Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular Oxygen
Abstract
:1. Introduction
2. Experimental
2.1. General Information
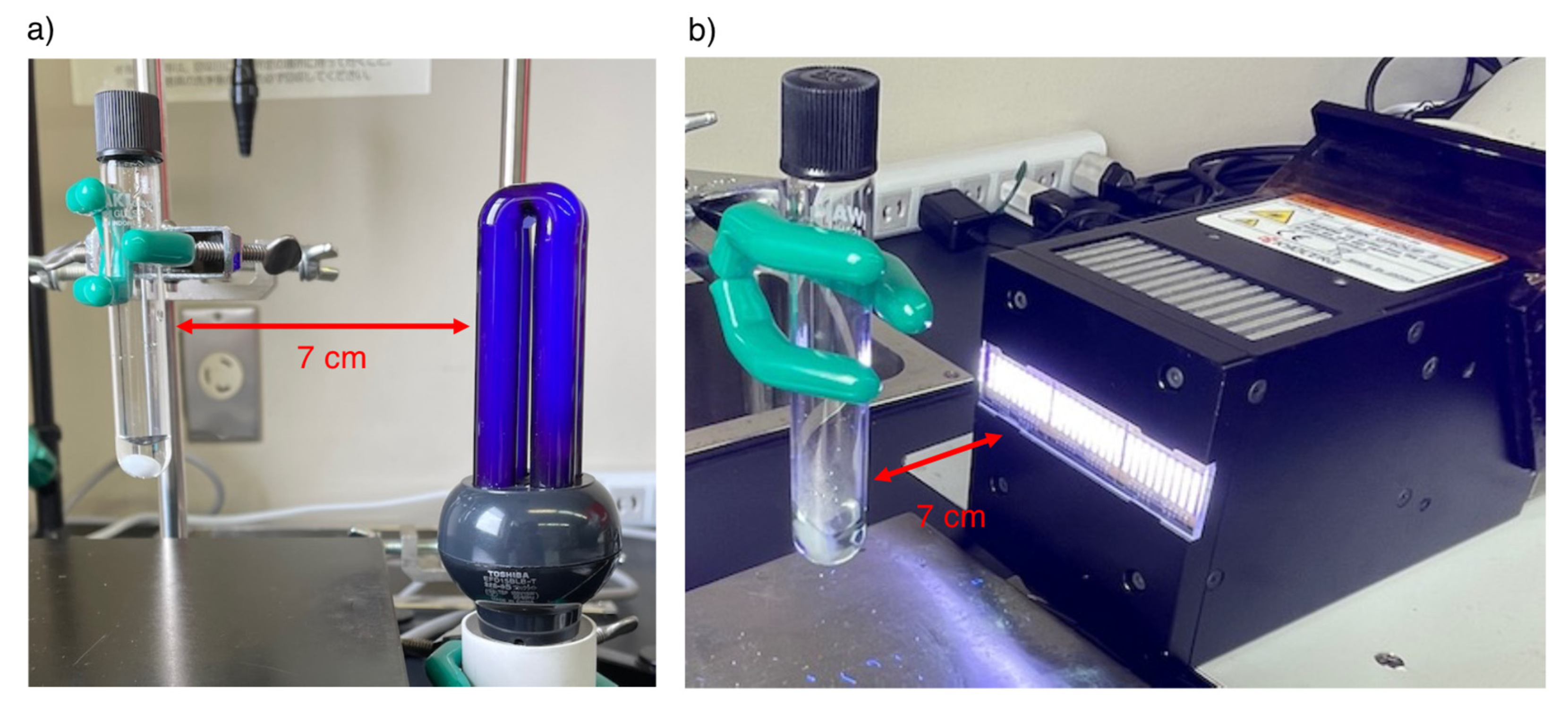
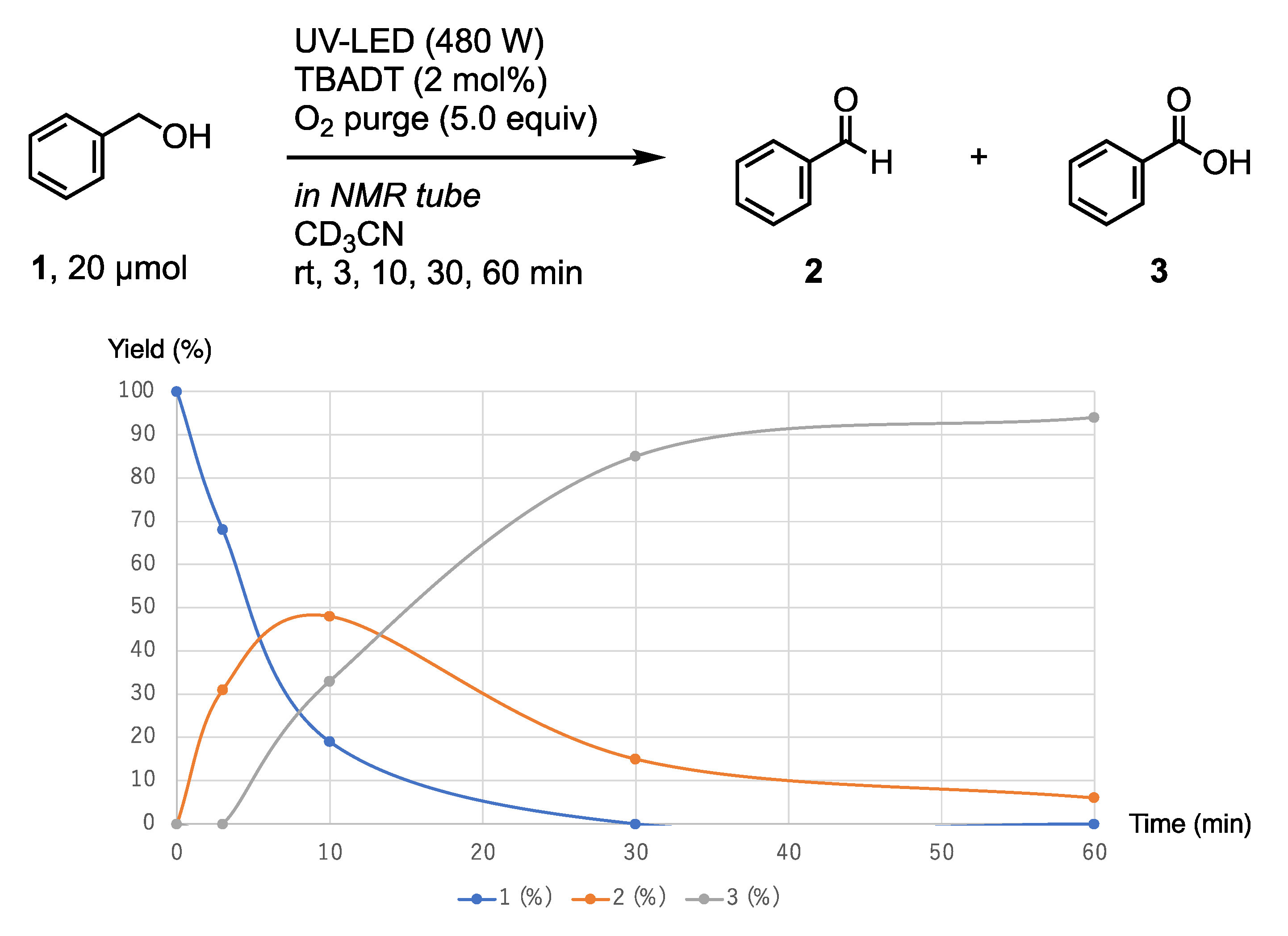
2.2. Typical Procedure for the Oxidation of Benzyl Alcohol 1
2.3. The Procedure for the Isolation of Benzoic Acid 3
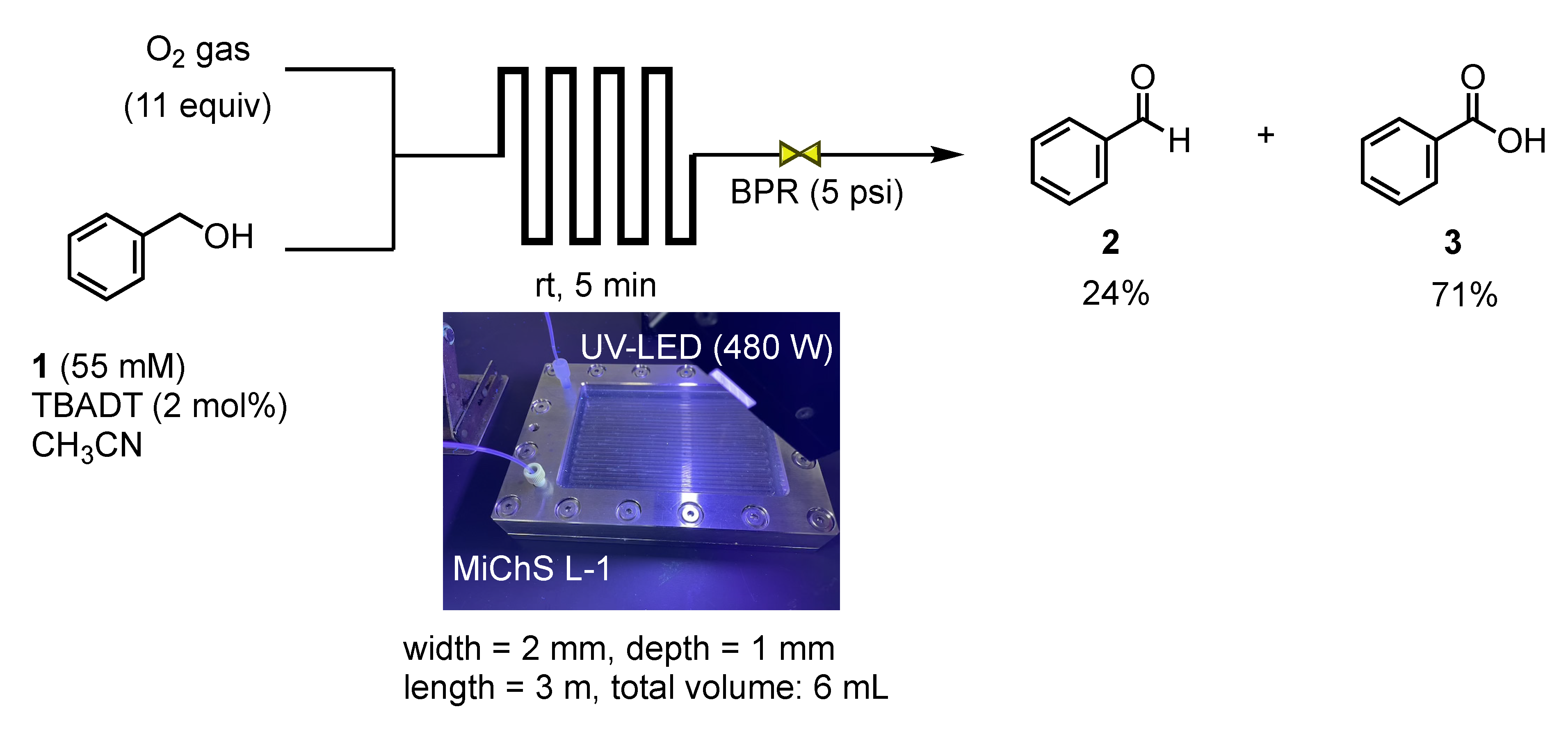
2.4. The Procedure for the Flow Oxidation of Benzyl Alcohol 1
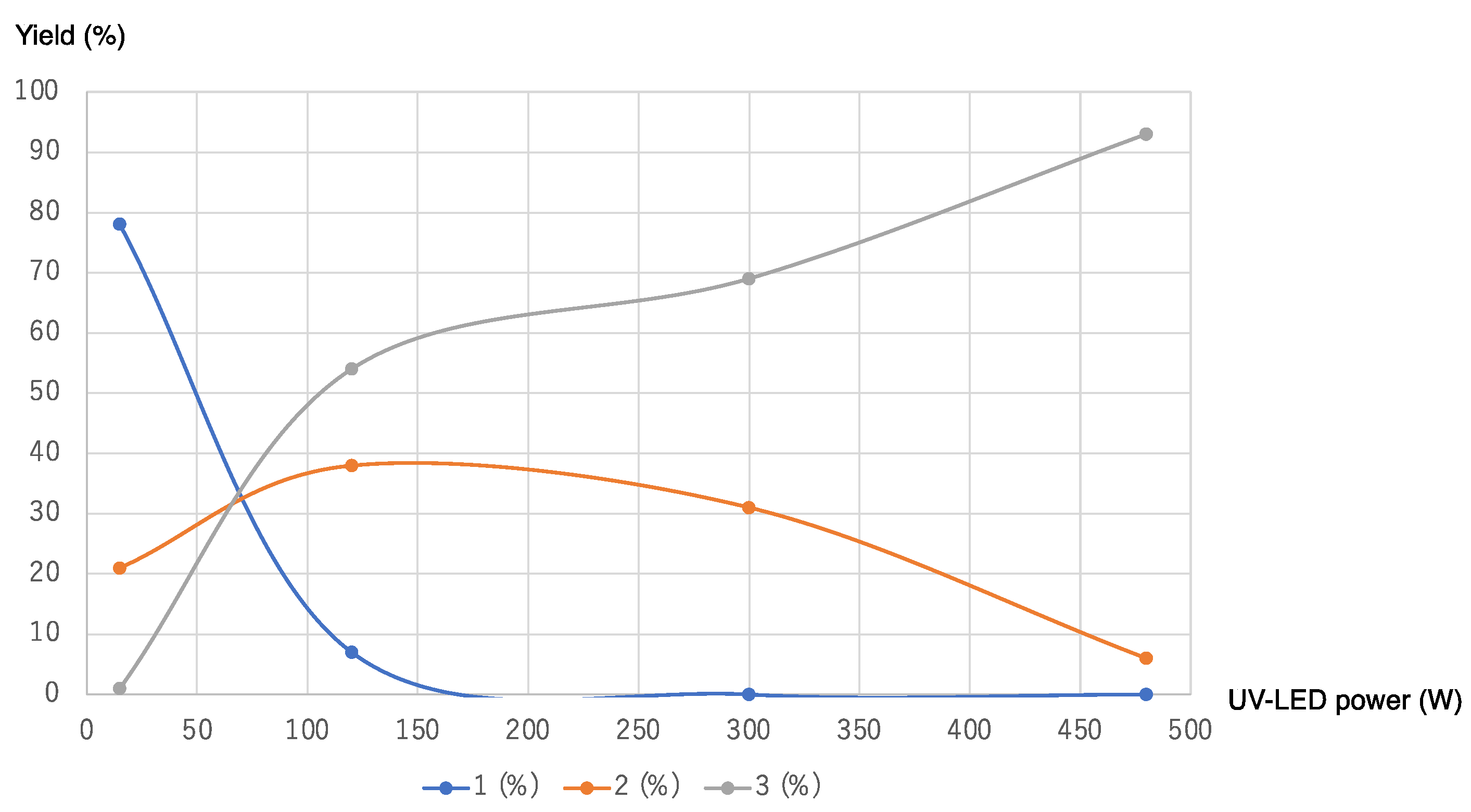
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hill, C.L. Introduction of functionality into unactivated carbon-hydrogen bonds. Catalytic generation and nonconventional utilization of organic radicals. Synlett 1995, 1995, 127–132. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Lykakis, I.N.; Orfanopoulos, M. Decatungstate as an efficient photocatalyst in organic chemistry. Chem. Soc. Rev. 2009, 38, 2609–2621. [Google Scholar] [CrossRef] [PubMed]
- Protti, S.; Fagnoni, M.; Ravelli, D. Photocatalytic C-H activation by hydrogen-atom transfer in synthesis. ChemCatChem 2015, 7, 1516–1523. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate anion for photocatalyzed “window ledge” reactions. Acc. Chem. Res. 2016, 49, 2232–2242. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-selective C–H functionalization by decatungstate anion photocatalysis: Synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 2018, 8, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, J.; Giannotti, C. Photo-oxygenation of 1,8-cineole by molecular oxygen catalysed by (Bu4N)4W10O32. J. Photochem. Photobiol. A Chem. 1992, 63, 173–177. [Google Scholar] [CrossRef]
