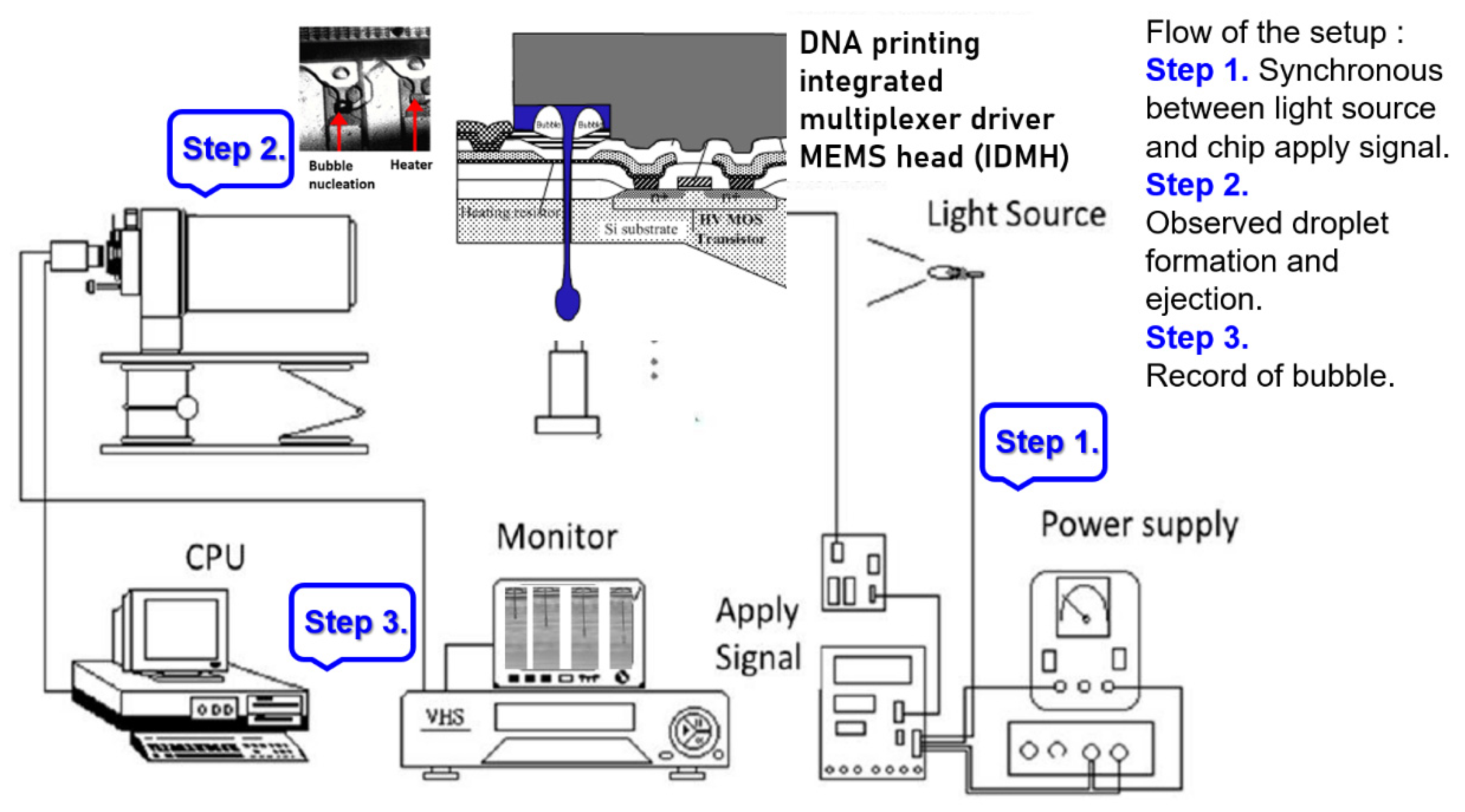
We employed Flow-3D to simulate the internal flow field and flow rate distribution in a 3D spray room. The simulation procedure was used to calculate the time and pressure to generate thermal bubbles. Also, the corresponding mean outlet speed state of the fluid was calculated. The “outlet speed state” function in Flow-3D served as the power source for spraying liquid into a monomer. The actual chip generation process and the starting voltage curve were measured and analyzed. Finally, this study presents the flow rate experiment as well as the results and discussion.
3.1. Flow-3D Simulation
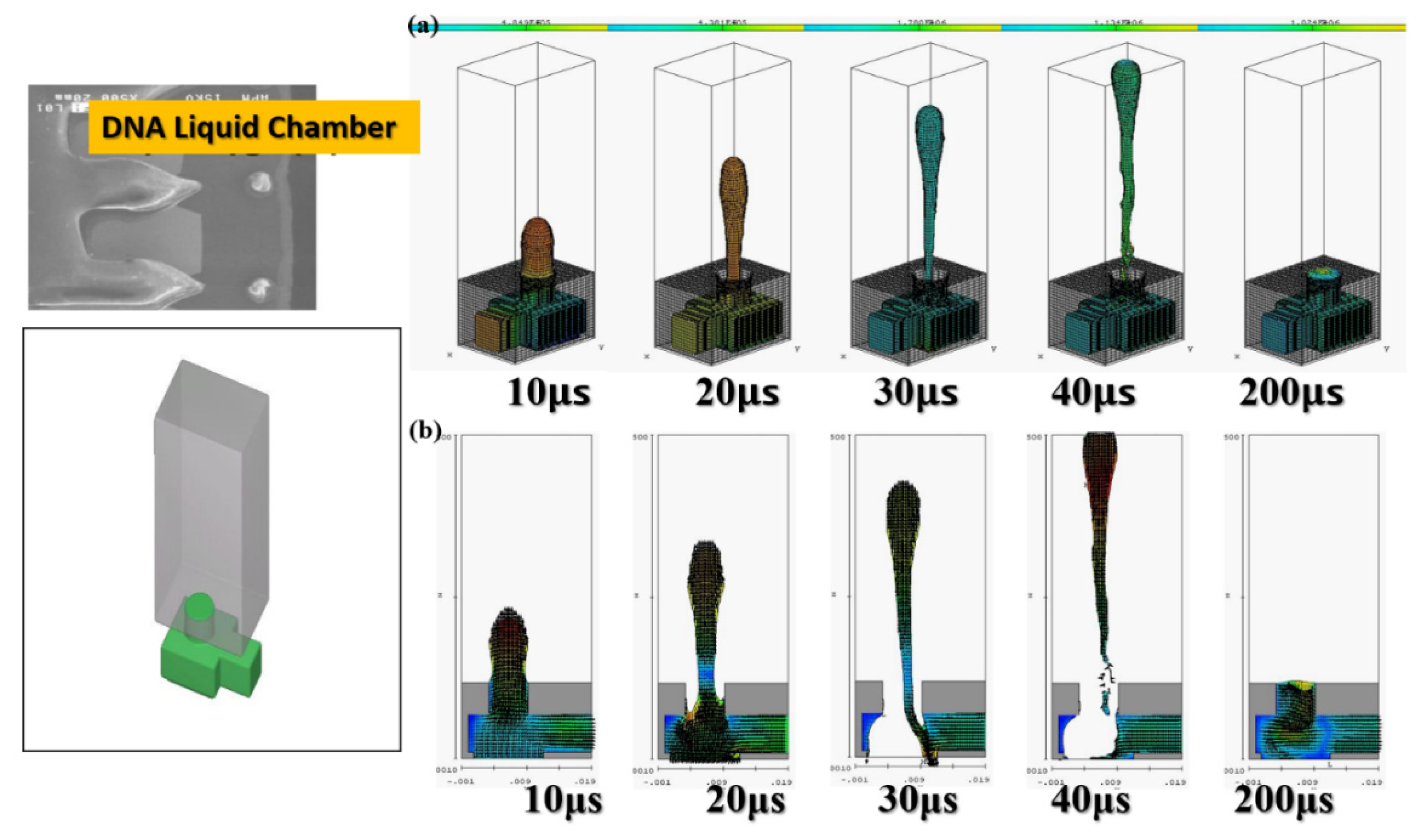
The software simulation was based on the actual DNA liquid cavity calculation to simulate the ejection cavity with single-channel supplying liquid. The geometric shape of the ejection cavity is illustrated in
Figure 3.
