Single-Cell Mechanophenotyping in Microfluidics to Evaluate Behavior of U87 Glioma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
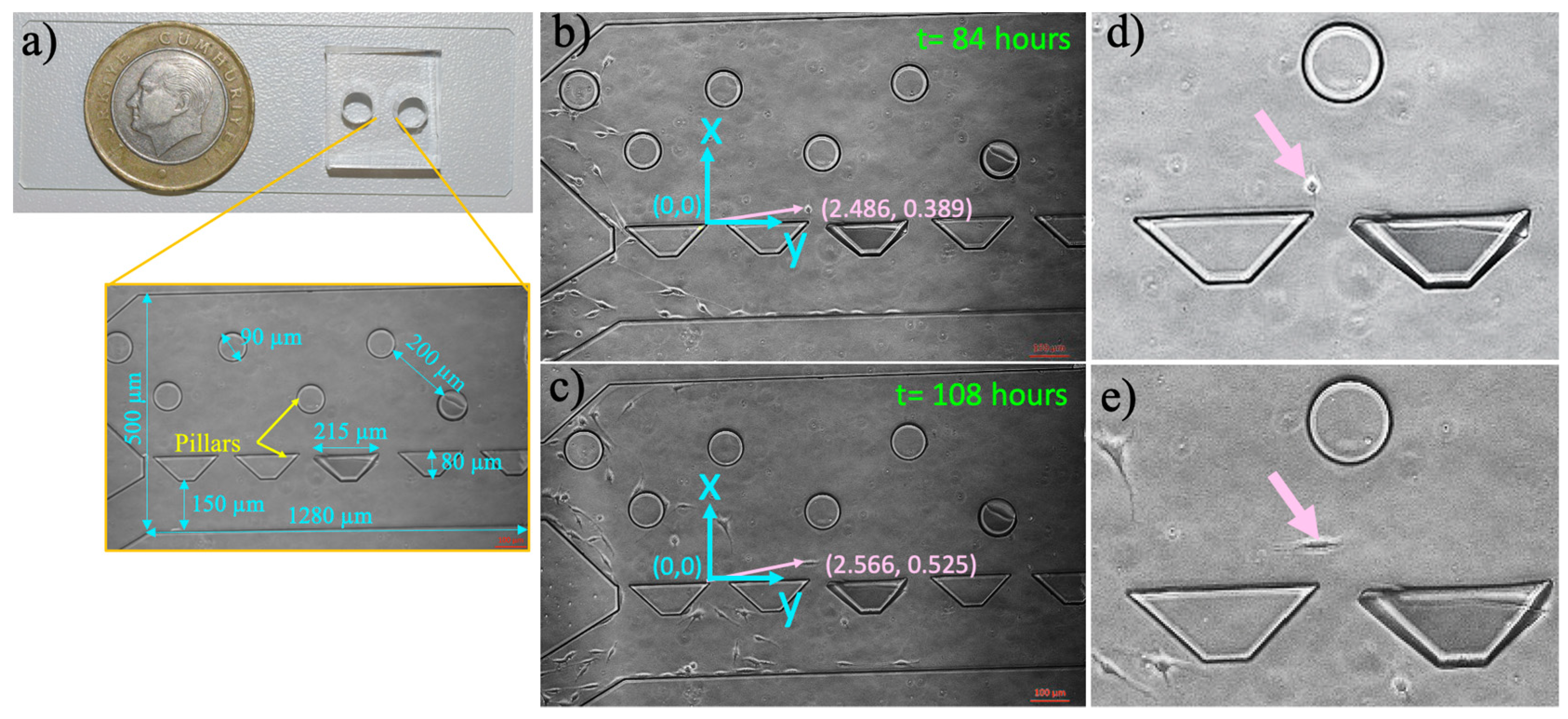
2.2. Microfluidic Chip Fabrication