- Lykakis, I.N.; Tanielian, C.; Orfanopoulos, M. Decatungstate photocatalyzed oxidation of aryl alkanols. electron transfer or hydrogen abstraction mechanism? Org. Lett. 2003, 5, 2875–2878. [Google Scholar] [CrossRef]
- Maldotti, A.; Amadelli, R.; Vitali, I.; Borgatti, L.; Molinari, A. CH2Cl2-assisted functionalization of cycloalkenes by photoexcited (nBu4N)4W10O32 heterogenized on SiO2. J. Mol. Catal. A Chem. 2003, 204–205, 703–711. [Google Scholar] [CrossRef]
- Lykakis, I.N.; Orfanopoulos, M. Photooxidation of aryl alkanes by a decatungstate/triethylsilane system in the presence of molecular oxygen. Tetrahedron Lett. 2004, 45, 7645–7649. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Lykakis, I.N.; Panagiotou, G.D.; Bourikas, K.; Lycourghiotis, A.L.; Kordulis, C.; Orfanopoulos, M. Decatungstate catalyst supported on silica and γ-alumina: Efficient photocatalytic oxidation of benzyl alcohols. J. Catal. 2007, 252, 178–189. [Google Scholar] [CrossRef]
- Laudadio, G.; Govaerts, S.; Wang, Y.; Ravelli, D.; Koolman, H.F.; Fagnoni, M.; Djuric, S.W.; Noel, T. Selective C(sp3)–H aerobic oxidation enabled by decatungstate photocatalysis in flow. Angew. Chem. Int. Ed. 2018, 57, 4078–4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamase, T.; Usami, Y. Photocatalytic dimerization of olefins by decatungstate, [W10O32]4–, in acetonitrile and magnetic resonance studies of photoreduced species. J. Chem. Soc. Dalton Trans. 1998, 183–190. [Google Scholar]
