Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble–Bubble Interactions
Abstract
:1. Introduction
2. Theory and Methods
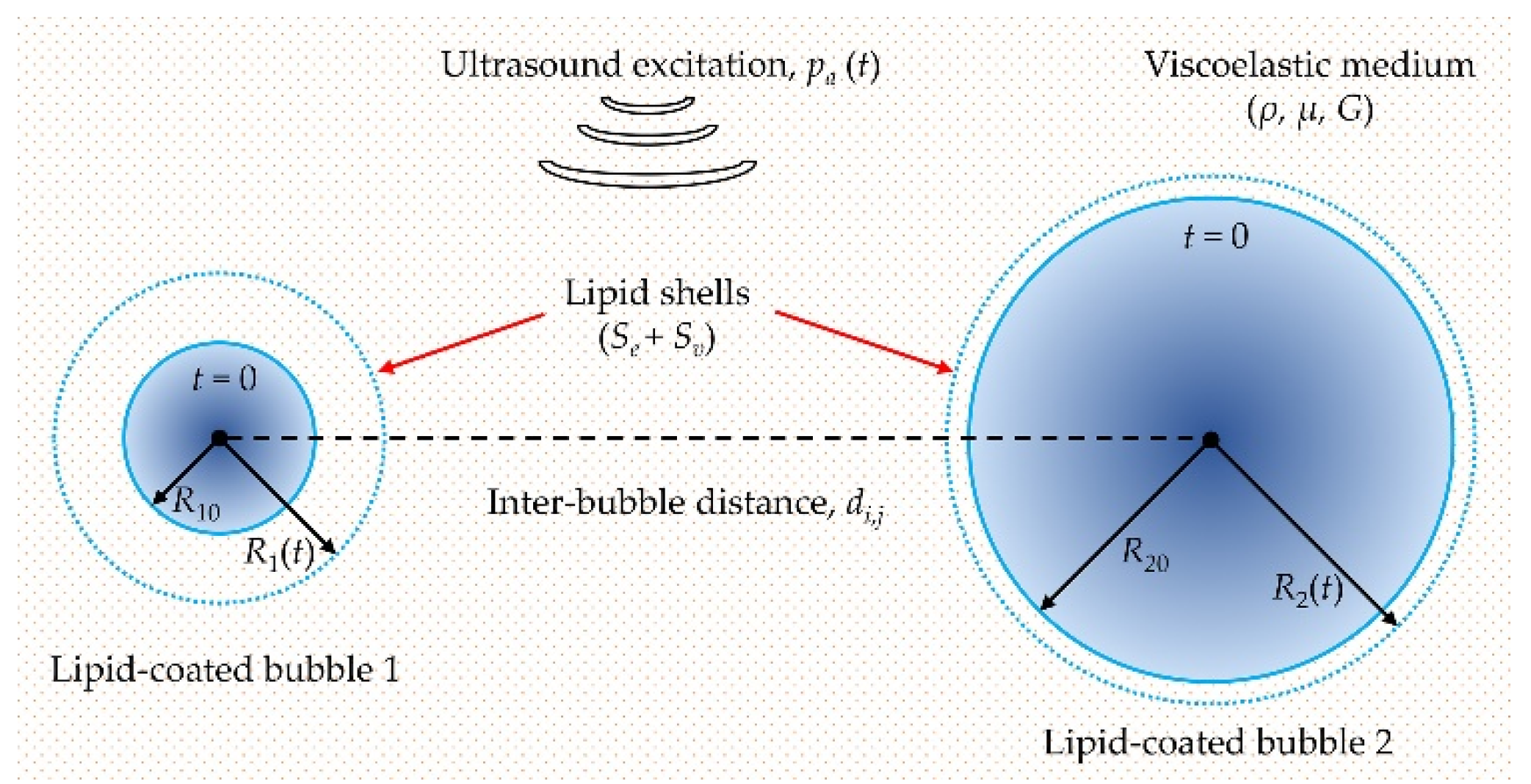
2.1. Modelling the Cavitation Dynamics of Encapsulated Microbubbles in Viscoelastic Media with Considering Bubble–Bubble Interactions
2.2. Viscoelastic Model for the Surrounding Medium
2.3. Modeling the Shell Properties of the Lipid-Coated Microbubbles
2.4. Numerical Simulation Conditions
3. Results
3.1. Dynamics of Two Interacting Encapsulated Microbubbles in the Viscoelastic Medium
3.2. Effects of Shell Properties on the Bubble Dynamics and Inertial Cavitation Threshold
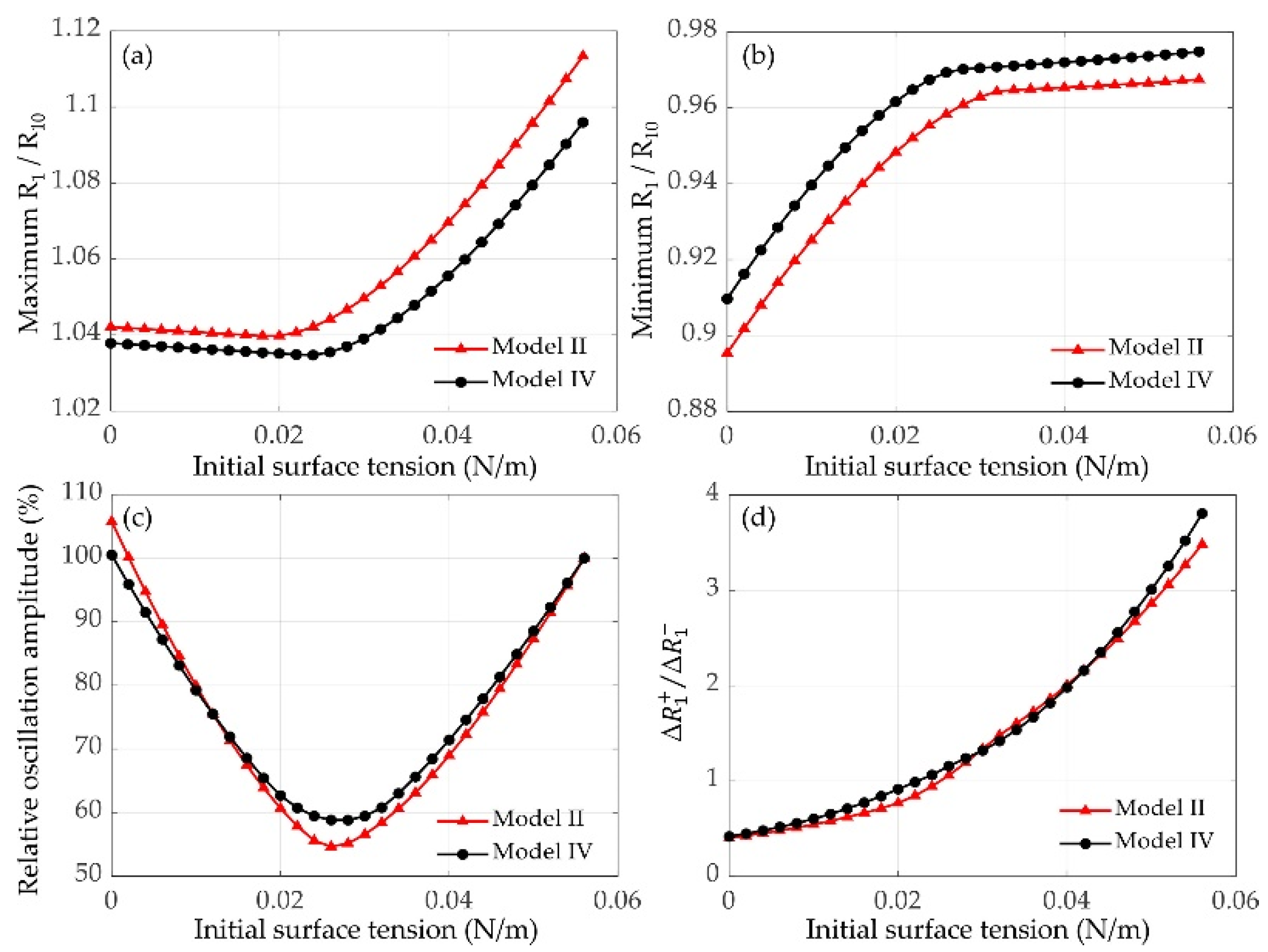
3.2.1. Effects of Initial Surface Tension
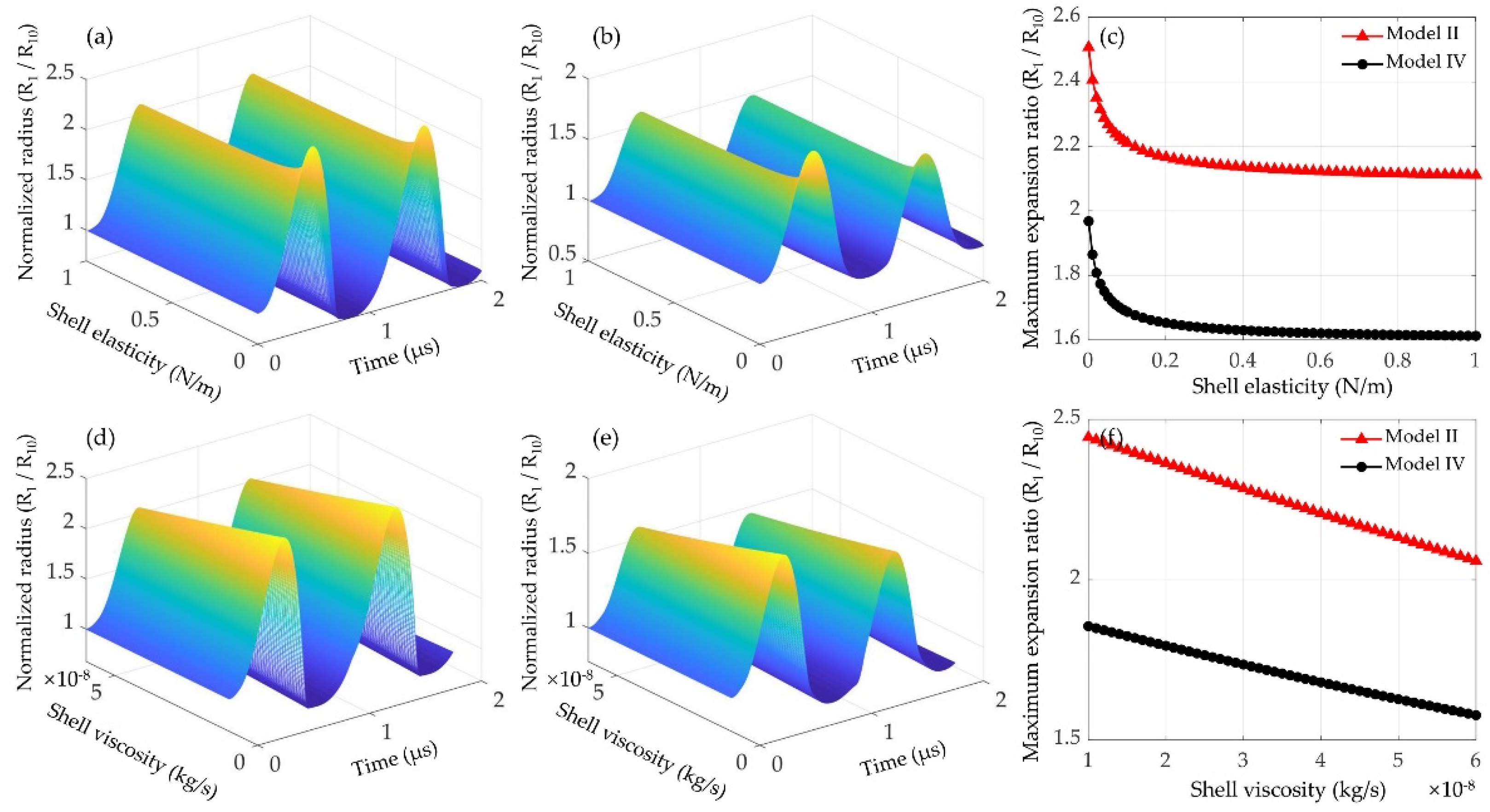
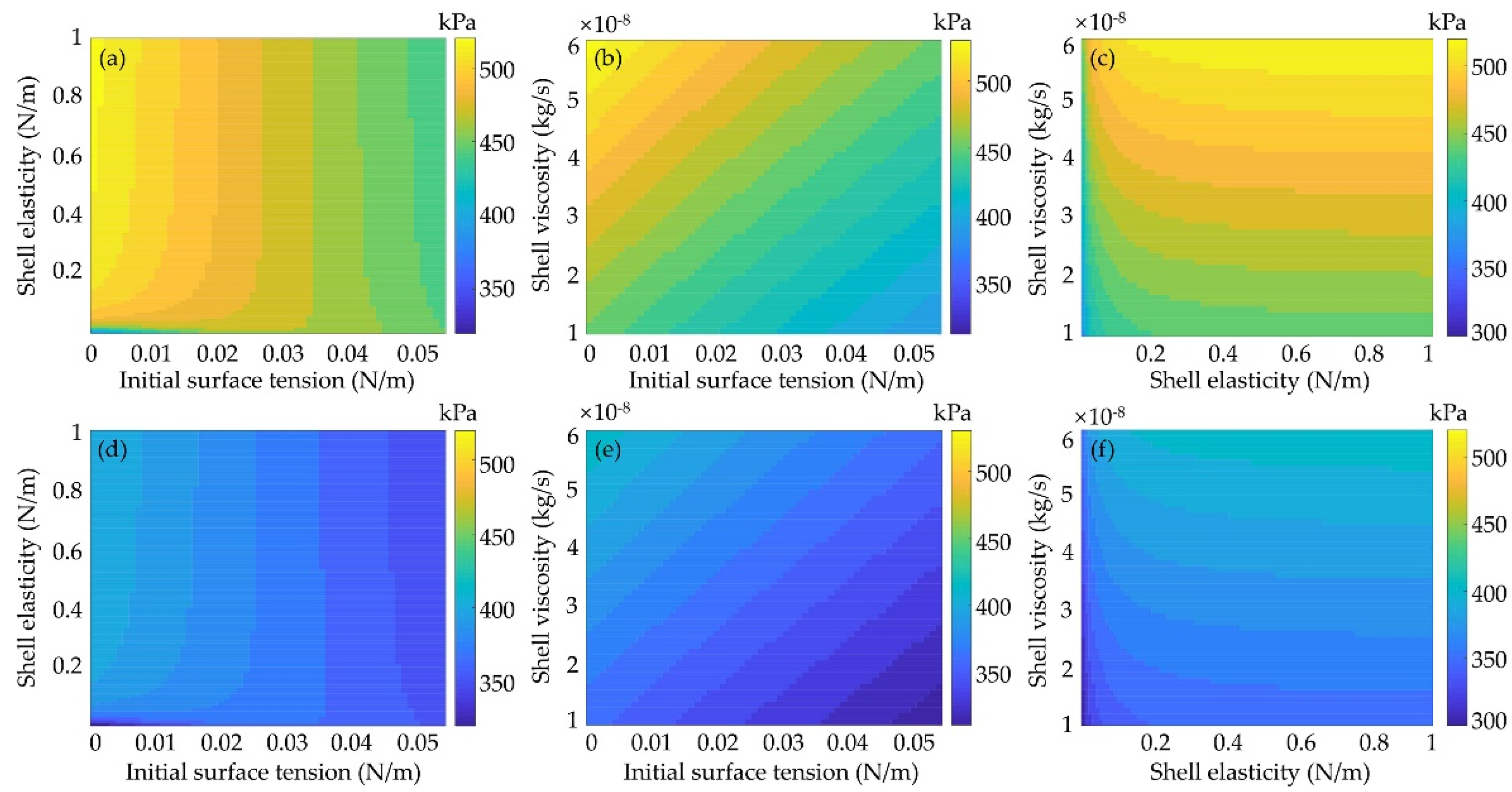
3.2.2. Effects of Shell Elasticity and Viscosity
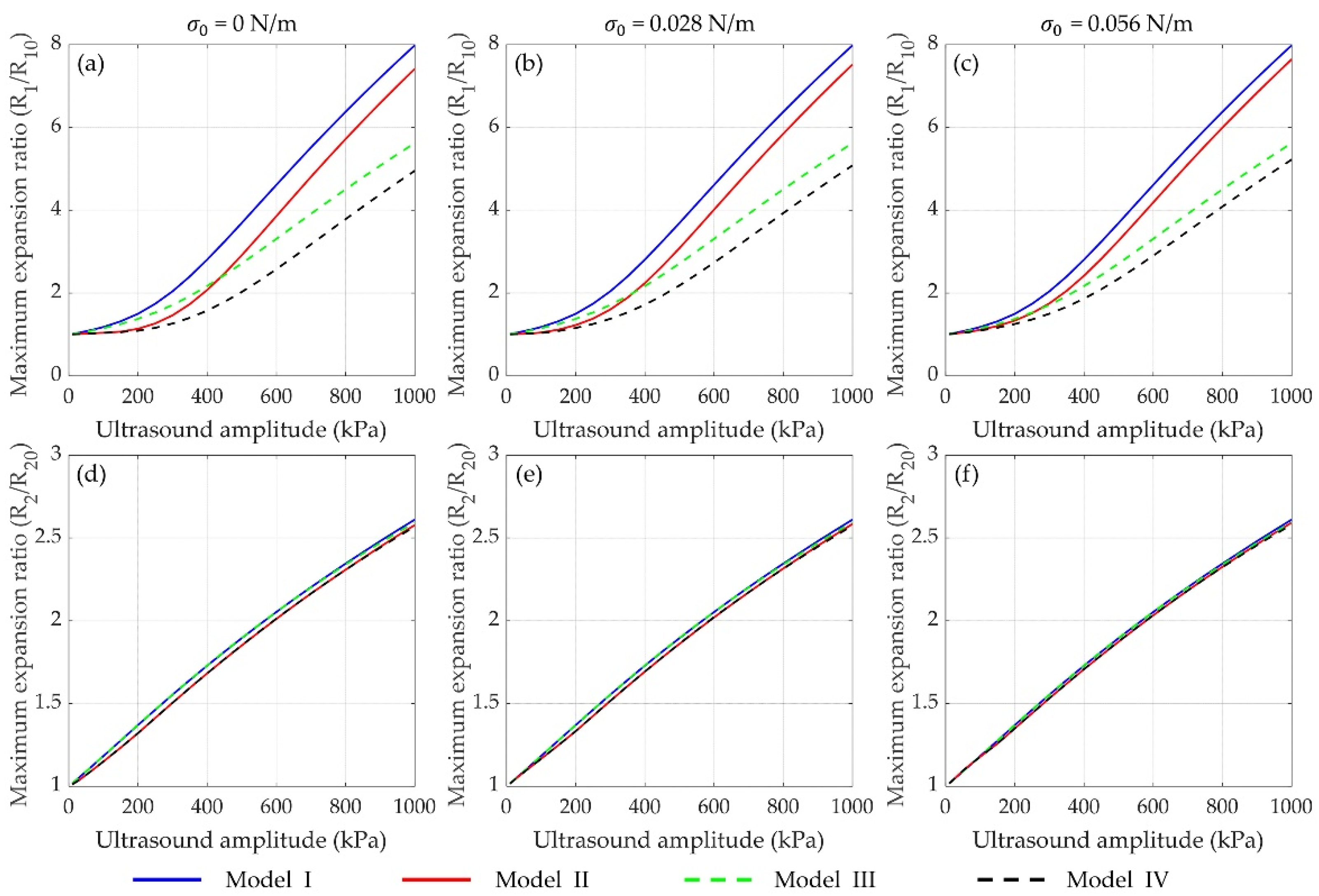
3.2.3. Effects of Shell Properties on Inertial Cavitation Threshold
3.3. Effects of Inter-Bubble Interactions on the Bubble Dynamics and Inertial Cavitation Threshold
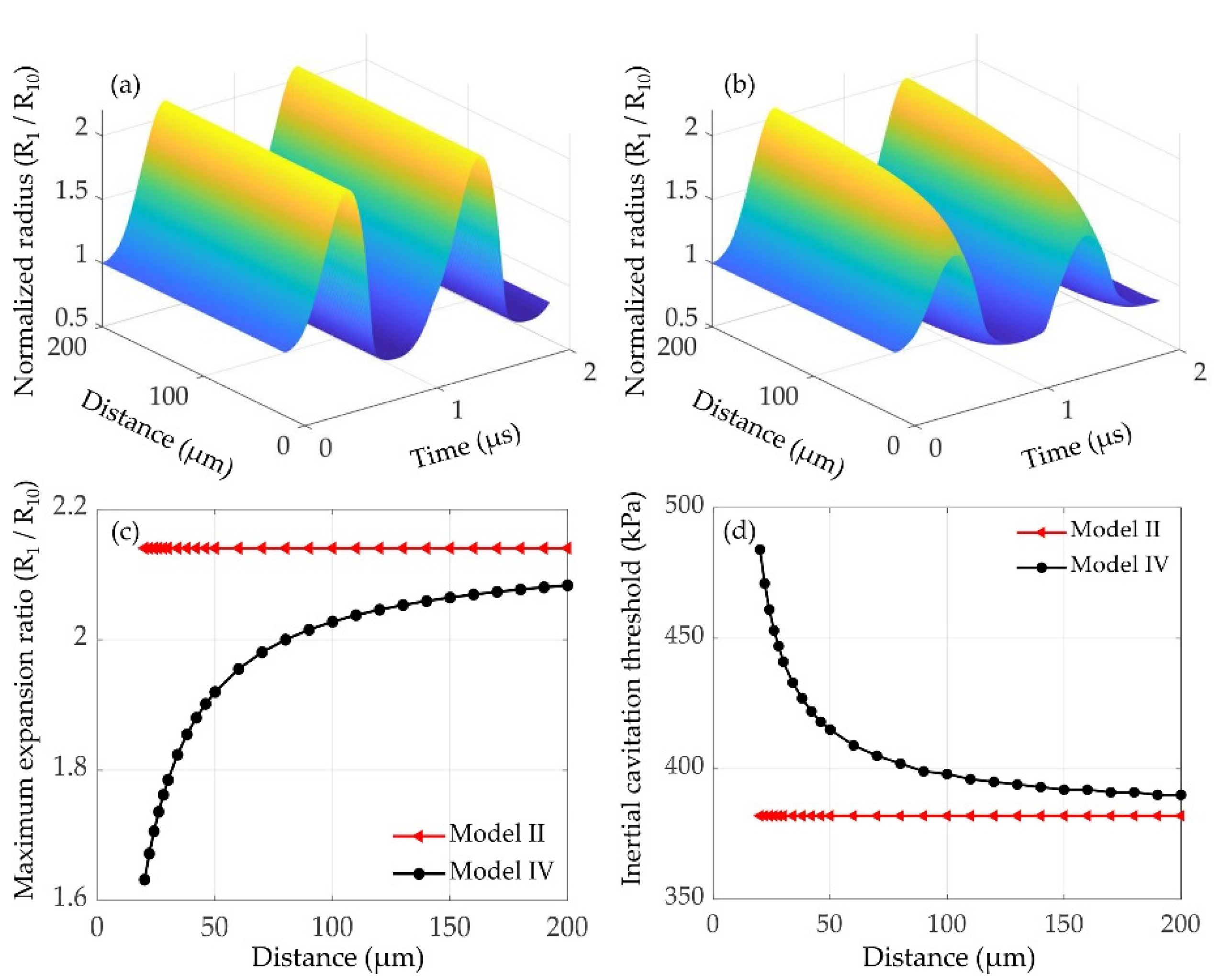
3.3.1. Effects of the Inter-Bubble Distance
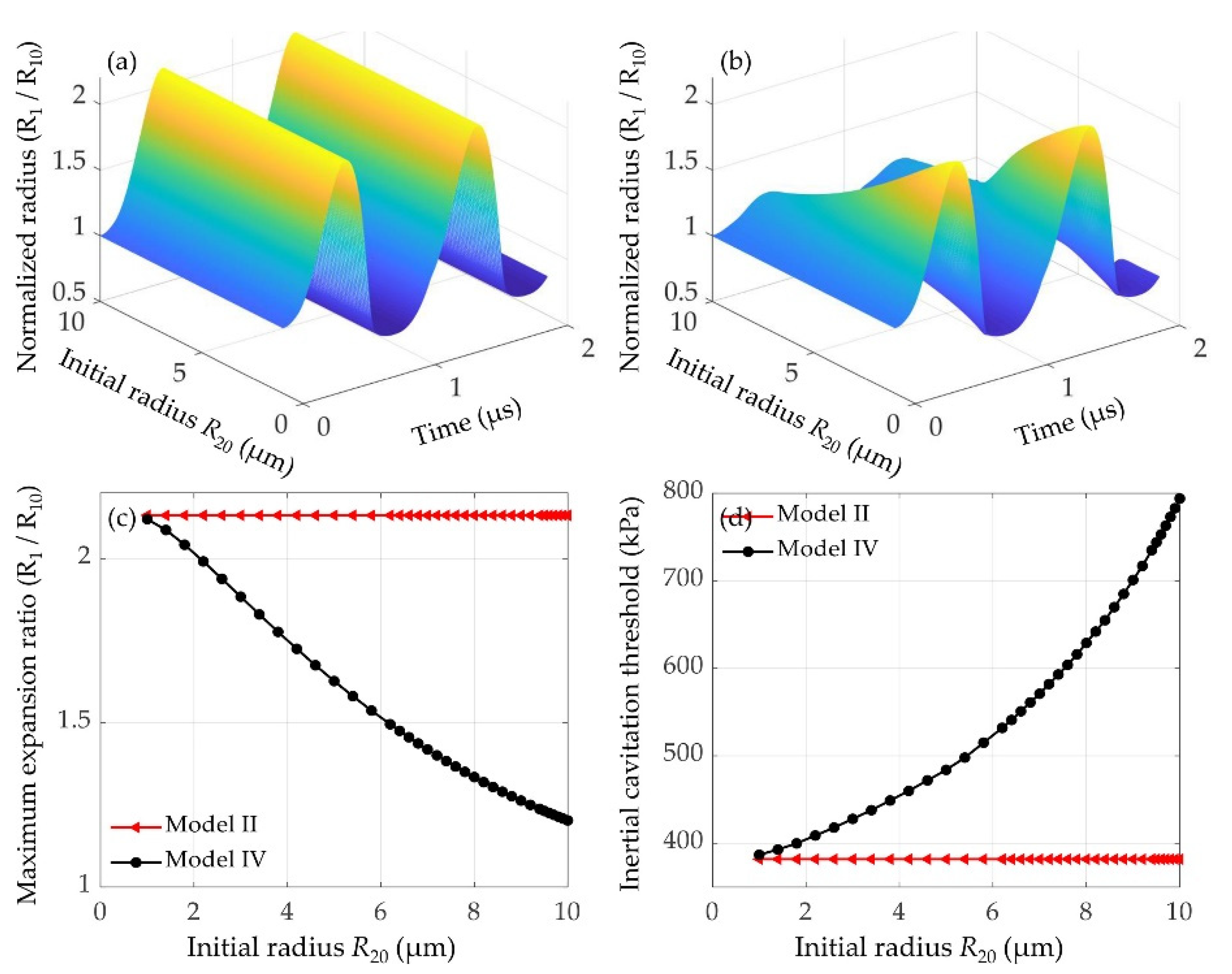
3.3.2. Effects of the Initial Radius of the Nearby Microbubble
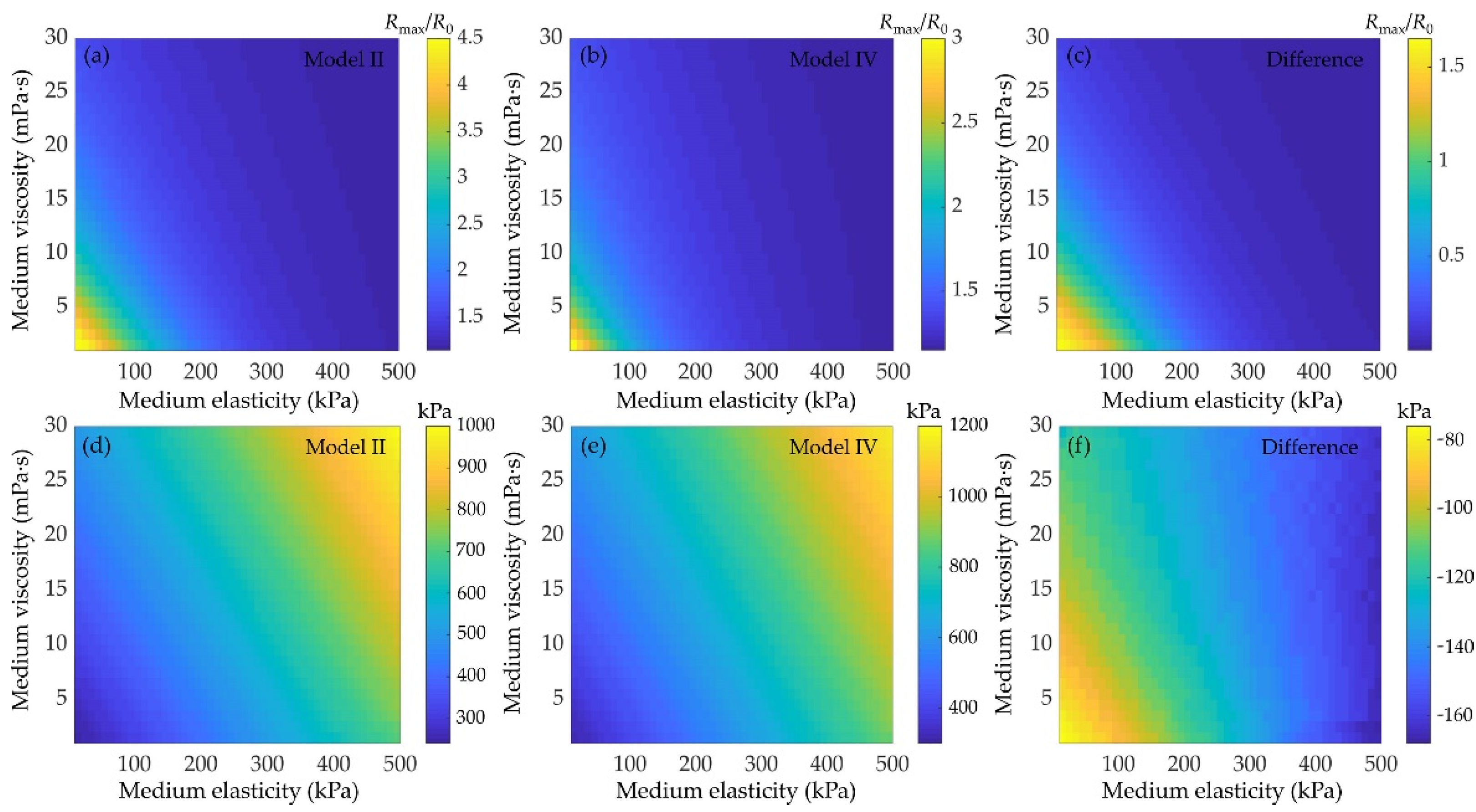
3.4. Effects of Medium Viscoelasticity on the Bubble Dynamics and Inertial Cavitation Threshold
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindner, J.R. Microbubbles in medical imaging: Current applications and future directions. Nat. Rev. Drug Discov. 2004, 3, 527–533. [Google Scholar] [CrossRef]
- Liu, H.L.; Fan, C.H.; Ting, C.Y.; Yeh, C.K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432–444. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Encapsulated ultrasound microbubbles: Therapeutic application in drug/gene delivery. J. Control. Release 2006, 114, 89–99. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Devel. Ther. 2013, 7, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; Smedt, S.C.D.; Lentacker, I. The role of ultrasound-driven microbubble dynamics in drug delivery: From microbubble fundamentals to clinical translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Dauba, A.; Delalande, A.; Kamimura, H.A.; Conti, A.; Larrat, B.; Tsapis, N.; Novell, A. Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics 2020, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Omata, D.; Unga, J.; Suzuki, R.; Maruyama, K. Lipid-based microbubbles and ultrasound for therapeutic application. Adv. Drug Deliv. Rev. 2020, 154, 236–244. [Google Scholar] [CrossRef]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble agents: New directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhang, L.; Huang, X.; Farooq, U.; Pang, N.; Zhou, W.; Qi, L.; Xu, L.; Niu, L. Cell lysis based on an oscillating microbubble array. Micromachines 2020, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lim, W.C.D. Influence of high-intensity focused ultrasound (HIFU) ablation on arteries: Ex vivo studies. Micromachines 2021, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, L.; Cheng, M.; Jin, L.; Du, L.; Han, T.; Xu, L.; Alfred, C.; Qin, P. Effect of acoustic parameters on the cavitation behavior of SonoVue microbubbles induced by pulsed ultrasound. Ultrason. Sonochem. 2017, 35, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, F.; Han, T.; Alfred, C.; Qin, P. Effects of ultrasound pulse parameters on cavitation properties of flowing microbubbles under physiologically relevant conditions. Ultrason. Sonochem. 2019, 52, 512–521. [Google Scholar] [CrossRef]
- Flynn, H.; Church, C.C. Transient pulsations of small gas bubbles in water. J. Acoust. Soc. Am. 1988, 84, 1863–1876. [Google Scholar] [CrossRef]
