Enhanced HIFU Theranostics with Dual-Frequency-Ring Focused Ultrasound and Activatable Perfluoropentane-Loaded Polymer Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
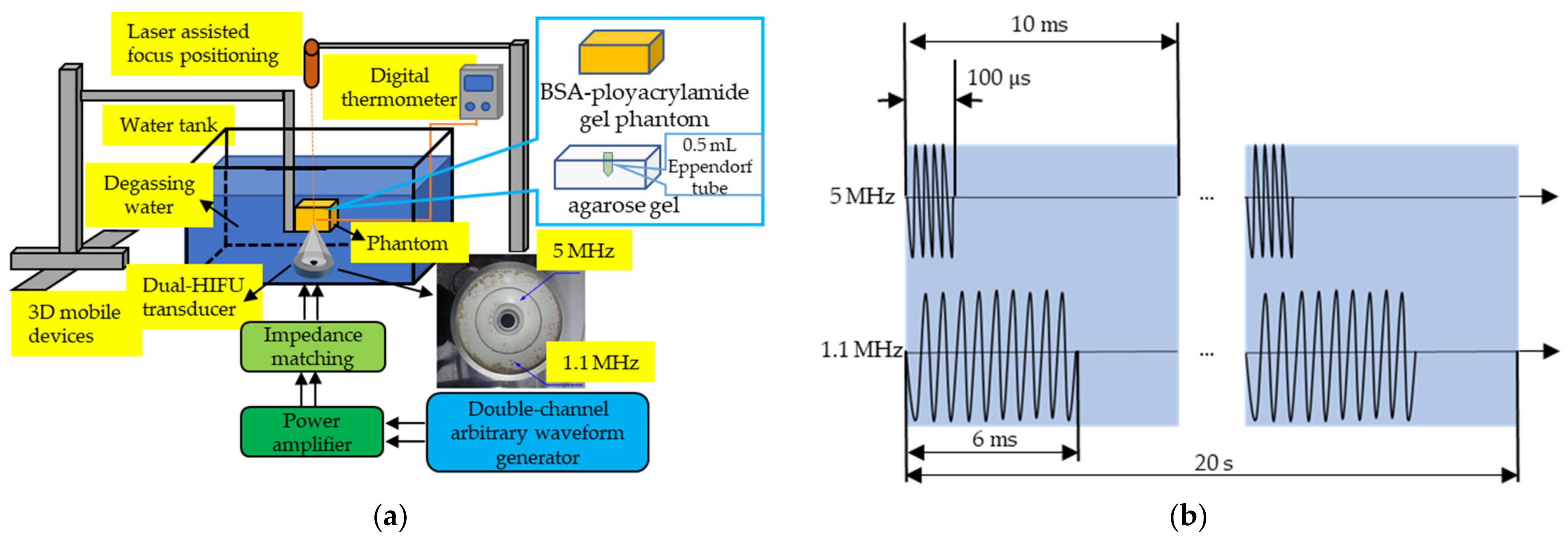
2.2. Ultrasound Apparatus
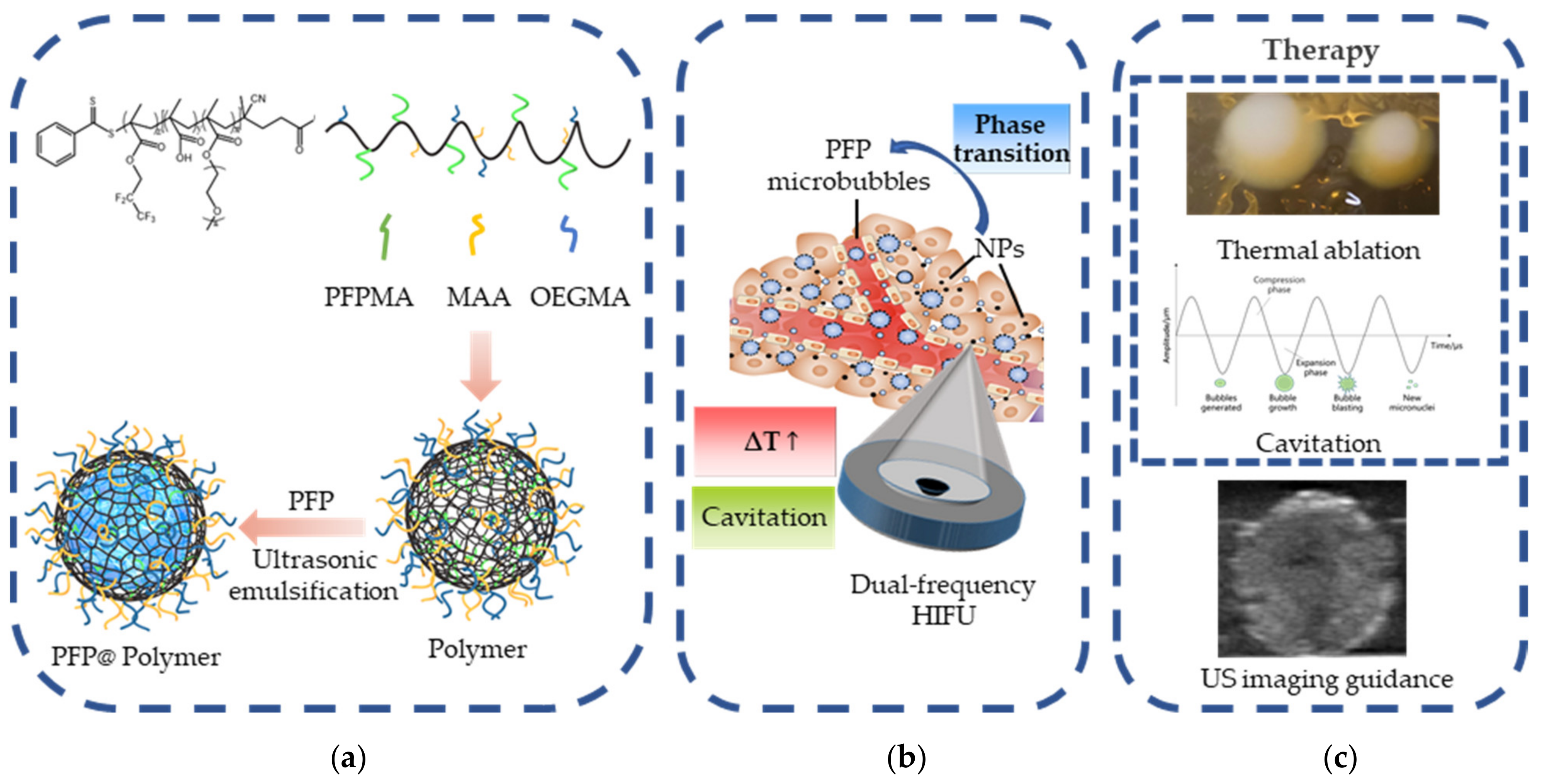
2.3. Synthesis and Characterization of Activatable PFP@Polymer Nanoparticles
2.4. Cell Culture
2.5. In Vitro Cytotoxicity Evaluation
2.6. Evaluation of Thermal and Cavitation Effects in the Biomimetic Phantom
2.7. Evaluation of Anti-Tumor Efficiency of Dual-Frequency HIFU and Activatable PFP@Polymer NPs
2.8. Evaluation of the Enhanced Ultrasound Imaging In Vitro
2.9. Statistical Analysis
3. Results
3.1. Characterization of Activatable PFP@Polymer Nanoparticles
3.2. Evaluation of Thermal and Cavitation Effects in the Biomimetic Phantom
3.3. Evaluation of Anti-Tumor Efficiency of Dual-Frequency HIFU and Activatable PFP@Polymer NPs
3.4. Evaluation of Enhanced Ultrasound Imaging In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Peek, M.C.L.; Wu, F. High-intensity focused ultrasound in the treatment of breast tumours. Ecancermedicalscience 2018, 12, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, Y.-H.; Kuo, S.-J.; Tsai, H.-D.; Chou, M.-C.; Yeh, G.-P. Clinical Application of High-intensity Focused Ultrasound in Cancer Therapy. J. Cancer 2016, 7, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempany, C.M.C.; McDannold, N.J.; Hynynen, K.; Jolesz, F.A. Focused Ultrasound Surgery in Oncology: Overview and Principles. Radiology 2011, 259, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Ma, K.W.; She, W.H.; Chu, F.; Lau, V.; Lam, S.W.; Cheung, T.T.; Lo, C.M. High-intensity focused ultrasound ablation of liver tumors in difficult locations. Int. J. Hyperth. 2021, 38, 56–64. [Google Scholar] [CrossRef]
- Schmitt, A.; Mougenot, C.; Chopra, R. Spatiotemporal filtering of MR-temperature artifacts arising from bowel motion during transurethral MR-HIFU. Med. Phys. 2014, 41, 113302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynynen, K. MRIgHIFU: A tool for image-guided therapeutics. J. Magn. Reson. Imaging 2011, 34, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Ebbini, E.; ter Haar, G. Ultrasound-guided therapeutic focused ultrasound: Current status and future directions. Int. J. Hyperth. 2015, 31, 77–89. [Google Scholar] [CrossRef]
