NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Non-Treated Materials
2.2. Chemical Treatment
2.3. Preparation of Sensing Material
2.4. Characterization of MWNTs
2.4.1. Transmission (TEM) and Scanning Electron Microscopy (SEM)
2.4.2. Raman Spectroscopy
2.4.3. X-ray Diffraction (XRD)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Photoelectron Spectroscopy (XPS)
2.5. Sensing Measurement
3. Results and Discussion
3.1. Characterization of MWNTs
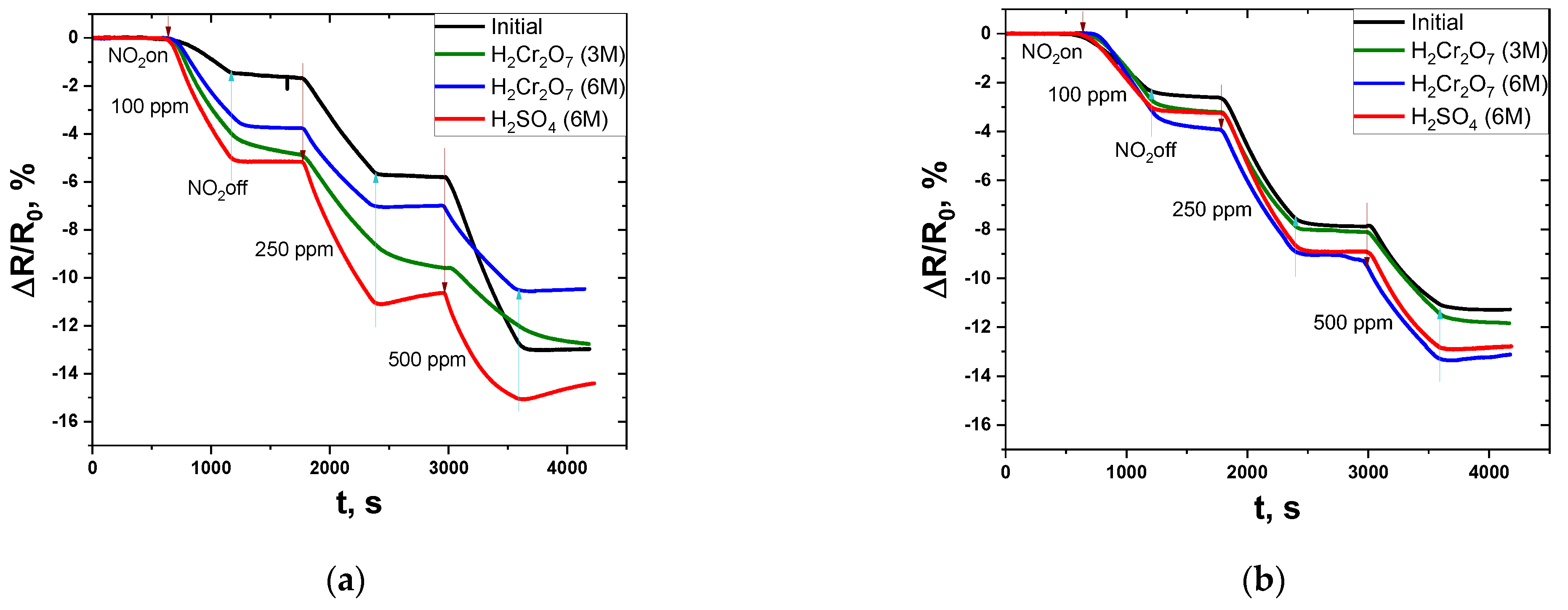
3.2. Gas Sensing Behavior
4. Conclusions
- (1)
- Generation of functional groups that act as sorption centers.
- (2)
- Formation of new defects.