- Yang, X.; Church, C.C. A model for the dynamics of gas bubbles in soft tissue. J. Acoust. Soc. Am. 2005, 118, 3595–3606. [Google Scholar] [CrossRef] [Green Version]
- Leighton, T. The Acoustic Bubble; Academic press: London, UK, 2012; pp. 308–312. [Google Scholar]
- Sojahrood, A.J.; Falou, O.; Earl, R.; Karshafian, R.; Kolios, M.C. Influence of the pressure-dependent resonance frequency on the bifurcation structure and backscattered pressure of ultrasound contrast agents: A numerical investigation. Nonlinear Dyn. 2015, 80, 889–904. [Google Scholar] [CrossRef]
- Sojahrood, A.J.; Earl, R.; Kolios, M.C.; Karshafian, R. Investigation of the 1/2 order subharmonic emissions of the period-2 oscillations of an ultrasonically excited bubble. Phys. Lett. A 2020, 384, 126446. [Google Scholar] [CrossRef]
- Apfel, R. Some new results on cavitation threshold prediction and bubble dynamics. In Cavitation and Inhomogeneities in Underwater Acoustics; Springer: Berlin/Heidelberg, Germany, 1980; pp. 79–83. [Google Scholar]
- Plesset, M.S.; Mitchell, T. On the stability of the spherical shape of a vapor cavity in a liquid. Q. Appl. Math. 1956, 13, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Chomas, J.E.; Dayton, P.A.; May, D.; Allen, J.; Klibanov, A.; Ferrara, K. Optical observation of contrast agent destruction. Appl. Phys. Lett. 2000, 77, 1056–1058. [Google Scholar] [CrossRef]
- Chomas, J.E.; Dayton, P.A.; May, D.J.; Ferrara, K.W. Threshold of fragmentation for ultrasonic contrast agents. J. Biomed. Opt. 2001, 6, 141–150. [Google Scholar] [CrossRef]
- Hegedűs, F. Stable bubble oscillations beyond Blake’s critical threshold. Ultrasonics 2014, 54, 1113–1121. [Google Scholar] [CrossRef]