- He, K.; Ran, H.; Su, Z.; Wang, Z.; Li, M.; Hao, L. Perfluorohexane-encapsulated fullerene nanospheres for dual-modality US/CT imaging and synergistic high-intensity focused ultrasound ablation. Int. J. Nanomed. 2019, 14, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Hu, Z.; He, Y.; Jin, H.; Yang, Y.; Chen, L.; Chen, Q.; Luo, Q.; Liu, J. Enhancement Effect of Microbubble-Enhanced Ultrasound in Microwave Ablation in Rabbit VX2 Liver Tumors. BioMed Res. Int. 2020, 2020, 3050148. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Wu, M.-F.; Zhang, Y.-X.; Shen, K.; Xia, G.-L. Treatment of hepatic carcinoma by low-frequency ultrasound and microbubbles: A case report. Oncol. Lett. 2015, 9, 1249–1253. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H.; Song, J.; Huang, J.; Yang, K.; Tan, B.; Wang, D.; Yang, N.; Wang, Z.; Li, X. Mitochondria-Targeted and Ultrasound-Activated Nanodroplets for Enhanced Deep-Penetration Sono-dynamic Cancer Therapy. ACS Appl. Mater. Inter. 2019, 11, 9355–9366. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Luo, Y.; Qiao, B.; Wang, X.; Zhang, L.; Chen, Q.; Cao, Y.; Wang, Z.; Ran, H. Dual ultrasound-activatable nanodroplets for highly-penetrative and efficient ovarian cancer theranostics. J. Mater. Chem. B 2020, 8, 380–390. [Google Scholar] [CrossRef]
- Loskutova, K.; Grishenkov, D.; Ghorbani, M. Review on Acoustic Droplet Vaporization in Ultrasound Diagnostics and Therapeutics. BioMed Res. Int. 2019, 2019, 480193. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kim, J.-H.; Rhim, H.; Lim, H.K.; Keserci, B.; Bae, D.-S.; Kim, B.-G.; Lee, J.-W.; Kim, T.-J.; Choi, C.H. Volumetric MR-guided High-Intensity Focused Ultrasound Ablation with a One-Layer Strategy to Treat Large Uterine Fibroids: Initial Clinical Outcomes. Radiology 2012, 263, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Xu, G.-L.; Gu, M.-F.; Luo, G.-Y.; Rong, Z.; Wu, P.-H.; Xia, J.-C. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J. Gastroenterol. 2007, 13, 2747–2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-K.; Kim, D.; Lee, M.-K.; Lee, C.-R.; Kang, S.-Y.; Chung, Y.-J.; Cho, H.-H.; Kim, J.-H.; Kim, M.-R. Three cases of complications after high-intensity focused ultrasound treatment in unmarried women. Obstet. Gynecol. Sci. 2015, 58, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Hong, G.-S.; Lee, C.W.; Kim, G.H. Complication Following Ultrasound-Guided High-Intensity Focused Ultrasound for the Treatment of Uterine Adenomyosis: Case Report of CT Imaging Features. J. Korean Soc. Radiol. 2019, 80, 579–584. [Google Scholar] [CrossRef]
- Coussios, C.; Farny, C.; ter Haar, G.; Roy, R.A. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int. J. Hyperth. 2007, 23, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Bonilla, S.; Suo, D.; Shapira, Y.; Averkiou, M. Microbubble-Enhanced Heating: Exploring the Effect of Microbubble Concentration and Pressure Amplitude on High-Intensity Focused Ultrasound Treatments. Ultrasound Med. Biol. 2021, 47, 2296–2309. [Google Scholar] [CrossRef]