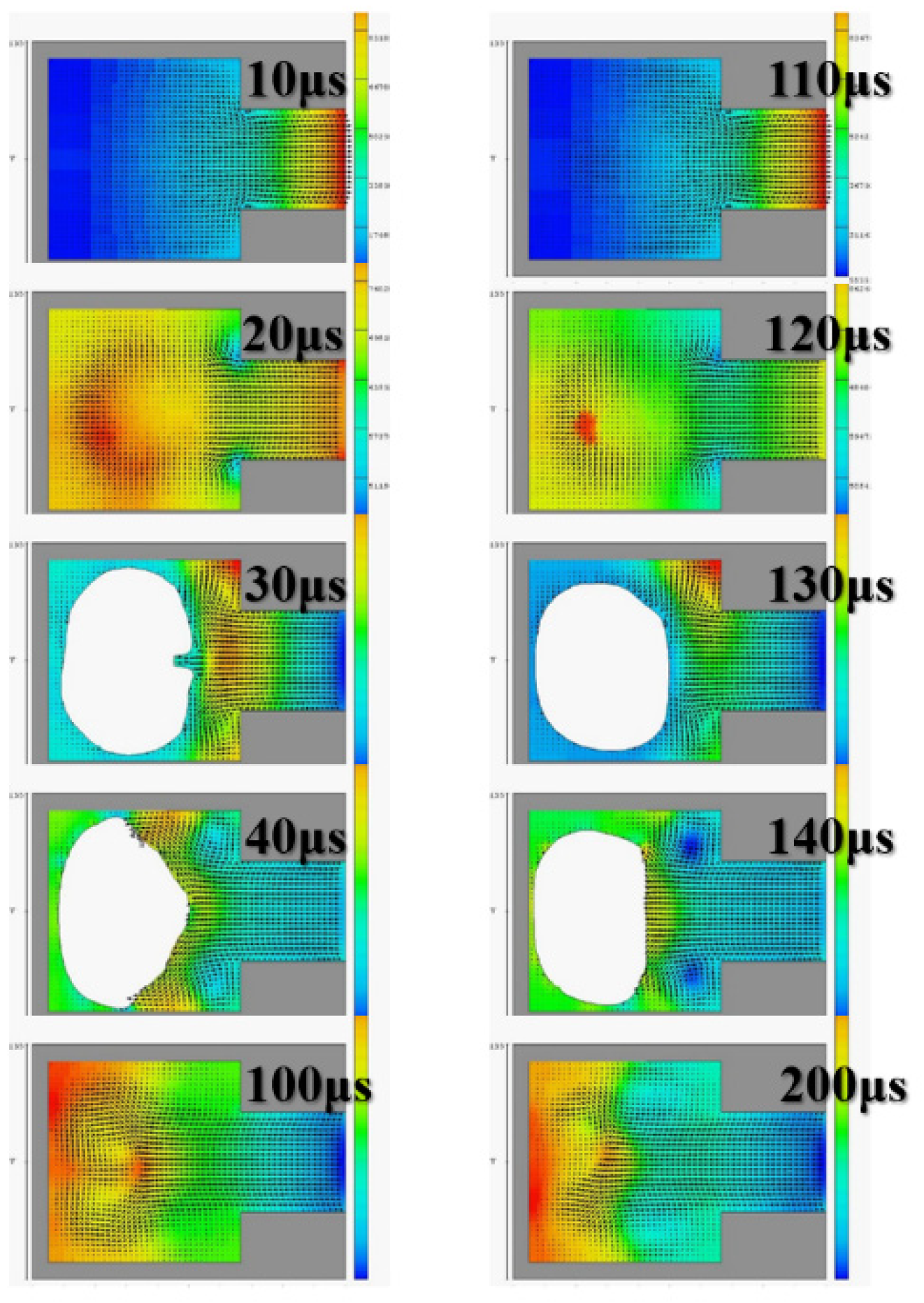
The diameter of the ejection outlet was 60 μm. The thickness of the ejection orifice (volcano) was 50 μm. The process thickness of the dry film layer was 60 μm. The bottom design of the ejection cavity was 120 μm × 120 μm. The area of the heater was 105 µm × 105 µm. Under the operating frequency of 5 KHz, the 3D side view and the X-Z two-dimensional (2D) section view were analyzed. Simulation spraying was conducted for 10, 20, 30, 40, and 200 µs. This simulation calculated the area of the top of the thermal resistance. Initially, the mean droplet tip speed was estimated at 10 m/s, and the length of the tail of a droplet was approximately 300 μm. A 200-μm X-Z section view revealed the simulation results. The liquid clearly reached the fluid outlet. The liquid was in a stable state, indicating that the liquid had filled up the cavity as expected. The cavity had a uniquely designed shape. The simulation results revealed the behavior of the geometric shape of the 3D ejection cavity. The simulation results under a 5-kHz operating frequency revealed no shortage of DNA liquid. Therefore, the tail of the DNA droplet had the lowest quality and was easily affected by the asymmetry of the flow field force, resulting in an off-track flying trajectory.
Figure 3 reveals an obvious deflection in the droplet tail of the supplied liquid at 40 μs. As the flight time increased, the horizontal displacement deflection became more substantial. In inkjet printers or other industrial-use inkjet printing processed, the asymmetrical geometric shape of the relative ejection direction (Z-axis direction) must be redesigned to restore the ejection cavity. The direction-related problem did not affect the realization of the planned goals.
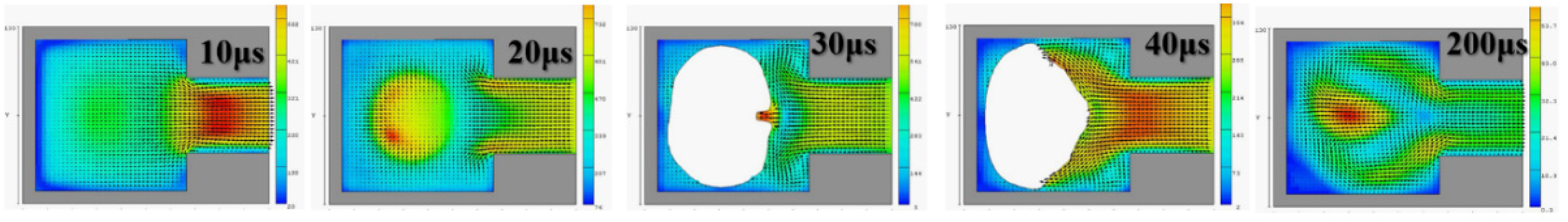
Figure 4 reveals that, with an orifice diameter of 60 μm and a thickness of 50 μm, the 2D X-Y cross section was higher. The 2D cross-sections before and after the fluid ejection cavity was ejected under instant flow field could be clearly observed. In addition to the orifice, liquid droplets were ejected. The fluid ejection cavity pushes the liquid from the single channel. The results revealed that at 20 μs, the thermal air bubbles enter a state of dissemination. During that stage, fluid starts to refill, and the speed vector passes inside the cavity and points at the outlet channel. During the 30-μs and 40-μs stage, because liquid has been ejected from the orifice, a certain amount of liquid enters the nozzle, and the cavity is not completely filled with liquid. Under this circumstance, external air passes the orifice and enters the cavity while waiting for the fluid to pass the refilled object, and then wait to flow out through the outlet. If air is not completely ejected, it may block the bubbles. Bubbles may also occupy the injection cavity; hence, during the next injection, an adequate volume of liquid cannot be ejected. In addition, because fluid attenuates the tremendous amount of heat generated from the heater, it has a cooling effect. Therefore, the injection cavity inside the chip may accumulate heat. This harmful cycle reduces the ejection performance, finally resulting in the inability to eject liquid droplets; the excessive internal heat also damages the chip. Microfluid channels are not ideal for the injection cavity design. This may be because the inside retains bubbles, resulting in reduced performance. If the heater does not immediately cease operation, the excessive temperature can damage the chip. A major part of the overall design parameters is focused on addressing this concern.