- Marmottant, P.; Meer, S.V.D.; Emmer, M.; Versluis, M.; Jong, N.D.; Hilgenfeldt, S.; Lohse, D. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. J. Acoust. Soc. Am. 2005, 118, 3499–3505. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, A.; Sarkar, K.; Jain, P. Effects of encapsulation elasticity on the stability of an encapsulated microbubble. J. Colloid Interface Sci. 2009, 336, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Guan, J.; Qiu, Y.; Matula, T.J. Estimating the shell parameters of SonoVue® microbubbles using light scattering. J. Acoust. Soc. Am. 2009, 126, 2954–2962. [Google Scholar] [CrossRef] [Green Version]
- Doinikov, A.A.; Bouakaz, A. Theoretical investigation of shear stress generated by a contrast microbubble on the cell membrane as a mechanism for sonoporation. J. Acoust. Soc. Am. 2010, 128, 11–19. [Google Scholar] [CrossRef]
- Qin, S.; Ferrara, K.W. A model for the dynamics of ultrasound contrast agents in vivo. J. Acoust. Soc. Am. 2010, 128, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Forbes, M.M.; O’Brien, W.D.J. Development of a theoretical model describing sonoporation activity of cells exposed to ultrasound in the presence of contrast agents. J. Acoust. Soc. Am. 2012, 131, 2723–2729. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Li, Q.; Zhang, Z.; Zhang, D.; Tu, J. Investigation on the inertial cavitation threshold and shell properties of commercialized ultrasound contrast agent microbubbles. J. Acoust. Soc. Am. 2013, 134, 1622–1631. [Google Scholar] [CrossRef]
- Harfield, C.; Ovenden, N.; Memoli, G.; Jones, P.; Stride, E. Theoretical characterisation of the radial and translational motion of coated microbubbles under acoustic excitation. In Proceedings of the 11th Anglo-French Physical Acoustics Conference (AFPAC 2012), Brighton, UK, 18–20 January 2012; p. 012001. [Google Scholar]