2.3. Microfluidic Chip Preparation and Culturing Cells in the Microfluidic Device
2.4. Cell Growth in a 12-Well Cell Culture Plate
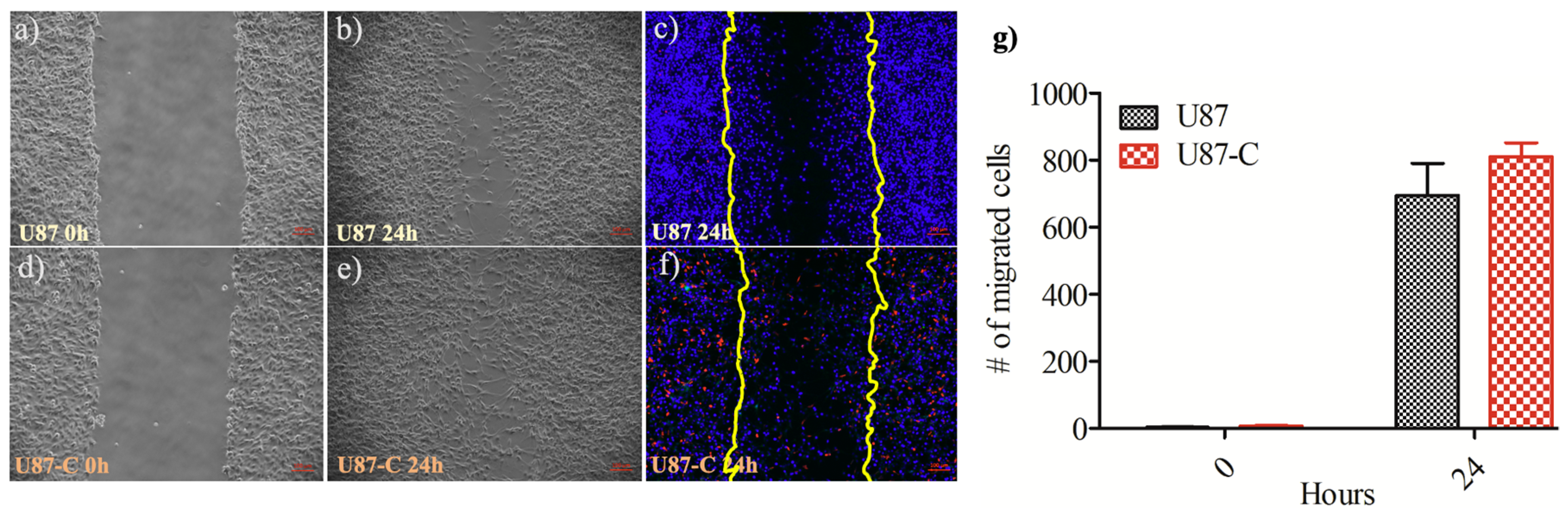
2.5. Cell Migration by Wound Healing in a 12-Well Cell Culture Plate