- (3)
- Adsorption of ions on the surface of MWNTs.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- McGinn, C.K.; Lamport, Z.A.; Kymissis, I. Review of Gravimetric Sensing of Volatile Organic Compounds. ACS Sens. 2020, 5, 1514–1534. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.-H.; Kumar, P.; Jeon, B.-H.; Kim, J.-C. Functional hybrid nanostructure materials: Advanced strategies for sensing applications toward volatile organic compounds. Coord. Chem. Rev. 2017, 342, 80–105. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef]
- Lee, J.; Jung, M.; Barthwal, S.; Lee, S.; Lim, S.-H. MEMS gas preconcentrator filled with CNT foam for exhaled VOC gas detection. BioChip J. 2015, 9, 44–49. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Wang, T.; Qi, D.; Yang, H.; Liu, Z.; Wang, M.; Leow, W.R.; Chen, G.; Yu, J.; He, K.; Cheng, H.; et al. Tactile Chemomechanical Transduction Based on an Elastic Microstructured Array to Enhance the Sensitivity of Portable Biosensors. Adv. Mater. 2019, 31, 1803883. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Manakhov, A.; Márik, M.; Nečas, D.; Zajíčková, L. High-Performance Ammonia Gas Sensors Based on Plasma Treated Carbon Nanostructures. IEEE Sens. J. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon nanostructures as a multi-functional platform for sensing applications. Chemosensors 2018, 6, 60. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, H.; Li, X.; Liu, B.; Liu, R.; Komarneni, S. Nanocomposite of halloysite nanotubes/multi-walled carbon nanotubes for methyl parathion electrochemical sensor application. Appl. Clay Sci. 2021, 200, 105907. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, Y.; Liu, R.; Li, B.; Li, F.; Zhang, F.; Shi, M.; Zhou, L.; Li, X. Facile synthesis of Vulcan XC-72 nanoparticles-decorated halloysite nanotubes for the highly sensitive electrochemical determination of niclosamide. Food Chem. 2021, 343, 128484. [Google Scholar] [CrossRef]
- Zhao, H.; Li, B.; Liu, R.; Chang, Y.; Wang, H.; Zhou, L.; Komarneni, S. Ultrasonic-assisted preparation of halloysite nanotubes/zirconia/carbon black nanocomposite for the highly sensitive determination of methyl parathion. Mater. Sci. Eng. C 2021, 123, 111982. [Google Scholar] [CrossRef]
- Mouhib, M.; Antonucci, A.; Reggente, M.; Amirjani, A.; Gillen, A.J.; Boghossian, A.A. Enhancing bioelectricity generation in microbial fuel cells and biophotovoltaics using nanomaterials. Nano Res. 2019, 12, 2184–2199. [Google Scholar] [CrossRef]
- Şenocak, A.; Göl, C.; Basova, T.V.; Demirbaş, E.; Durmuş, M.; Al-Sagur, H.; Kadem, B.; Hassan, A. Preparation of single walled carbon nanotube-pyrene 3D hybrid nanomaterial and its sensor response to ammonia. Sens. Actuators B Chem. 2018, 256, 853–860. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Abdulla, S.; Ponnuvelu, D.V.; Pullithadathil, B. Rapid, Trace-Level Ammonia Gas Sensor Based on Surface-Engineered Ag Nanoclusters@Polyaniline/Multiwalled Carbon Nanotubes and Insights into Their Mechanistic Pathways. ChemistrySelect 2017, 2, 4277–4289. [Google Scholar] [CrossRef]
- Caetano, K.d.S.; da Rosa, D.S.; Pizzolato, T.M.; dos Santos, P.A.M.; Hinrichs, R.; Benvenutti, E.V.; Dias, S.L.P.; Arenas, L.T.; Costa, T.M.H. MWCNT/zirconia porous composite applied as electrochemical sensor for determination of methyl parathion. Microporous Mesoporous Mater. 2020, 309, 110583. [Google Scholar] [CrossRef]