- Bailey, M.R.; Khokhlova, V.A.; Sapozhnikov, O.; Kargl, S.G.; Crum, L.A. Physical mechanisms of the therapeutic effect of ultrasound (a review). Acoust. Phys. 2003, 49, 369–388. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, X.; Sun, L.; Li, H.; Su, C.; Zhang, Y.; Xu, G.; Li, X.; Zhao, C.; Chen, Y.; et al. Continuous inertial cavitation evokes massive ROS for reinforcing sonodynamic therapy and immunogenic cell death against breast carcinoma. Nano Today 2021, 36, 101009. [Google Scholar] [CrossRef]
- Suo, D.; Govind, B.; Zhang, S.; Jing, Y. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrason. Sonochemistry 2018, 41, 419–426. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Chang, Y.-C.; Yeh, C.-K. Improving Nanoparticle Penetration in Tumors by Vascular Disruption with Acoustic Droplet Vaporization. Theranostics 2016, 6, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Chen, Y.; Shen, H.; Luo, Z.; Li, A.; Wang, Q.; Ran, H.; Li, P.; Song, W.; et al. Microbubbles from Gas-Generating Perfluorohexane Nanoemulsions for Targeted Temperature-Sensitive Ultrasonography and Synergistic HIFU Ablation of Tumors. Adv. Mater. 2013, 25, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Aliabouzar, M.; Kripfgans, O.D.; Wang, W.Y.; Baker, B.M.; Fowlkes, J.B.; Fabiilli, M.L. Stable and transient bubble formation in acoustically-responsive scaffolds by acoustic droplet vapor-ization: Theory and application in sequential release. Ultrason. Sonochemistry 2021, 72, 105430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Application of acoustic droplet vaporization in ultrasound therapy. J. Ther. Ultrasound 2015, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Pitt, W.G. Acoustic Droplet Vaporization in Biology and Medicine. BioMed Res. Int. 2013, 2013, 404361. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Zhang, L.; Zhu, H.; Chen, J.; Wu, D.; Bouakaz, A.; Wan, M.; Feng, Y. A Highly Efficient One-for-All Nanodroplet for Ultrasound Imaging-Guided and Cavitation-Enhanced Pho-tothermal Therapy. Int. J. Nanomed. 2021, 16, 3105–3119. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.; Kim, J.; Kong, W.H.; Kim, K.S.; Kim, C.; Hahn, S.K. Multifunctional Nanodroplets Encapsulating Naphthalocyanine and Perfluorohexane for Bimodal Im-age-Guided Therapy. Biomacromolecules 2019, 20, 3767–3777. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Gil You, D.; Kim, S.; Um, W.; Jeon, J.; Kim, C.H.; Joo, H.; Yi, G.; Park, J.H. Cavitation-Inducible Mesoporous Silica–Titania Nanoparticles for Cancer Sonotheranostics. Adv. Health Mater. 2020, 9, 2000877. [Google Scholar] [CrossRef]
- Xu, S.; Chang, N.; Wang, R.; Liu, X.; Guo, S.; Wang, S.; Zong, Y.; Wan, M. Acoustic droplet vaporization and inertial cavitation thresholds and efficiencies of nanodroplets emulsions inside the focused region using a dual-frequency ring focused ultrasound. Ultrason. Sonochemistry 2018, 48, 532–537. [Google Scholar] [CrossRef]