Figure 5 depicts the calculation results of the 2D X-Z cross section. At 100 μs and 200 μs, the fluid injection orifice did not completely fill the chamber. This may be because the size of the single-channel injection cavity was unsuitable for the highest operating frequency of 10 KHz. Thus, subsequent calculation simulations employed 5 KHz as the reference operating frequency. The calculation simulation results were calculated according to the operating frequency of the impact.
Figure 6 illustrates the injection cavity height as 60 μm and 30 μm and reveals the 2D X-Y cross section. At 100 μs and 200 μs, the fluid injection orifice did not completely fill the chamber. In those stages, the fluid was still filling the chamber, and the flow field was not yet stable.
3.2. Starting Voltage Curve
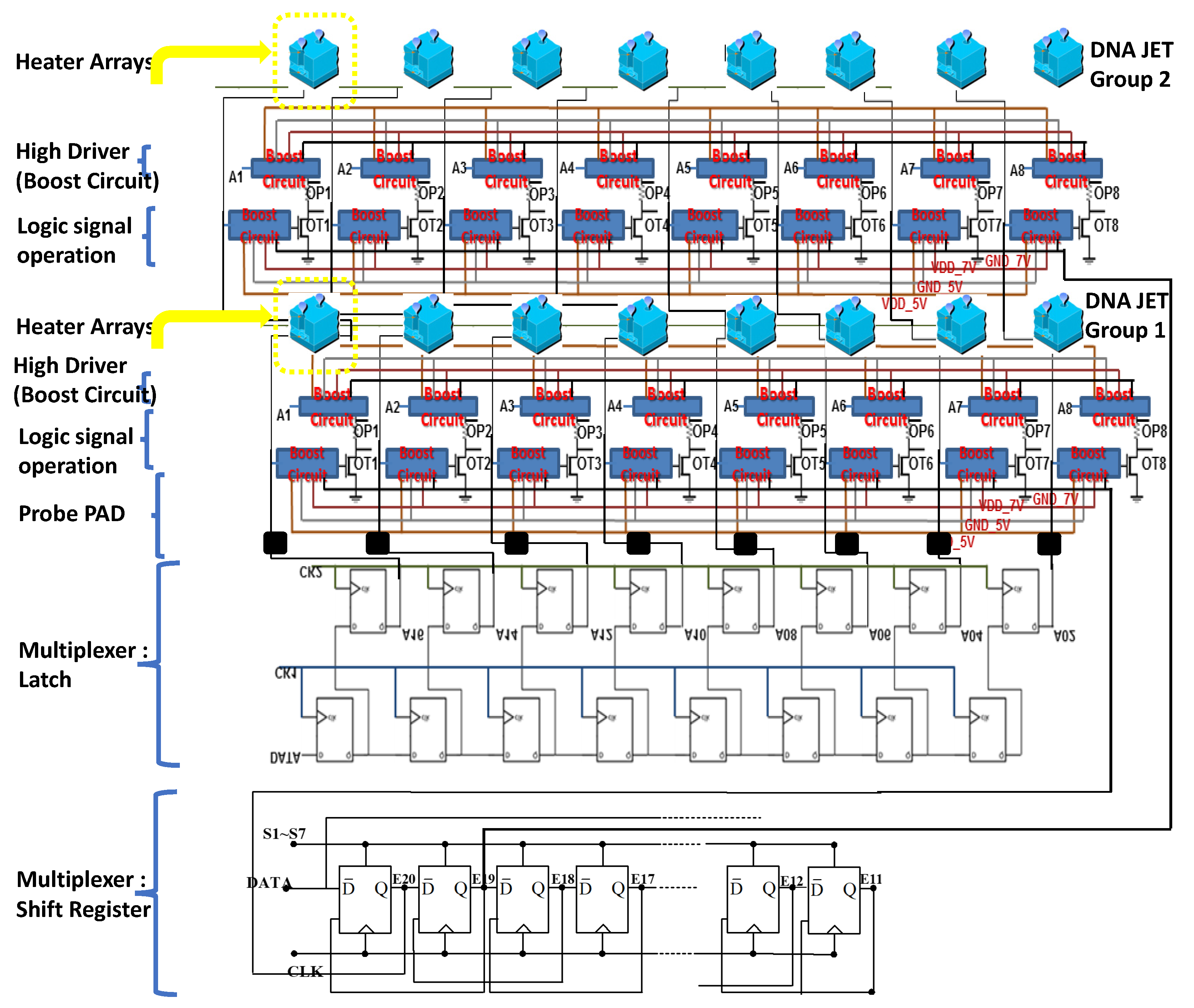
The DNA printing integrated multiplexer driver MEMS head (IDMH) follows the 0.35-um 2P2M processing model standard complementary metal–oxide–semiconductor (CMOS) design rules to establish the model (
Figure 7). This study involved high-pressure processing. For systems based on the 0.35-um DPDM 12 V/12 V or 12 V/5 V mixed model processing model design and allocating high voltage circuit, this study design may serve as a basic allocation guide. This study investigated the differences from the original 0.35-um CMOS mixed-mode (DPDM, 5 V) design principles. That is, during this process, we specified NDD, PDD, HV_OX rules, and high voltage equipment rules. Basically, the low voltage (LV) part adopts the 5-V logic, and its allocation rules are the same as those for the Taiwan Semiconductor Manufacturing Company common 0.6-um DPDM (including the PO1/PO2 capacitor options) ASIC design rules.
The measurement of the starting voltage curve was conducted on the ASIC on the actual manufactured MEMS chip.
Figure 8 and
Figure 9 reveal the starting voltage and energy of the Type A chip, respectively. That chip was designed using dual heaters in series. The figures reveal that the starting voltage of the chip decreased as the pulse width increased. From the energy perspective, an increase in pulse width increased the required energy.
Figure 8 depicts the starting voltage graphs of Type B and Type E chips. The nozzle density of these two chips was 50, and their resistance was 35 Ω. As the pulse width increased, the starting voltage of Type B and Type E chip decreased. From the energy perspective, an increase in pulse width increased the required energy (
Figure 9). The Type E design required more energy than did Type B. Although the two designs have the same circuit resistance and the same heater resistance (with an L/W value of 1), the area of Type E is greater than that of Type B. Therefore, regarding the power density of the work performed by the heater, when the same driving pulse voltage was input into these two heater designs, the power density of the work performed by the Type E heater was higher than that of Type B. Type B was designed very high; thus, the starting voltage curve of Type E is higher than that of Type B.
Figure 8 illustrates the starting voltage of chip Type C and Type D. Both chips have a long heater design, and they have the same heater area. The L/W value of these two models was 0.75 and 1.33, respectively. The figure indicates that as the pulse width increased, the starting voltage of Type C and Type D declined. The starting voltage of Type D was higher than that of Type C. Concerning energy, an increase in pulse width increased the required energy (
Figure 9). The energy required for the Type C design is higher than that of Type D.