- Fukuyama, T.; Nishikawa, T.; Ryu, I. Site-Selective C(sp3)–H functionalization of fluorinated alkanes driven by polar effects using a tungstate photocatalyst. Eur. J. Org. Chem. 2020, 2020, 1424–1428. [Google Scholar] [CrossRef]
- Bonassi, F.; Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate photocatalyzed acylations and alkylations in flow via hydrogen atom transfer. Adv. Synth. Catal. 2015, 357, 3687–3695. [Google Scholar] [CrossRef]
- Schultz, D.M.; Levesque, F.; DiRocco, D.A.; Reibarkh, M.; Ji, Y.; Joyce, L.A.; Dropinski, J.F.; Sheng, H.; Sherry, B.D.; Davies, I.W. Oxyfunctionalization of the remote C–H bonds of aliphatic amines by decatungstate photocatalysis. Angew. Chem. Int. Ed. 2017, 56, 15274–15278. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, G.; Deng, Y.; van del Wal, K.; Ravelli, D.; Nuno, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noel, T. C(sp3)–H functionalizations of light hydrocarbons using decatungstate photocatalysis in flow. Science 2020, 369, 92–96. [Google Scholar] [CrossRef]
- Bonciolini, S.; Filippo, M.D.; Baumann, M. A scalable continuous photochemical process for the generation of aminopropylsulfones. Org. Biomol. Chem. 2020, 18, 9428–9432. [Google Scholar] [CrossRef]