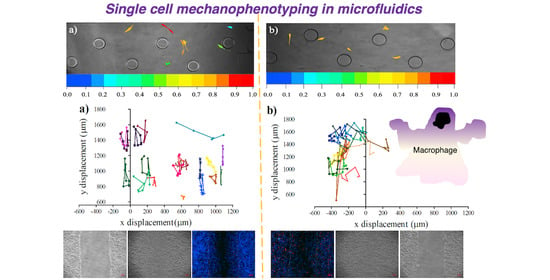
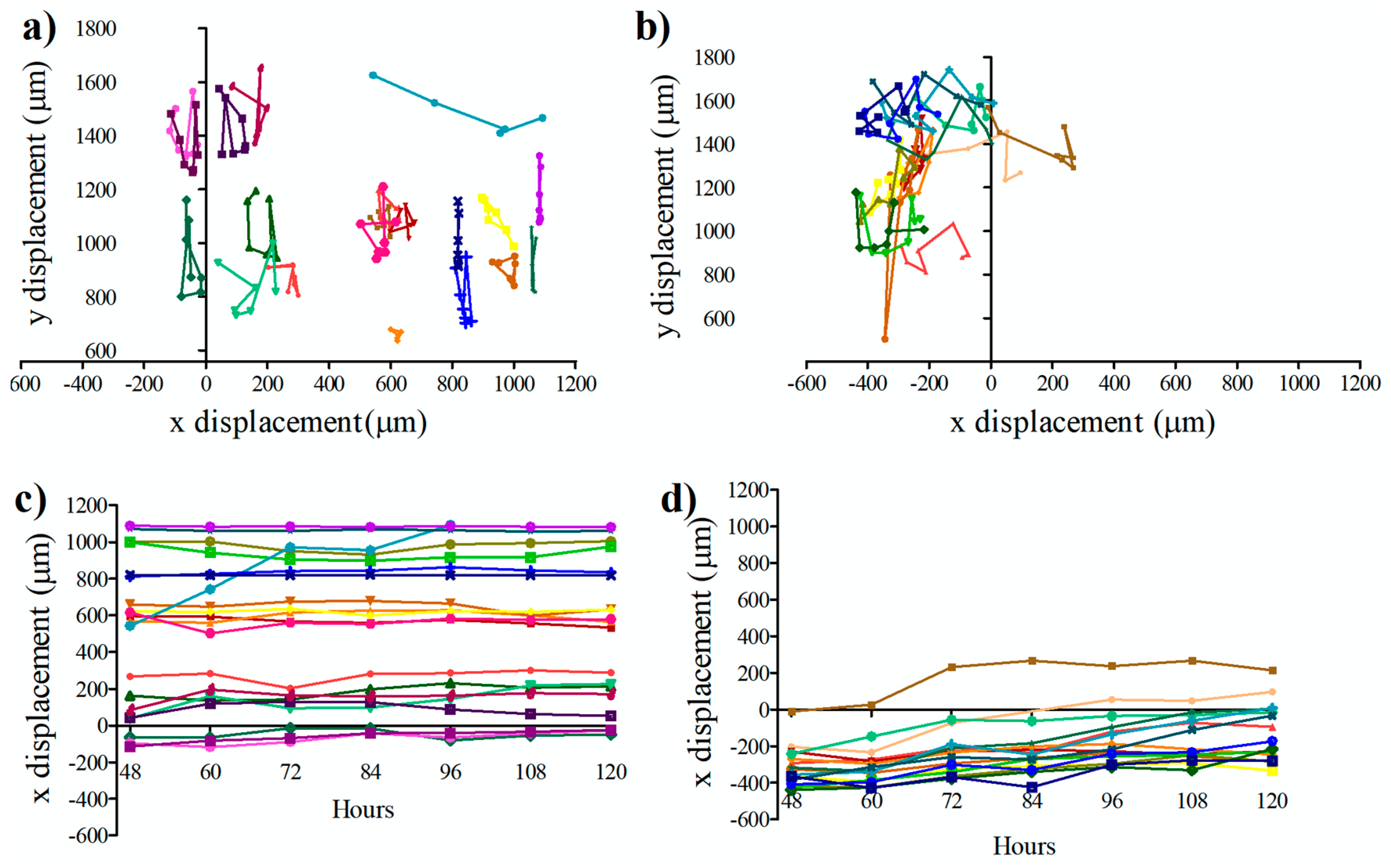
2.6. Measurement of Single-Cell Migration in the Microfluidic Device
2.7. Immunohistochemistry
3. Results
3.1. Influence of Conditioned Medium