3.3. Flow Experiment and Results Discussion
This study endeavored to estimate the amount of DNA being ejected and the amount of the DNA sample for testing. Therefore, we conducted tests on power conditions and DNA flow.
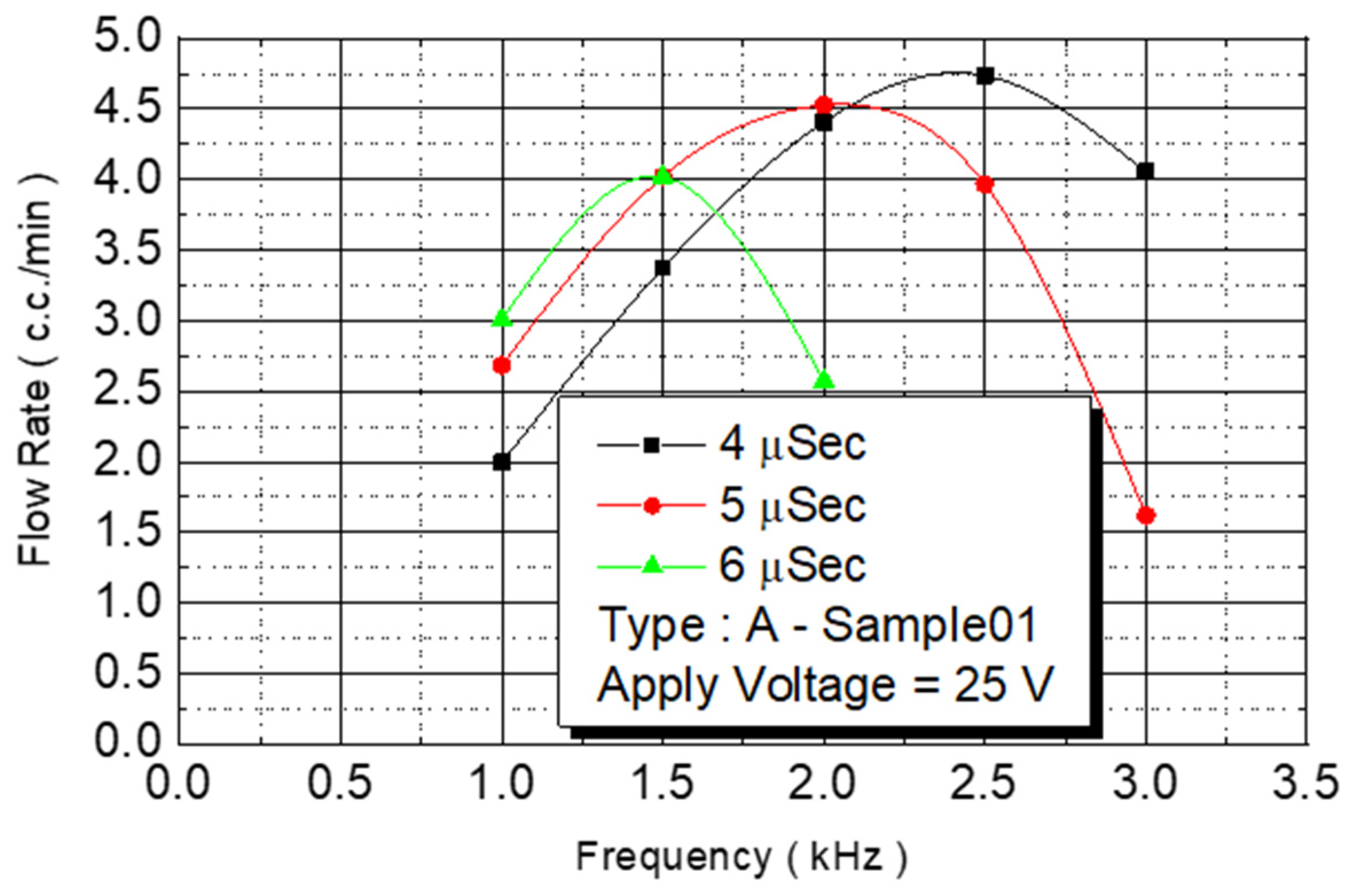
The spray DNA chip composed of 50 nozzles could be placed rapidly, and the heater area was increased. The maximum flow rate was 3.5 cc/min, and the DNA chip ejection speed was increased. In this study, we increased the density of the unit area nozzles without increasing the size of the chip, and the microchannel design method was used to expand the original two-line nozzles into four-line nozzles. The number of nozzles used on the spray DNA chip was substantially increased from 50 to 100. The increase in nozzle number was for testing maximum flow. The Type A chip had an increased density and 100 nozzles. On a chip with increased nozzle density, we first used chip No. B2-01 to conduct a flow test. We fixed the driving voltage at 25 V for the experiment, and we observed the flow curve under various pulse widths and operating frequencies (
Figure 10).
An experiment was conducted to analyze different pulse widths. When the pulse width was 4 μs, the flow rate of the injected monomer linearly increased at a frequency of 1–2 KHz. The linear slope was 2.22. When the frequency was 2.5 KHz, the flow rate no longer increased linearly. When the operating frequency was increased to 3 KHz, the flow rate began to decrease. When the pulse width was 5 μs, the linearly increased frequency was only 1.5 KHz, and the linear slope was 2.68. When the pulse width was increased to 6 μs, the linearly increased frequency was smaller than 1.5 KHz, and the linear slope was 3.
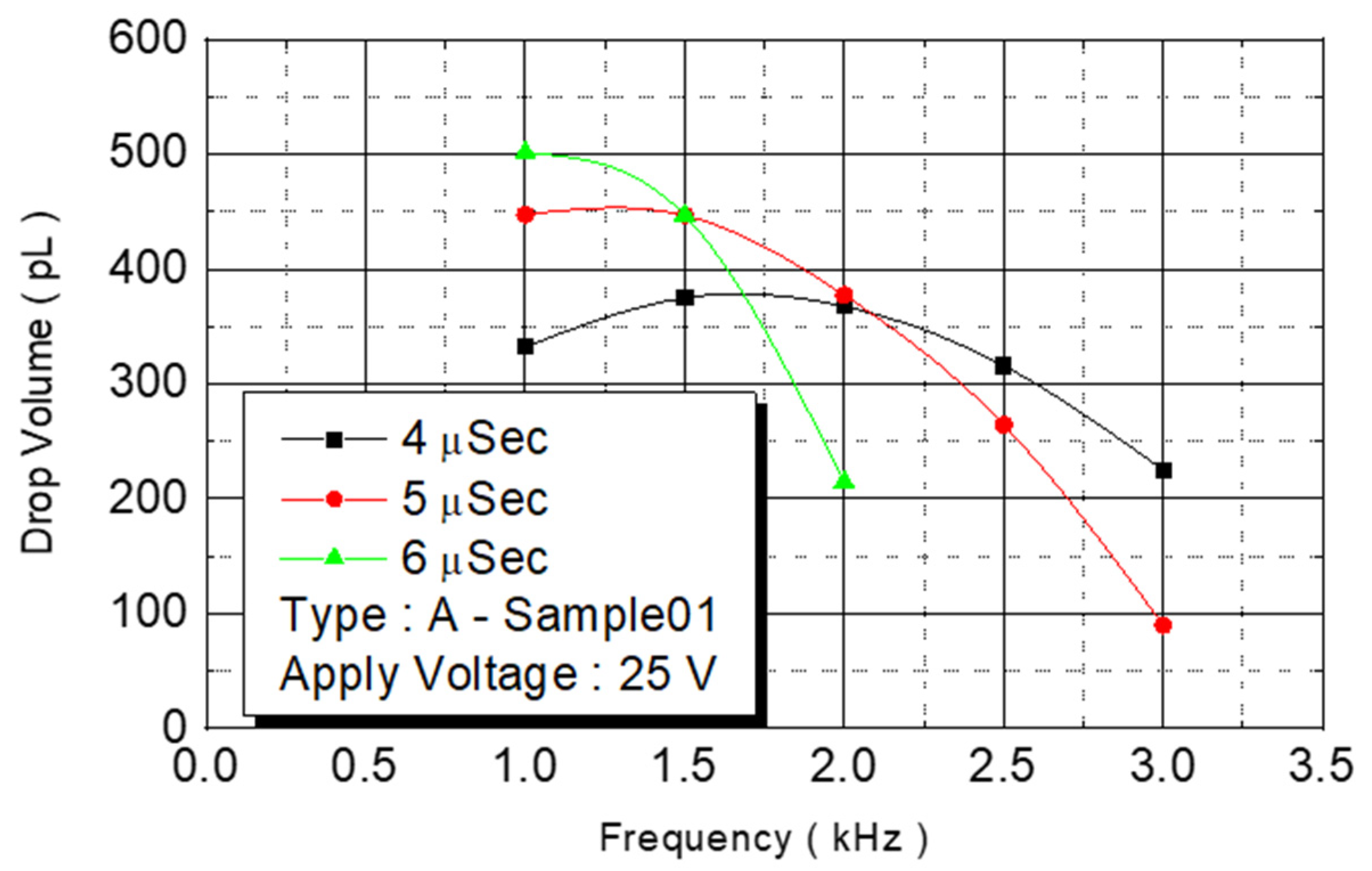
We observed the experimental results under an operating frequency of 15 KHz (
Figure 11). The volume of the spray dots increased from 330 pl under a pulse width of 4 μs to 500 pl. At 6 μs, the volume increased with the pulse width. The ejection rate of the ejection point’s volume, in contrast to the chamber capacity, increased from 40% to 58%. The experimental results indicated that an increase in energy increased the pressure of bubbles and more liquid was expelled.