- Law, S.K.B.; Zhou, Y. High-Intensity Focused Ultrasound Ablation by the Dual-Frequency Excitation. IEEE Trans. Ultrason. Ferroelectr. 2019, 66, 18–25. [Google Scholar]
- Guo, S.; Jing, Y.; Jiang, X. Temperature rise in tissue ablation using multi-frequency ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 1699–1707. [Google Scholar] [CrossRef]
- Lafon, C.; Zderic, V.; Noble, M.L.; Yuen, J.C.; Kaczkowski, P.J.; Sapozhnikov, O.A.; Chavrier, F.; Crum, L.A.; Vaezy, S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med. Biol. 2005, 31, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Guntur, S.R.; Lee, K.I.; Paeng, D.G.; Coleman, A. A Tissue Mimicking Polyacrylamide Hydrogel Phantom for Visualizing Thermal Lesions Generated by High Intensity Focused Ultrasound. Ultrasound Med. Biol. 2013, 39, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ilovitsh, T.; Feng, Y.; Foiret, J.; Kheirolomoom, A.; Zhang, H.; Ingham, E.S.; Ilovitsh, A.; Tumbale, S.K.; Fite, B.Z.; Wu, B.; et al. Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites. Proc. Natl. Acad. Sci. USA 2020, 117, 12674–12685. [Google Scholar] [CrossRef] [PubMed]
- He, P.Z.; Xia, R.M.; Duan, S.M.; Shou, W.D.; Qian, D.C. The affection on the tissue lesions of difference frequency in dual-frequency high-intensity focused ultrasound (HIFU). Ultrason. Sonochemistry 2006, 13, 339–344. [Google Scholar] [CrossRef]
- Tung, Y.-S.; Liu, H.-L.; Wu, C.-C.; Ju, K.-C.; Chen, W.-S.; Lin, W.-L. Contrast-agent-enhanced ultrasound thermal ablation. Ultrasound Med. Biol. 2006, 32, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Cho, S.H.; Lee, J.M.; Hahn, S.-T. Effect of microbubble contrast agent during high intensity focused ultrasound ablation on rabbit liver in vivo. Eur. J. Radiol. 2012, 81, e519–e523. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (Ceus) in the Liver-Update 2012 a Wfumb-Efsumb Initiative in Cooperation with Representatives of Afsumb, Aium, Asum, Flaus and Icus. Ultrasound. Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef]
- Choi, W.; Choi, H.; Kim, J.; Kim, C.; Hahn, S.K. Surface-crosslinked multi-functional nanodroplets for photoacoustic/ultrasound image-guided high intensity focused ultrasound therapy. In Photons Plus Ultrasound: Imaging and Sensing 2020; SPIE: Bellingham, WA, USA, 2020; Volume 11240, p. 1124040. [Google Scholar]
- Pang, E.H.T.; Chan, A.; Ho, S.G.; Harris, A.C. Contrast-Enhanced Ultrasound of the Liver: Optimizing Technique and Clinical Applications. Am. J. Roentgenol. 2018, 210, 320–332. [Google Scholar] [CrossRef]
