, M.; Katikireddy, K.R.; Mittal, S.K.; Chauhan, S.K. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol. Ther. 2017, 25, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Salabarria, A.; Braun, G.; Heykants, M.; Koch, M.; Reuten, R.; Mahabir, E.; Cursiefen, C.; Bock, F. Local VEGF -A blockade modulates the microenvironment of the corneal graft bed. Am. J. Transplant. 2019, 19, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, C.; Cai, Q.; Bao, X.; Tang, L.; Ao, H.; Liu, J.; Jin, M.; Zhou, Y.; Wan, Y.; et al. Studies on bacterial cellulose/poly(vinyl alcohol) hydrogel composites as tissue-engineered corneal stroma. Biomed. Mater. 2020, 15, 035022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; You, J.; Liu, X.; Cooper, S.; Hodge, C.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomaterials for corneal bioengineering. Biomed. Mater. 2018, 13, 032002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019, 636. [Google Scholar] [CrossRef] [Green Version]
- McNally, A.K.; Jones, J.A.; MacEwan, S.R.; Colton, E.; Anderson, J.M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. Part A 2008. [Google Scholar] [CrossRef] [PubMed]
- Jenney, C.R.; Anderson, J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. 2000. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Bridges, A.W.; Burns, K.L.; Tate, C.C.; Babensee, J.E.; LaPlaca, M.C.; García, A.J. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Karamichos, D. Modeling the cornea in 3-dimensions: Current and future perspectives. Exp. Eye Res. 2020, 197, 108127. [Google Scholar] [CrossRef] [PubMed]
- RGouveia, M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Que, R.; Wang, S.W.; Liu, W.F. Modification of Biomaterials with a Self-Protein Inhibits the Macrophage Response. Adv. Healthc. Mater. 2014. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004. [Google Scholar] [CrossRef]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Ravensbergen, K.; Alabanza, A.; Soldin, D.; Hahm, J.I. Distinct adsorption configurations and self-Assembly characteristics of fibrinogen on chemically uniform and alternating surfaces including block copolymer nanodomains. ACS Nano 2014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Parajuli, O.; Gupta, A.; Hahm, J.I. Elucidation of protein adsorption behavior on polymeric surfaces: Toward high-density, high-payload protein templates. Langmuir 2008. [Google Scholar] [CrossRef] [PubMed]
- Ouberai, M.M.; Xu, K.; Welland, M.E. Effect of the interplay between protein and surface on the properties of adsorbed protein layers. Biomaterials 2014. [Google Scholar] [CrossRef] [Green Version]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Biocompatibility and antifouling: Is there really a linkα. Trends Biotechnol. 2014. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Singh, S.; Czaja, K.; Bornemann, J.; Bockstaller, M.; Moeller, M.; Zwadlo-Klarwasser, G.; Groll, J. Rapid uptake of gold nanorods by primary human blood phagocytes and immunomodulatory effects of surface chemistry. ACS Nano 2010. [Google Scholar] [CrossRef]
- Wen, Y.; Waltman, A.; Han, H.; Collier, J.H. Switching the Immunogenicity of Peptide Assemblies Using Surface Properties. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hulander, M.; Lundgren, A.; Faxälv, L.; Lindahl, T.L.; Palmquist, A.; Berglin, M.; Elwing, H. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surfaces B Biointerfaces 2013. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Ruch, D.S.; Little, D.; Guilak, F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014. [Google Scholar] [CrossRef]
- Tan, K.S.; Qian, L.; Rosado, R.; Flood, P.M.; Cooper, L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 2006. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Gao, H.C.; Qin, L.; Jia, Y.G.; Ren, L. Engineering topography: Effects on corneal cell behavior and integration into corneal tissue engineering. Bioact. Mater. 2019, 4, 293–302. [Google Scholar] [CrossRef]
- Myrna, K.E.; Mendonsa, R.; Russell, P.; Pot, S.A.; Liliensiek, S.J.; Jester, J.V.; Nealey, P.F.; Brown, D.; Murphy, C.J. Substratum topography modulates corneal fibroblast to Myofibroblast transformation. Investig. Ophthalmol. Vis. Sci. 2012. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yokohama, S.; Yoneda, M.; Okamoto, S.; Tamaki, Y.; Ito, T.; Okada, M.; Aso, K.; Makino, I. High, But not low, Molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J. Gastroenterol. 2004. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg. Med. Chem. 2006. [Google Scholar] [CrossRef]
- Coco, G.; Foulsham, W.; Nakao, T.; Yin, J.; Amouzegar, A.; Taketani, Y.; Chauhan, S.K.; Dana, R. Regulatory T cells promote corneal endothelial cell survival following transplantation via interleukin-10. Am. J. Transplant. 2020, 20, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Kisaalita, W. Exploring cellular adhesion and differentiation in a micro-/nano-hybrid polymer scaffold. Biotechnol. Prog. 2010, 26, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 1908996. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-de Santiago, G.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2019, 197, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Raghu, P.K.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of nanotechnology in delivering drugs to eyes, skin and wounds via topical route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef]