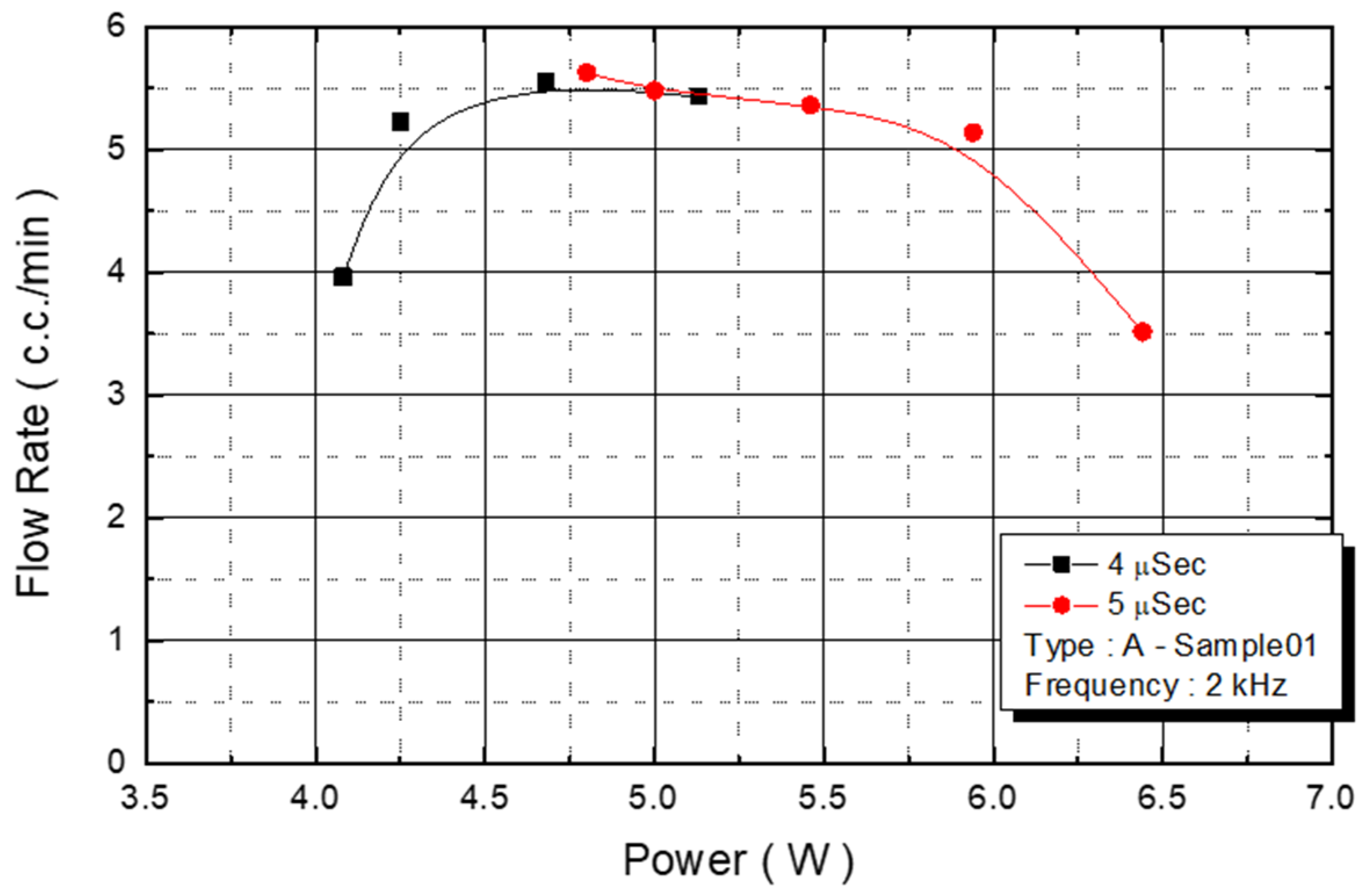
We observed the flow rate changes under various power conditions, with an operating frequency of 2 KHz and a pulse width of 4 and 5 μs. With power ranging from 4.3 W to 5.7 W, the flow rate of the injected monomer was 5.5 cc/min (
Figure 12). The experimental parameter was the maximum yield of the DNA liquid.