- Üğe, A.; Koyuncu Zeybek, D.; Zeybek, B. An electrochemical sensor for sensitive detection of dopamine based on MWCNTs/CeO2-PEDOT composite. J. Electroanal. Chem. 2018, 813, 134–142. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernández, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Al-Makram, N.M.; Saleh, W.R. Functionalized multi-walled carbon nanotubes network sensor for NO2 gas detection at room temperature. AIP Conf. Proc. 2020, 2290, 050031. [Google Scholar] [CrossRef]
- Ma, D.; Su, Y.; Tian, T.; Yin, H.; Huo, T.; Shao, F.; Yang, Z.; Hu, N.; Zhang, Y. Highly Sensitive Room-Temperature NO2 Gas Sensors Based on Three-Dimensional Multiwalled Carbon Nanotube Networks on SiO2 Nanospheres. ACS Sustain. Chem. Eng. 2020, 8, 13915–13923. [Google Scholar] [CrossRef]
- Kumar, R.; Mamta; Singh, B.P.; Singh, V.N. Exploring the possibility of using MWCNTs sheets as an electrode for flexible room temperature NO2 detection. Micro Nanostruct. 2022, 164, 107165. [Google Scholar] [CrossRef]
- Amirjani, A.; Tsoulos, T.V.; Sajjadi, S.H.; Antonucci, A.; Wu, S.J.; Tagliabue, G.; Haghshenas, D.F.; Boghossian, A.A. Plasmon-induced near-infrared fluorescence enhancement of single-walled carbon nanotubes. Carbon N. Y. 2022, 194, 162–175. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.H.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Fei, H.; Wu, G.; Cheng, W.Y.; Yan, W.; Xu, H.; Zhang, D.; Zhao, Y.; Lv, Y.; Chen, Y.; Zhang, L.; et al. Enhanced NO2 Sensing at Room Temperature with Graphene via Monodisperse Polystyrene Bead Decoration. ACS Omega 2019, 4, 3812–3819. [Google Scholar] [CrossRef] [Green Version]
- Lim, N.; Kim, H.; Pak, Y.; Byun, Y.T. Enhanced NO2 sensing performance of graphene with thermally induced defects. Materials 2021, 14, 2347. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Lee, D.S.; Choi, H.K.; Lee, D.H.; Kim, J.E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S.Y. Flexible room-temperature NO2 gas sensors based on carbon nanotubes/reduced graphene hybrid films. Appl. Phys. Lett. 2010, 96, 213105. [Google Scholar] [CrossRef]
- Barthwal, S.; Singh, B.; Singh, N.B. ZnO-SWCNT Nanocomposite as NO2 gas sensor. Mater. Today Proc. 2018, 5, 15439–15444. [Google Scholar] [CrossRef]
- Teker, T.; Aslanoglu, M. Sensitive and selective determination of paracetamol using a composite of carbon nanotubes and nanoparticles of samarium oxide and zirconium oxide. Microchem. J. 2020, 158, 105234. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Prášek, J.; Buršík, J.; Zajíčková, L. Enhanced ammonia adsorption on directly deposited nanofibrous carbon films. J. Sens. 2018, 2018, 7497619. [Google Scholar] [CrossRef]
- Majzlíková, P.; Sedláček, J.; Prášek, J.; Pekárek, J.; Svatoš, V.; Bannov, A.G.; Jašek, O.; Synek, P.; Eliáš, M.; Zajíčková, L.; et al. Sensing properties of multiwalled carbon nanotubes grown in MW plasma torch: Electronic and electrochemical behavior, Gas sensing, Field emission, IR absorption. Sensors 2015, 15, 2644–2661. [Google Scholar] [CrossRef]