- Patra, H.K.; Azharuddin, M.; Islam, M.M.; Papapavlou, G.; Deb, S.; Osterrieth, J.; Zhu, G.H.; Romu, T.; Dhara, A.K.; Jafari, M.J.; et al. Rational Nanotoolbox with Theranostic Potential for Medicated Pro-Regenerative Corneal Implants. Adv. Funct. Mater. 2019, 29, 1903760. [Google Scholar] [CrossRef]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Ther. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Motealleh, A.; Kehr, N.S. Nanocomposite Hydrogels and Their Applications in Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1600938. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Taniguchi, J. Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul. Surf. 2017, 15, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, U.V.; Devi, V.K.; Jain, N. Formulation and optimization of mucoadhesive nanodrug delivery system of acyclovir. J. Young Pharm. 2011, 3, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Vichare, R.; Garner, I.; Paulson, R.J.; Tzekov, R.; Sahiner, N.; Panguluri, S.K.; Mohapatra, S.; Mohapatra, S.S.; Ayyala, R.; Sneed, K.B.; et al. Biofabrication of chitosan-based nanomedicines and its potential use for translational ophthalmic applications. Appl. Sci. 2020, 10, 4189. [Google Scholar] [CrossRef]
- Chang, M.-C.; Kuo, Y.-J.; Hung, K.-H.; Peng, C.-L.; Chen, K.-Y.; Yeh, L.-K. Liposomal dexamethasone-moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed. Mater. 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target 2011, 19, 409–417. [Google Scholar] [CrossRef]
- Ulyanov, V.A.; Makarova, M.B.; Molchaniuk, N.I.; Ulyanova, N.A.; Skobeeva, V.M.; Chernezhenko, E.A. Effect of colloidal silver nanoparticle solution instillation on the ultrastructure of the corneal epithelium and stroma. J. Ophthalmol. (Ukraine) 2017, 3, 63–69. [Google Scholar] [CrossRef]
- Chen, M.; Bao, L.; Zhao, M.; Cao, J.; Zheng, H. Progress in Research on the Role of FGF in the Formation and Treatment of Corneal Neovascularization. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Salama, A.H.; Shamma, R.N. Tri/tetra-block co-polymeric nanocarriers as a potential ocular delivery system of lornoxicam: In-vitro characterization, and in-vivo estimation of corneal permeation. Int. J. Pharm. 2015, 492, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Üstündaǧ-Okur, N.; Gökçe, E.H.; Bozbiyik, D.I.; Eǧrilmez, S.; Özer, Ö.; Ertan, G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Iriyama, A.; Oba, M.; Ishii, T.; Nishiyama, N.; Kataoka, K.; Tamaki, Y.; Yanagi, Y. Gene transfer using micellar nanovectors inhibits choroidal neovascularization in vivo. PLoS ONE. 2011. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Singh, P.R.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Cho, W.K.; Kang, S.; Choi, H.; Rho, C.R. Topically administered gold nanoparticles inhibit experimental corneal neovascularization in mice. Cornea 2015, 34, 456–459. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Wang, Y.; Song, C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int. J. Nanomed. 2009, 4, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, W.; Chen, Y.; Liu, S.; Ren, L. Collagen-based materials combined with microRNA for repairing cornea wounds and inhibiting scar formation. Biomater. Sci. 2019, 7, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Ahmadian, E.; Fard, J.K.; Khosroushahi, A.Y. Biomacromolecule based nanoscaffolds for cell therapy. J. Drug Deliv. Sci. Technol. 2017, 37, 61–66. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Merayo, J.; Durán, J.; Orive, G. Plasma Rich in Growth Factors for the Treatment of Ocular Surface Diseases. Curr. Eye Res. 2016, 41, 875–882. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijanović, O.; Pylaev, T.; Nikitkina, A.; Artyukhova, M.; Branković, A.; Peshkova, M.; Bikmulina, P.; Turk, B.; Bolevich, S.; Avetisov, S.; et al. Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration. Micromachines 2021, 12, 1336. https://doi.org/10.3390/mi12111336
Mijanović O, Pylaev T, Nikitkina A, Artyukhova M, Branković A, Peshkova M, Bikmulina P, Turk B, Bolevich S, Avetisov S, et al. Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration. Micromachines. 2021; 12(11):1336. https://doi.org/10.3390/mi12111336
Chicago/Turabian StyleMijanović, Olja, Timofey Pylaev, Angelina Nikitkina, Margarita Artyukhova, Ana Branković, Maria Peshkova, Polina Bikmulina, Boris Turk, Sergey Bolevich, Sergei Avetisov, and et al. 2021. "Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration" Micromachines 12, no. 11: 1336. https://doi.org/10.3390/mi12111336
APA StyleMijanović, O., Pylaev, T., Nikitkina, A., Artyukhova, M., Branković, A., Peshkova, M., Bikmulina, P., Turk, B., Bolevich, S., Avetisov, S., & Timashev, P. (2021). Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration. Micromachines, 12(11), 1336. https://doi.org/10.3390/mi12111336