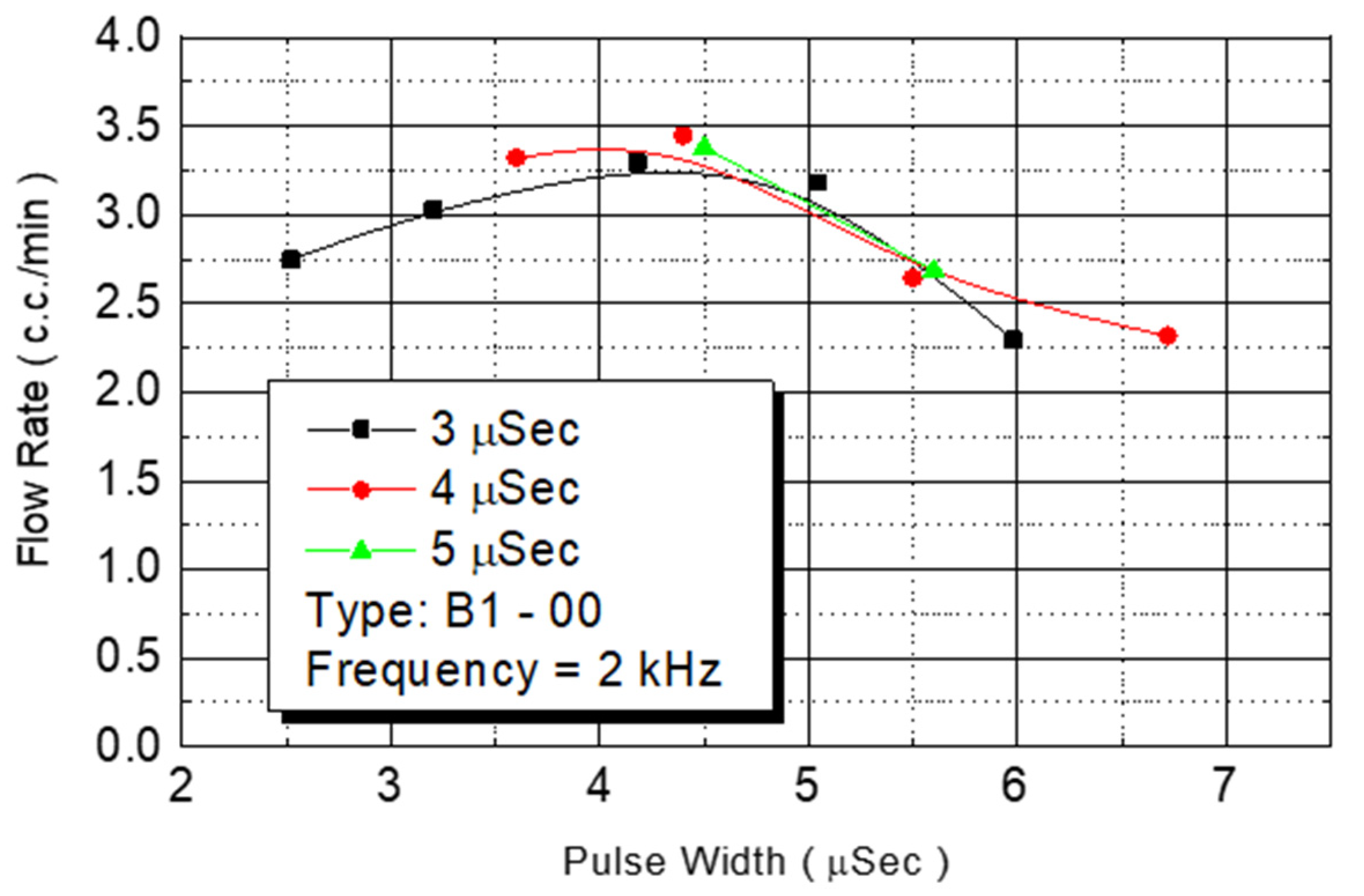
This study tested the total flow rate of the ejected DNA chip. First, we injected a monomer in chips in the B1-oo series. Under an operating frequency of 2 KHz, various operating voltages were employed to test the flow rate. The results revealed that the amount of injected monomer was closely related to the provided power from the outside environment (
Figure 13). Although the injected monomer had a different pulse width energy, under the same power condition, the energy obtained from injecting the monomer was the same. The bubbles generated on the heater had the same instant pressure. Therefore, the introduced flow rate was similar. The figure revealed that the flow rate increased with the added power. At a power condition of 4.5 W, the system had a maximum flow rate of 3.5 cc/min. When the power exceeded that level, the flow rate decreased.
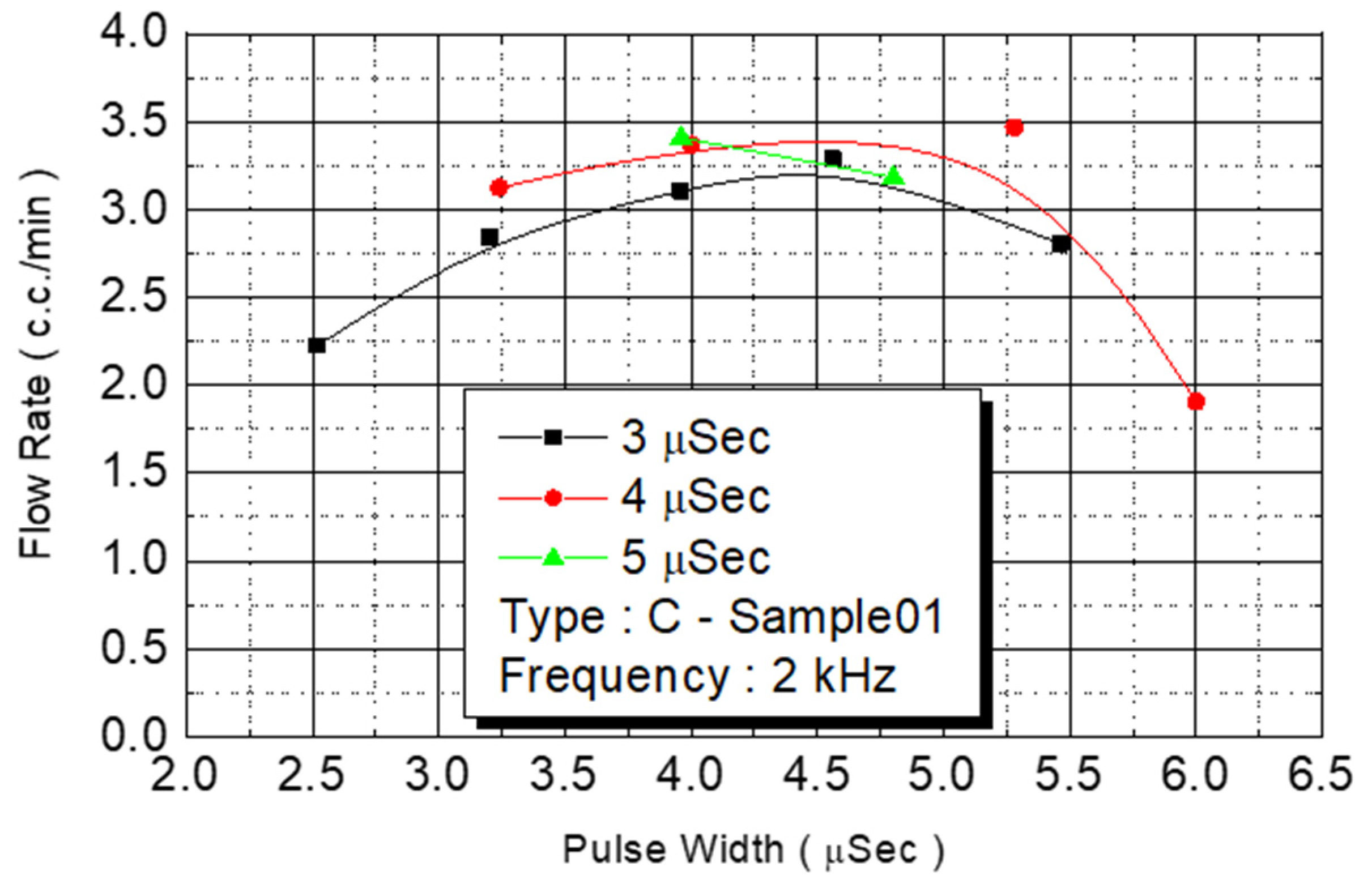
We employed the same experimental procedures for C Type-01 and D Type-02 flow rate test chips. The results are presented in
Figure 14 and
Figure 15, respectively. Under the condition of a fixed operating frequency of 2 KHz, the amount of monomer injected changed according to the external power (
Figure 14). The maximum flow rate (3.5 cc/min) of the injected monomer appeared when the power was 4.5 W.