- Espinosa, E.H.; Ionescu, R.; Bittencourt, C.; Felten, a.; Erni, R.; Van Tendeloo, G.; Pireaux, J.-J.; Llobet, E. Metal-decorated multi-wall carbon nanotubes for low temperature gas sensing. Thin Solid Films 2007, 515, 8322–8327. [Google Scholar] [CrossRef]
- Xue, L.; Wang, W.; Guo, Y.; Liu, G.; Wan, P. Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors. Sens. Actuators B Chem. 2017, 244, 47–53. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Bulavskiy, M.O.; Pinakov, D.V.; Chekhova, G.N.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L.G.; Okotrub, A.V. Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials 2020, 13, 3538. [Google Scholar] [CrossRef]
- Lapekin, N.I.; Shestakov, A.A.; Brester, A.E.; Popov, M.V.; Bannov, A.G. Investigation of the Electrical Properties of Carbon Nanofibers–Thermally Expanded Graphite Compacted Composites. Dokl. Chem. 2021, 500, 219–223. [Google Scholar] [CrossRef]
- Mori, H.; Ogura, Y.; Enomoto, K.; Hara, M.; Maurstad, G.; Stokke, B.T.; Kitamura, S. Dense carbon-nanotube coating scaffolds stimulate osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2020, 15, e0225589. [Google Scholar] [CrossRef] [PubMed]
- Kolacyak, D.; Ihde, J.; Merten, C.; Hartwig, A.; Lommatzsch, U. Fast functionalization of multi-walled carbon nanotubes by an atmospheric pressure plasma jet. J. Colloid Interface Sci. 2011, 359, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.; Espinosa, E.H.; Sotter, E.; Llobet, E.; Vilanova, X.; Correig, X.; Felten, A.; Bittencourt, C.; Lier, G.V.; Charlier, J.-C.; et al. Oxygen functionalisation of MWNT and their use as gas sensitive thick-film layers. Sens. Actuators B Chem. 2006, 113, 36–46. [Google Scholar] [CrossRef]
- Ran, M.; Sun, W.; Liu, Y.; Chu, W.; Jiang, C. Functionalization of multi-walled carbon nanotubes using water-assisted chemical vapor deposition. J. Solid State Chem. 2013, 197, 517–522. [Google Scholar] [CrossRef]
- Wang, Z.; Shirley, M.D.; Meikle, S.T.; Whitby, R.L.D.; Mikhalovsky, S.V. The surface acidity of acid oxidised multi-walled carbon nanotubes and the influence of in-situ generated fulvic acids on their stability in aqueous dispersions. Carbon N. Y. 2009, 47, 73–79. [Google Scholar] [CrossRef]
- Lin, T.; Bajpai, V.; Ji, T.; Dai, L. Chemistry of carbon nanotubes. Aust. J. Chem. 2003, 56, 635–651. [Google Scholar] [CrossRef]
- Valdés-Madrigal, M.A.; Montejo-Alvaro, F.; Cernas-Ruiz, A.S.; Rojas-Chávez, H.; Román-Doval, R.; Cruz-Martinez, H.; Medina, D.I. Role of defect engineering and surface functionalization in the design of carbon nanotube-based nitrogen oxide sensors. Int. J. Mol. Sci. 2021, 22, 12968. [Google Scholar] [CrossRef]
- Brester, A.E.; Golovakhin, V.V.; Novgorodtseva, O.N.; Lapekin, N.I.; Shestakov, A.A.; Ukhina, A.V.; Prosanov, I.Y.; Maksimovskii, E.A.; Popov, M.V.; Bannov, A.G. Chemically Treated Carbon Nanofiber Materials for Supercapacitors. Dokl. Chem. 2021, 501, 264–269. [Google Scholar] [CrossRef]
- Mishra, P.; Tai, N.H.; Harsh; Islam, S.S. Transfer of microstructure pattern of CNTs onto flexible substrate using hot press technique for sensing applications. Mater. Res. Bull. 2013, 48, 2804–2808. [Google Scholar] [CrossRef]
- Maire, J.; Mering, J. Chemistry and Physics of Carbon; Taylor & Francis: Boca Raton, FL, USA, 1970. [Google Scholar]