- Renneke, R.F.; Pasquali, M.; Hill, C.L. Polyoxometalate systems for the catalytic selective production of nonthermodynamic alkenes from alkanes. Nature of excited-state deactivation processes and control of subsequent thermal processes in polyoxometalate photoredox chemistry. J. Am. Chem. Soc. 1990, 112, 6585–6594. [Google Scholar] [CrossRef]
- Bahamonde, A.; Murphy, J.J.; Savarese, M.; Bremond, E.; Cavalli, A.; Melchiorre, P. Studies on the enantioselective iminium ion trapping of radicals triggerd by an electron-relay mechanism. J. Am. Chem. Soc. 2017, 139, 4559–4567. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Maheshwari, A.; Sambiagio, C.; Deng, Y.; Laudadio, G.; Aken, K.V.; Sun, Y.; Gemoets, H.P.L.; Noel, T. Optimization of a decatungstate-catalyzed C(sp3)–H alkylation using a continuous oscillatory milli structured photoreactor. Org. Process Res. Dev. 2020, 24, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Rahman, M.T.; Sato, M.; Ryu, I. Adventures in inner space: Microflow synthesis for practical organic synthesis. Synlett 2008, 2008, 151–163. [Google Scholar]
- Voyle, E.E.; Oelgemöller, M. Micro-photochemistry: Photochemistry in microstructured reactors. The new photochemistry of the future? Photochem. Photobiol. Sci. 2008, 7, 1313–1322. [Google Scholar]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noel, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. The use of molecular oxygen for liquid phase aerobic oxidations in continuous flow. Top. Curr. Chem. 2019, 377, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambacorta, G.; Sharley, J.S.; Baxendale, I.R. A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries. Beilstein J. Org. Chem. 2021, 17, 1181–1312. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Park, J.; Suenobu, T.; Lee, Y.-M.; Nam, W.; Fukuzumi, S. Mechanistic borderline of one-step hydrogen atom transfer versus stepwise Sc3+-coupled electron transfer from benzyl alcohol derivatives to a non-heme iron(IV)-oxo complex. Inorg. Chem. 2012, 51, 10025–10036. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lo, P.-K.; Lam, K.-C.; Lau, T.-C. A hydrogen-atom transfer mechanism in the oxidation of alcohols by [FeO4]2– in aqueous solution. Dalton Trans. 2018, 47, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kushch, O.V.; Hordieieva, I.O.; Kompanets, M.O.; Zosenko, O.O.; Opedia, I.A.; Shendrik, A.N. Hydrogen atom transfer from benzyl alcohol to N-oxyl radicals. Reactivity parameters. J. Org. Chem. 2021, 86, 3797–3799. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, C.; Lan, Z.; Lin, Z.; Zhang, L.; Wang, C. Photocatalytic hydroacylation of trifluoromethyl alkenes. Chem. Commun. 2019, 55, 12691–12694. [Google Scholar] [CrossRef]
- Kuang, Y.; Cao, H.; Junhong, H.; Chew, J.; Chen, W.; Shi, X.; Wu, J. Visible light driven deuteration of formyl C–H and hydridic C(sp3)–H bonds in feedstock chemicals and pharmaceutical molecules. Chem. Sci. 2020, 11, 8912–8918. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H.; Zhou, Z.; Kong, W. Three-component alkene difunctionalization by direct and selective activation of aliphatic C–H bonds. Angew. Chem. Int. Ed. 2021, 60, 7405–7411. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, V.; Ganguly, A.; Karthika, A.; Rasappan, R. C–H alkylation of aldehydes by merging TBADT hydrogen atom transfer with nickel catalysis. Org. Lett. 2021, 23, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; Nowicka, E.; Carter, E.; Murphy, D.M.; Knight, D.W.; Bethell, D.; Hutchings, G.J. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nat. Commun. 2014, 5, 3332. [Google Scholar] [CrossRef]
- Feng, W.; Wu, G.; Li, L.; Guan, N. Solvent-free selective photocatalytic oxidation of benzyl alcohol over modified TiO2. Green Chem. 2011, 13, 3265–3272. [Google Scholar] [CrossRef]
- Shimada, Y.; Hattori, K.; Tada, N.; Miura, T.; Itoh, A. Facile aerobic photooxidation of alcohols using 2-chloroanthraquinone visible light irradiation. Synthesis 2013, 45, 2684–2690. [Google Scholar]
- Nikitas, N.F.; Tzaras, D.I.; Triandafillidi, I.; Kokotos, C.G. Photochemical oxidation of benzylic primary and secondary alcohols utilizing air as the oxidant. Green Chem. 2020, 22, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, Y.; Tanba, K.; Tada, N.; Yamaguchi, E.; Itoh, A. A Study of aerobic photooxidation with a continuous flow microreactor. Synlett 2015, 26, 412–415. [Google Scholar]
- Tanielian, C. Decatungstate photocatalysis. Coord. Chem. Rev. 1998, 178–180, 1165–1181. [Google Scholar] [CrossRef]



 | ||||||
| Entry | Wavelength (nm) | Irradiation Power (W) | Time (min) | 1 (%) | 2 (%) | 3 (%) |