- Li, Q.; Matula, T.J.; Tu, J.; Guo, X.; Zhang, D. Modeling complicated rheological behaviors in encapsulating shells of lipid-coated microbubbles accounting for nonlinear changes of both shell viscosity and elasticity. Phys. Med. Biol. 2013, 58, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, S. A model to calculate microstreaming-shear stress generated by oscillating microbubbles on the cell membrane in sonoporation. Biomed. Mater. Eng. 2014, 24, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, Y.; Wang, L. Effects of liquid compressibility on the dynamics of ultrasound contrast agent microbubbles. Fluid Dyn. Res. 2020, 52, 055507. [Google Scholar] [CrossRef]
- Gümmer, J.; Schenke, S.; Denner, F. Modelling lipid-coated microbubbles in focused ultrasound applications at subresonance frequencies. Ultrasound Med. Biol. 2021, 47, 2958–2979. [Google Scholar] [CrossRef]
- Qin, D.; Zou, Q.; Li, Z.; Wang, W.; Wan, M.; Feng, Y. Acoustic cavitation of an encapsulated microbubble and its mechanical effect in soft tissue. Acta Phys. Sin. 2021, 70, 154701. [Google Scholar]
- Sojahrood, A.J.; Haghi, H.; Karshafian, R.; Kolios, M.C. Nonlinear dynamics and bifurcation structure of ultrasonically excited lipid coated microbubbles. Ultrason. Sonochem. 2021, 72, 105405. [Google Scholar] [CrossRef]
- Sojahrood, A.J.; Leon, A.C.D.; Lee, R.; Cooley, M.; Abenojar, E.C.; Kolios, M.C.; Exner, A.A. Toward precisely controllable acoustic response of shell-stabilized nanobubbles: High yield and narrow dispersity. ACS Nano 2021, 15, 4901–4915. [Google Scholar] [CrossRef]
- Sojahrood, A.J.; Haghi, H.; Porter, T.M.; Karshafian, R.; Kolios, M.C. Experimental and numerical evidence of intensified non-linearity at the microscale: The lipid coated acoustic bubble. Phys. Fluids 2021, 33, 072006. [Google Scholar] [CrossRef]
- Doinikov, A.A.; Haac, J.F.; Dayton, P.A. Modeling of nonlinear viscous stress in encapsulating shells of lipid-coated contrast agent microbubbles. Ultrasonics 2009, 49, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Zilonova, E.; Solovchuk, M.; Sheu, T. Bubble dynamics in viscoelastic soft tissue in high-intensity focal ultrasound thermal therapy. Ultrason. Sonochem. 2018, 40, 900–911. [Google Scholar] [CrossRef]