- Duco, W.; Grosso, V.; Zaccari, D.; Soltermann, A.T. Generation of ROS mediated by mechanical waves (ultrasound) and its possible applications. Methods 2016, 109, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Mountford, P.A.; Borden, M.A. On the thermodynamics and kinetics of superheated fluorocarbon phase-change agents. Adv. Colloid Interface Sci. 2016, 237, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.J.; Cady, G.H. Vapor Pressures of Perfluoropentanes. J. Phys. Chem. 1956, 60, 504–505. [Google Scholar] [CrossRef]
- Kee, A.L.Y.; Teo, B.M. Biomedical applications of acoustically responsive phase shift nanodroplets: Current status and future directions. Ultrason. Sonochemistry 2019, 56, 37–45. [Google Scholar] [CrossRef] [PubMed]




| P * (W) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 |
| I1.1 MHz # ) | 23.8 | 43.1 | 62.4 | 81.7 | 101.0 | 120.3 | 139.6 | 158.9 | 178.2 | 197.6 | 216.9 | 236.2 | 255.5 | 275.8 |
| P * (W) | 10 | 20 | 30 | 40 | 50 | 60 |
| I5 MHz # ) | 0.35 | 0.85 | 1.55 | 2.48 | 3.61 | 4.96 |
| Power of Single Frequency 1.1 MHz HIFU (W) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 60 | 80 | 100 | 110 | 120 | 130 | |
| Without NPs (40 s) | × | × | × | × | × | × | × | ||
| With NPs (20 s) | × | ||||||||
| Power Gradient (W) | ||||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | |
| Dual-Frequency (5 MHz:10W; 1.1 MHz) | ||||||
| 1.1 MHz | × | × | ||||
| 5 MHz | × | × | × | × | × | × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Nan, Z.; Zhao, Y.; Zhang, L.; Zhu, H.; Wu, D.; Zong, Y.; Lu, M.; Ilovitsh, T.; Wan, M.; et al. Enhanced HIFU Theranostics with Dual-Frequency-Ring Focused Ultrasound and Activatable Perfluoropentane-Loaded Polymer Nanoparticles. Micromachines 2021, 12, 1324. https://doi.org/10.3390/mi12111324
Chen J, Nan Z, Zhao Y, Zhang L, Zhu H, Wu D, Zong Y, Lu M, Ilovitsh T, Wan M, et al. Enhanced HIFU Theranostics with Dual-Frequency-Ring Focused Ultrasound and Activatable Perfluoropentane-Loaded Polymer Nanoparticles. Micromachines. 2021; 12(11):1324. https://doi.org/10.3390/mi12111324
Chicago/Turabian StyleChen, Junjie, Zhezhu Nan, Yubo Zhao, Lei Zhang, Hongrui Zhu, Daocheng Wu, Yujin Zong, Mingzhu Lu, Tali Ilovitsh, Mingxi Wan, and et al. 2021. "Enhanced HIFU Theranostics with Dual-Frequency-Ring Focused Ultrasound and Activatable Perfluoropentane-Loaded Polymer Nanoparticles" Micromachines 12, no. 11: 1324. https://doi.org/10.3390/mi12111324
APA StyleChen, J., Nan, Z., Zhao, Y., Zhang, L., Zhu, H., Wu, D., Zong, Y., Lu, M., Ilovitsh, T., Wan, M., Yan, K., & Feng, Y. (2021). Enhanced HIFU Theranostics with Dual-Frequency-Ring Focused Ultrasound and Activatable Perfluoropentane-Loaded Polymer Nanoparticles. Micromachines, 12(11), 1324. https://doi.org/10.3390/mi12111324