|---|---|---|---|---|---|---|
| 1 a | 352 | 15 (blacklight) | 60 | 78 | 21 | 1 |
| 2 a | 352 | 15 (blacklight) | 360 | 28 | 49 | 22 |
| 3 a | 352 | 15 (blacklight) | 1200 | 0 | 3 | 97 |
| 4 | 365 | 120 (UV-LED) | 60 | 7 | 38 | 54 |
| 5 | 365 | 300 (UV-LED) | 60 | 0 | 31 | 69 |
| 6 | 365 | 480 (UV-LED) | 10 | 15 | 18 | 67 |
| 7 | 385 | 480 (UV-LED) | 10 | 13 | 18 | 69 |
| 8 | 395 | 480 (UV-LED) | 10 | 20 | 24 | 56 |
| 9 | 365 | 480 (UV-LED) | 30 | 0 | 7 | 92 |
| 10 | 365 | 480 (UV-LED) | 60 | 0 | 6 | 93 |
| 11 b | 365 | 480 (UV-LED) | 60 | 11 | 77 | 11 |
| 12 c | 365 | 480 (UV-LED) | 60 | 39 | 36 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyodo, M.; Iwano, H.; Kasakado, T.; Fukuyama, T.; Ryu, I. Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular Oxygen. Micromachines 2021, 12, 1307. https://doi.org/10.3390/mi12111307
Hyodo M, Iwano H, Kasakado T, Fukuyama T, Ryu I. Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular Oxygen. Micromachines. 2021; 12(11):1307. https://doi.org/10.3390/mi12111307
Chicago/Turabian StyleHyodo, Mamoru, Hitomi Iwano, Takayoshi Kasakado, Takahide Fukuyama, and Ilhyong Ryu. 2021. "Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular Oxygen" Micromachines 12, no. 11: 1307. https://doi.org/10.3390/mi12111307
APA StyleHyodo, M., Iwano, H., Kasakado, T., Fukuyama, T., & Ryu, I. (2021). Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular Oxygen. Micromachines, 12(11), 1307. https://doi.org/10.3390/mi12111307