- Nguyen, T.K.; Bannov, A.G.; Popov, M.V.; Yun, J.-W.; Nguyen, A.D.; Kim, Y.S. High-temperature-treated multiwall carbon nanotubes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 6526–6531. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 95–107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]










| Acid | Sample | I(D)/I(G) |
|---|---|---|
| - | MWNT-1020 | 1.04 |
| MWNT-4060 | 0.56 | |
| H2Cr2O7 (3 M) | MWNT-1020 | 0.85 |
| MWNT-4060 | 1.46 | |
| H2Cr2O7 (6 M) | MWNT-1020 | 0.78 |
| MWNT-4060 | 0.66 | |
| H2SO4 (6 M) | MWNT-1020 | 0.82 |
| MWNT-4060 | 0.74 |
| Acid | Sample | Interlayer Spacing d002, nm | Degree of Graphitization, % |
|---|---|---|---|
| - | MWNT-1020 | 0.34 | 46.5 |
| - | MWNT-4060 | 0.3387 | 61.6 |
| H2Cr2O7 (3 M) | MWNT-1020 | 0.33931 | 54.5 |
| MWNT-4060 | 0.34026 | 43.5 | |
| H2Cr2O7 (6 M) | MWNT-1020 | 0.34292 | 12.6 |
| MWNT-4060 | 0.33904 | 58.1 | |
| H2SO4 (6 M) | MWNT-1020 | 0.33967 | 50.3 |
| MWNT-4060 | 0.3386 | 62.8 |
| Acid | Sample | EDX | XPS | ||
|---|---|---|---|---|---|
| C:O (at.) | Other Elements | C:O (at.) | Other Elements | ||
| - | MWNT-1020 | Oxygen was not detected | Ni (0.16) | Oxygen was not detected | - |
| - | MWNT-4060 | Oxygen was not detected | Ni (0.34) | Oxygen was not detected | - |
| H2Cr2O7 (3 M) | MWNT-1020 | 14 | Cr (0.31) | n/a | n/a |
| MWNT-4060 | 33 | Ni (0.11), Cr (0.15) | n/a | n/a | |
| H2Cr2O7 (6 M) | MWNT-1020 | 10 | Cr (1.62) | 8.55 | Cr (0.18) |
| MWNT-4060 | 17 | Cr (0.48) | 8.53 | Cr (0.1) | |
| H2SO4 (6 M) | MWNT-1020 | 82 | S (0.07) | 12.84 | S (0.1) |
| MWNT-4060 | 127 | Ni (0.25), S (0.02) | 8.78 | S (1.15) | |
| Acid | Sample | ΔR/R0 (%) | R25 °C, Ω | ||
|---|---|---|---|---|---|
| 100 ppm | 250 ppm | 500 ppm | |||
| - | MWNT-1020 | 1.7 | 5.8 | 13.0 | 1.39 |
| MWNT-4060 | 2.6 | 7.9 | 11.3 | 2.52 | |
| H2Cr2O7 (3 M) | MWNT-1020 | 4.9 | 9.6 | 12.8 | 1.76 |
| MWNT-4060 | 3.2 | 8.1 | 11.8 | 3.27 | |
| H2Cr2O7 (6 M) | MWNT-1020 | 3.8 | 7.1 | 10.5 | 5.14 |
| MWNT-4060 | 3.9 | 9.3 | 13.3 | 4.57 | |
| H2SO4 (6 M) | MWNT-1020 | 5.2 | 11.1 | 15.1 | 1.62 |
| MWNT-4060 | 3.3 | 8.9 | 12.9 | 1.42 | |
| Acid | Sample | Response Time (s) | ||
|---|---|---|---|---|
| 100 ppm | 250 ppm | 500 ppm | ||
| - | MWNT-1020 | 902 | 535 | 511 |
| MWNT-4060 | 667 | 533 | 341 | |
| H2Cr2O7 (3 M) | MWNT-1020 | 687 | 619 | 487 |
| MWNT-4060 | 665 | 508 | 427 | |
| H2Cr2O7 (6 M) | MWNT-1020 | 590 | 437 | 374 |
| MWNT-4060 | 583 | 523 | 368 | |
| H2SO4 (6 M) | MWNT-1020 | 551 | 457 | 279 |
| MWNT-4060 | 590 | 514 | 347 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapekin, N.I.; Golovakhin, V.V.; Kim, E.Y.; Bannov, A.G. NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes. Micromachines 2022, 13, 1495. https://doi.org/10.3390/mi13091495
Lapekin NI, Golovakhin VV, Kim EY, Bannov AG. NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes. Micromachines. 2022; 13(9):1495. https://doi.org/10.3390/mi13091495
Chicago/Turabian StyleLapekin, Nikita I., Valeriy V. Golovakhin, Ekaterina Yu. Kim, and Alexander G. Bannov. 2022. "NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes" Micromachines 13, no. 9: 1495. https://doi.org/10.3390/mi13091495
APA StyleLapekin, N. I., Golovakhin, V. V., Kim, E. Y., & Bannov, A. G. (2022). NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes. Micromachines, 13(9), 1495. https://doi.org/10.3390/mi13091495