- Pahk, K.J.; de Andrade, M.O.; Gélat, P.; Kim, H.; Saffari, N. Mechanical damage induced by the appearance of rectified bubble growth in a viscoelastic medium during boiling histotripsy exposure. Ultrason. Sonochem. 2019, 53, 164–177. [Google Scholar] [CrossRef]
- Doinikov, A.A. Translational motion of two interacting bubbles in a strong acoustic field. Phys. Rev. E 2001, 64, 026301. [Google Scholar] [CrossRef]
- Yasui, K.; Lee, J.; Tuziuti, T.; Towata, A.; Kozuka, T.; Iida, Y. Influence of the bubble–bubble interaction on destruction of encapsulated microbubbles under ultrasound. J. Acoust. Soc. Am. 2009, 126, 973–982. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Tang, M.; Zhang, D. Effect of secondary radiation force on aggregation between encapsulated microbubbles. Chin. Phys. B 2011, 20, 114302. [Google Scholar] [CrossRef]
- Dzaharudin, F.; Suslov, S.A.; Manasseh, R.; Ooi, A. Effects of coupling, bubble size, and spatial arrangement on chaotic dynamics of microbubble cluster in ultrasonic fields. J. Acoust. Soc. Am. 2013, 134, 3425–3434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, S. The secondary Bjerknes force between two gas bubbles under dual-frequency acoustic excitation. Ultrason. Sonochem. 2016, 29, 129–145. [Google Scholar] [CrossRef]
- Jiang, L.; Ge, H.; Liu, F.; Chen, D. Investigations on dynamics of interacting cavitation bubbles in strong acoustic fields. Ultrason. Sonochem. 2017, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.L.; Yu, J.; Tu, J.; Guo, X.S.; Huang, P.T.; Zhang, D. Interaction between encapsulated microbubbles: A finite element modelling study. Chin. Phys. B 2018, 27, 084302. [Google Scholar] [CrossRef]