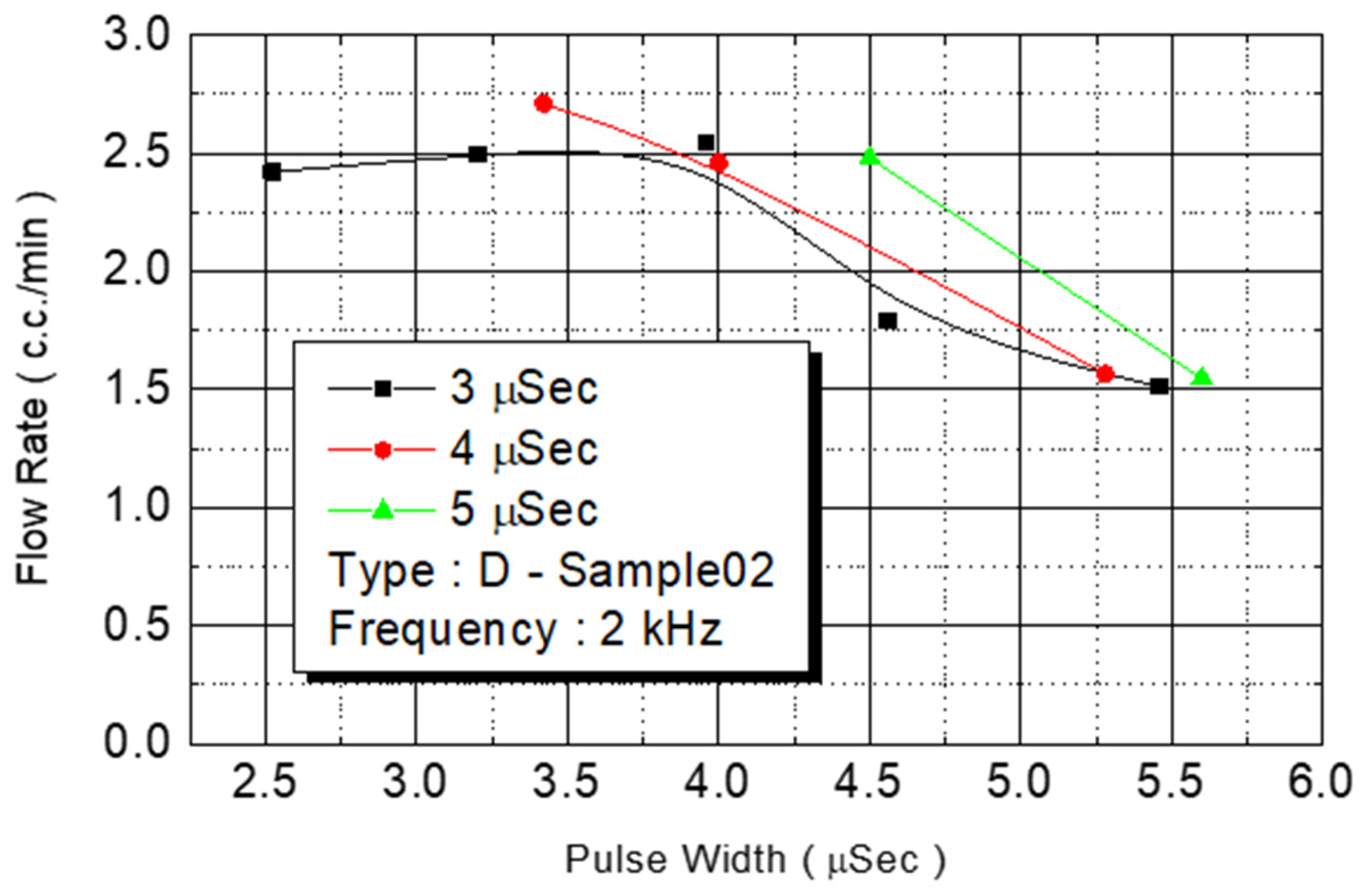
Figure 15 revealed the same result: The maximum flow rate (2.5 cc/min) of the injected monomer in D Type-02 was achieved under the power of 4.2 W. At 4-W added power, another D Type-02 chip achieved its maximum flow (3 cc/min).
In the aforementioned experiments, the chip types were heaters (size: 105 μm × 105 μm) with a nozzle density of 50. The nozzle outlet size was 80 μm for all chip designs. On the basis of the aforementioned results, the maximum flow rate for all B1 series chips was limited to 3.5 cc/min. The required power for generating the maximum flow rate was 3.5–4.5 W.
In an effort to increase the amount of monomer being injected, we first considered expanding the size of the heater. The unit heater size of chip No. E1-00 was increased from 105 μm × 105 μm to 132 μm × 132 μm. The size of the microfluid flow channel was increased from 120 μm × 120 μm to 150 μm × 150 μm. The injection cavity volume increased by 56%. The number of monomer injection nozzles was maintained at 50 for the experiment.
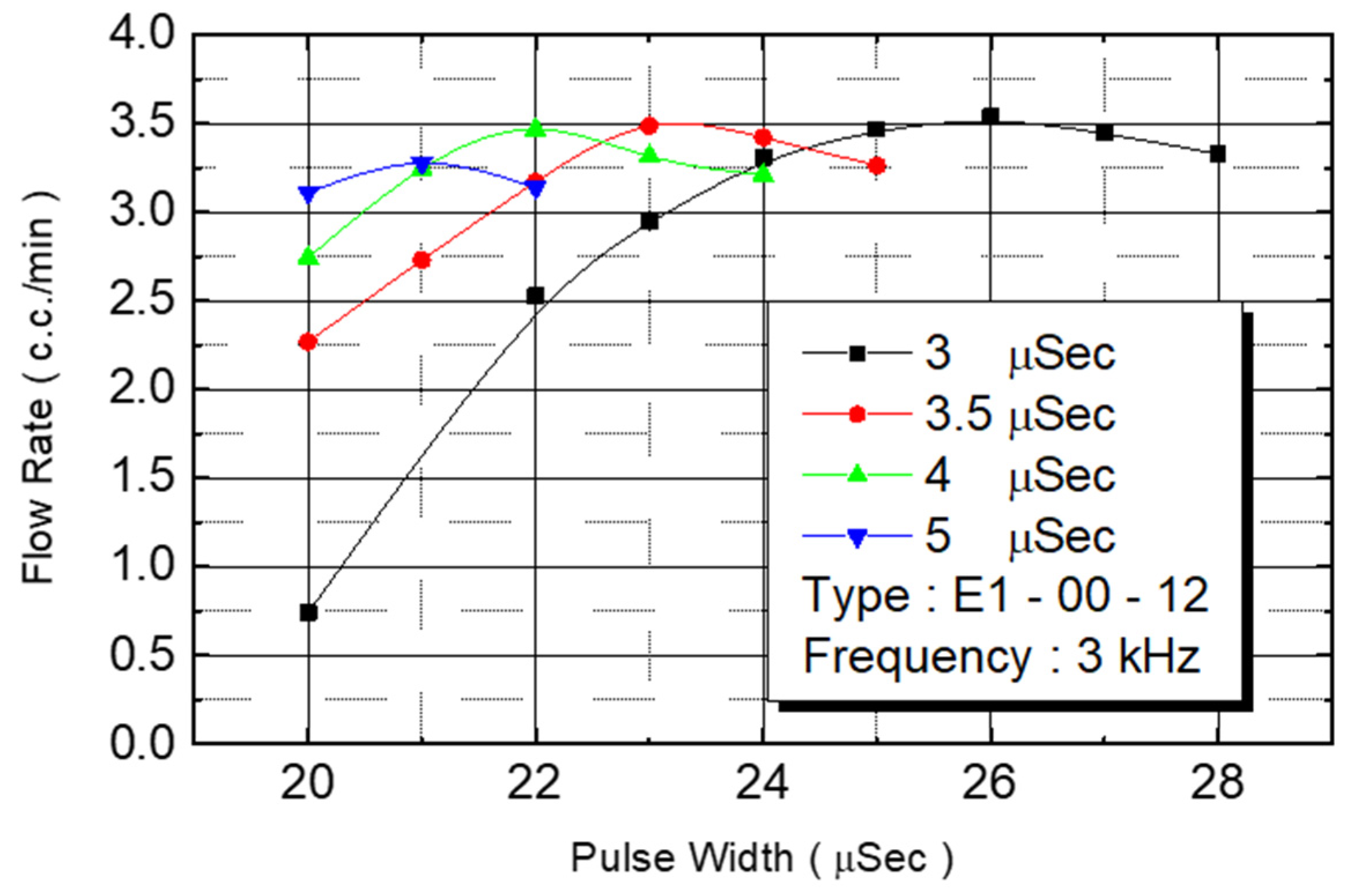
Figure 16 reveals the flow rate test results. Under an operating frequency of 3 KHz and pulse width of 3 μs, the flow rate of the nozzle under 20–25 V of driving voltage range could be observed. The flow rate increased with the voltage. In excess of 25 V, any further voltage increase reduced the flow rate. When the pulse width was changed to 3.5 μs, the flow rate’s increase range was reduced to 20–23 V. When the voltage exceeded 23 V, the flow rate began to decrease. The pulse width was contained to increase to 4 and 5 μs, and the increased voltage range was further reduced.
We observed flow performance under various pulse widths under 20 V of driving voltage. As the pulse width increased from 3 μs to 3.5 μs, 4 μs, and to 5 μs, the corresponding flow rate increased from 0.75 cc/min to 23 cc/min, 2.7 cc/min, and to 3.1 cc/min.
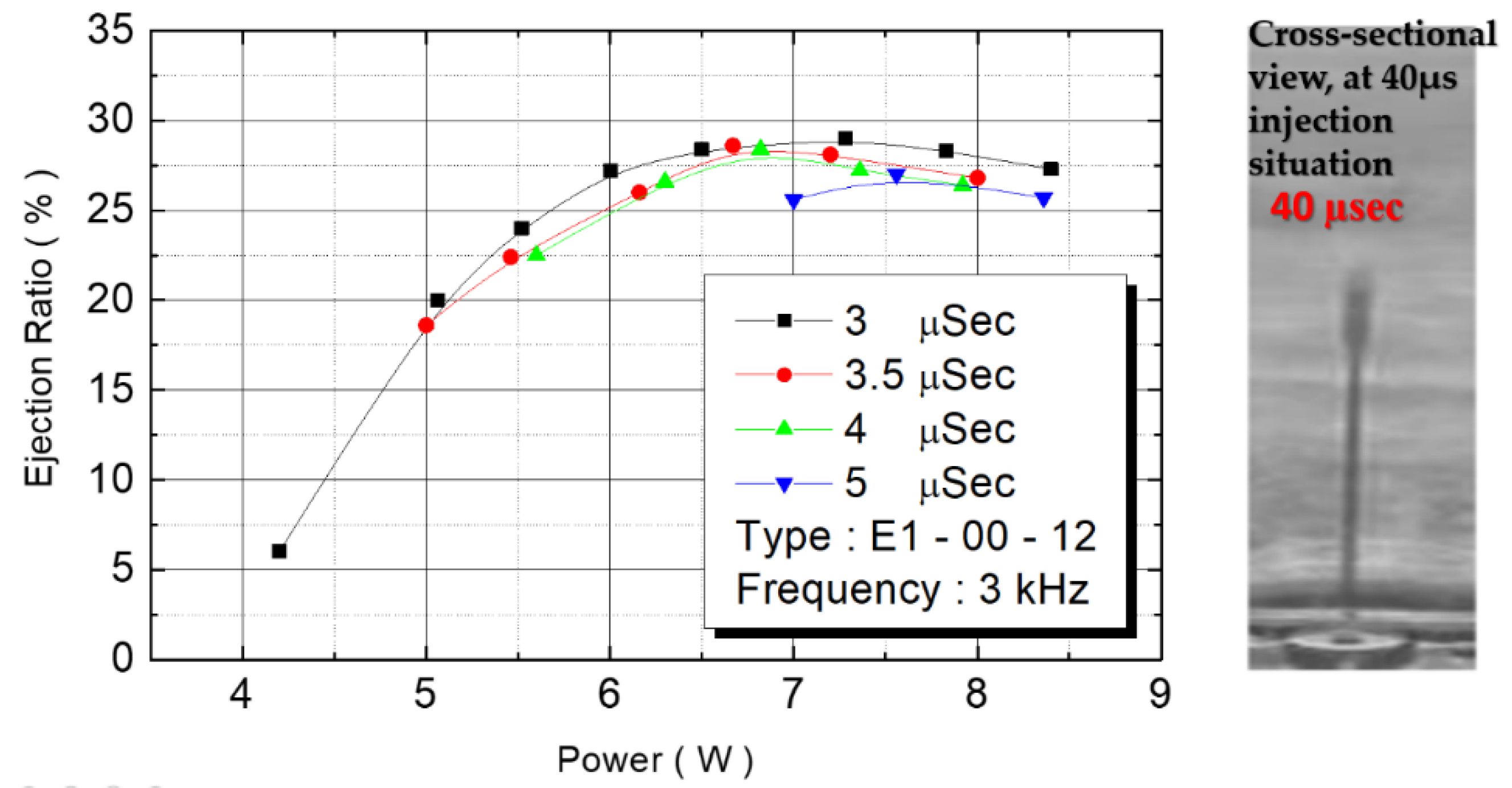
Figure 17 depicts the correlation between power and flow rate. Flow rate was still affected by power. The figure reveals that as the power increased, the flow rate increased from 0.75 cc/min to 3.5 cc/min until the power reached 6.5 W. When power increased further, the flow rate did not increase with the energy, revealing that the maximum flow rate of this design is 3.5 cc/min.
The summary of the experimental results is obtained from increasing the number of nozzles, increasing the input signal frequency, pulse width, the reduced heater size, and decreasing the input voltage (power) data to obtain the optimal DNA spray. DNA is not easy to obtain, it is estimated the amount of DNA sprayed outcomes from the data (increased number of nozzles, reduced heater size) while quickly deploying DNA according to the input parameters.
Spraying was conducted using a special 3D MEMS structure. DNA spraying technology can be employed to test the relationship between the flow rate of DNA on the glass slide and time. Because DNA cannot be obtained easily, rapidly deploying DNA while calculating the amount of DNA being sprayed is essential. Microarray biochip sample spots can probe clusters composed of DNA, RNA, peptide proteins, antibodies, cells, or human tissues. The sampling material was determined by the biological characteristics to be measured. For example, the DNA chips for testing the single nucleotide A, T, C, or G (single nucleotide polymorphism) are sample points of different single-stranded DNA sequence fragments being grouped; in this case, DNA sequences were used. The paring principle of AT and CG. When the designed single-stranded DNA sample spot can be paired with the sample and emit fluorescence, it indicates that the DNA sequence was changed by a single nucleotide, and the change in the DNA sequence can be used to explore the chromosome genome diversity of species (including humans). Another example is the protein biochip for detecting food-specific allergens. The conventional method is to place dozens to hundreds of different food allergen sampling point on a standard-size 1″ × 3″ glass. A probe design of 200–500 microns and its fluorescence strength can be used to screen and test dozens to hundreds of different food allergens.
Conventional experimental procedures require a long time to extract and purify DNA (DNA separation), removing polymerase chain reaction inhibitors to obtain DNA. Although DNA extraction is feasible, during the extraction process, precious samples may be lost. Human operation may lead to experimental errors and sample cross contamination. DNA nucleic acid is highly valuable. Through this experiment, we can precisely estimate the total amount of DNA required for thousands of spots on the microarray biochip sprayed with DNA.