- Haghi, H.; Sojahrood, A.J.; Kolios, M.C. Collective nonlinear behavior of interacting polydisperse microbubble clusters. Ultrason. Sonochem. 2019, 58, 104708. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, H.; Zhu, J.; Du, W. A simple model of bubble cluster dynamics in an acoustic field. Ultrason. Sonochem. 2020, 64, 104790. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, W.Z.; Zhang, Y.Y.; Wu, Y.R.; Wang, X.; Zhao, G.Y. Bubble translation driven by pulsation in a double-bubble system. Chin. Phys. B 2020, 29, 034303. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, L.; Wu, Y.; Chen, W. The role of the bubble–bubble interaction on radial pulsations of bubbles. Ultrason. Sonochem. 2021, 73, 105535. [Google Scholar] [CrossRef]
- Sojahrood, A.J.; Earl, R.; Haghi, H.; Li, Q.; Porter, T.M.; Kolios, M.C.; Karshafian, R. Nonlinear dynamics of acoustic bubbles excited by their pressure-dependent subharmonic resonance frequency: Influence of the pressure amplitude, frequency, encapsulation and multiple bubble interactions on oversaturation and enhancement of the subharmonic signal. Nonlinear Dyn. 2021, 103, 429–466. [Google Scholar]
- Zilonova, E.; Solovchuk, M.; Sheu, T. Dynamics of bubble–bubble interactions experiencing viscoelastic drag. Phys. Rev. E 2019, 99, 023109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lai, Z.; Chen, Z.; Li, Y. The secondary Bjerknes force between two oscillating bubbles in Kelvin-Voigt-type viscoelastic fluids driven by harmonic ultrasonic pressure. Ultrason. Sonochem. 2019, 52, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.; Zou, Q.; Lei, S.; Wang, W.; Li, Z. Nonlinear dynamics and acoustic emissions of interacting cavitation bubbles in viscoelastic tissues. Ultrason. Sonochem. 2021, 78, 105712. [Google Scholar] [CrossRef] [PubMed]
- King, D.A. Collapse Dynamics of Ultrasound Contrast Agent Microbubbles; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2012; pp. 84–90. [Google Scholar]
- Versluis, M.; Stride, E.; Lajoinie, G.; Dollet, B.; Segers, T. Ultrasound contrast agent modeling: A review. Ultrasound Med. Biol. 2020, 46, 2117–2144. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Naoe, T.; Futakawa, M. Suppression of cavitation inception by gas bubble injection: A numerical study focusing on bubble–bubble interaction. Phys. Rev. E 2007, 76, 046309. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, A.; Harker, A.; Ventikos, Y.; Edirisinghe, M. Novel preparation of monodisperse microbubbles by integrating oscillating electric fields with microfluidics. Micromachines 2018, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Helfield, B. A review of phospholipid encapsulated ultrasound contrast agent microbubble physics. Ultrasound Med. Biol. 2019, 45, 282–300. [Google Scholar] [CrossRef]
- Matsumi, C.T.; Da Silva, W.J.; Schneider, F.K.; Maia, J.M.; Morales, R.E.M.; Filho, W.D.A. Micropipette-based microfluidic device for monodisperse microbubbles generation. Micromachines 2018, 9, 387. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, D.; Zou, Q.; Lei, S.; Wang, W.; Li, Z. Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble–Bubble Interactions. Micromachines 2021, 12, 1125. https://doi.org/10.3390/mi12091125
Qin D, Zou Q, Lei S, Wang W, Li Z. Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble–Bubble Interactions. Micromachines. 2021; 12(9):1125. https://doi.org/10.3390/mi12091125
Chicago/Turabian StyleQin, Dui, Qingqin Zou, Shuang Lei, Wei Wang, and Zhangyong Li. 2021. "Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble–Bubble Interactions" Micromachines 12, no. 9: 1125. https://doi.org/10.3390/mi12091125
APA StyleQin, D., Zou, Q., Lei, S., Wang, W., & Li, Z. (2021). Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble–Bubble Interactions. Micromachines, 12(9), 1125. https://doi.org/10.3390/mi12091125