on U87 Proliferation in 12-Well Plate and Microfluidic Device
3.2. Influence of Conditional Medium on U87 Cell Migration by Wound Healing Assay
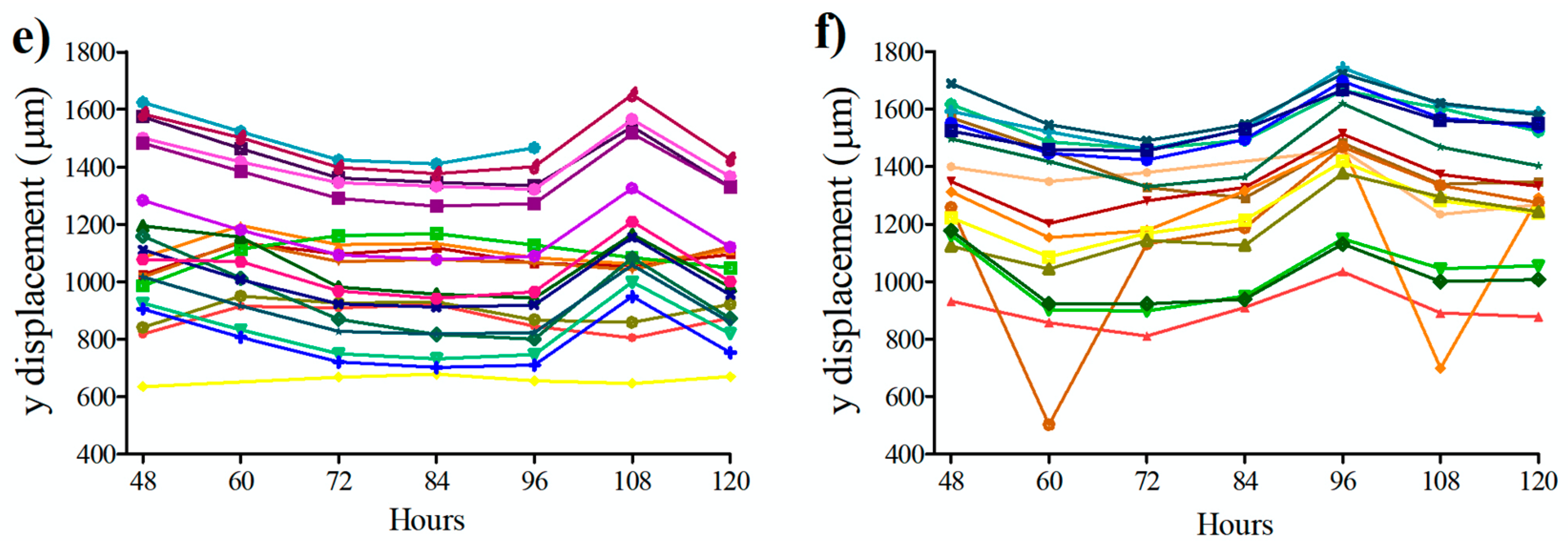
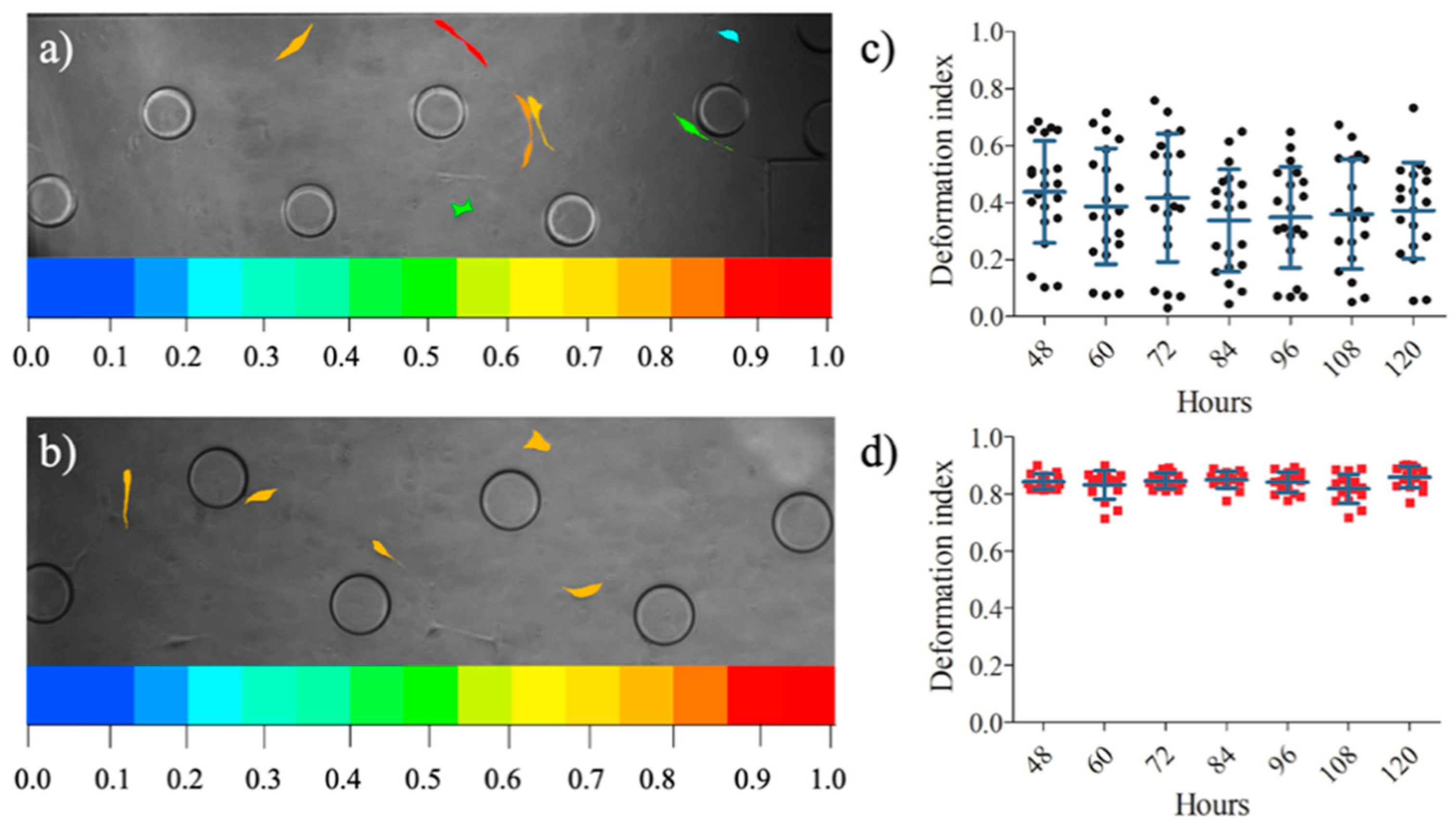
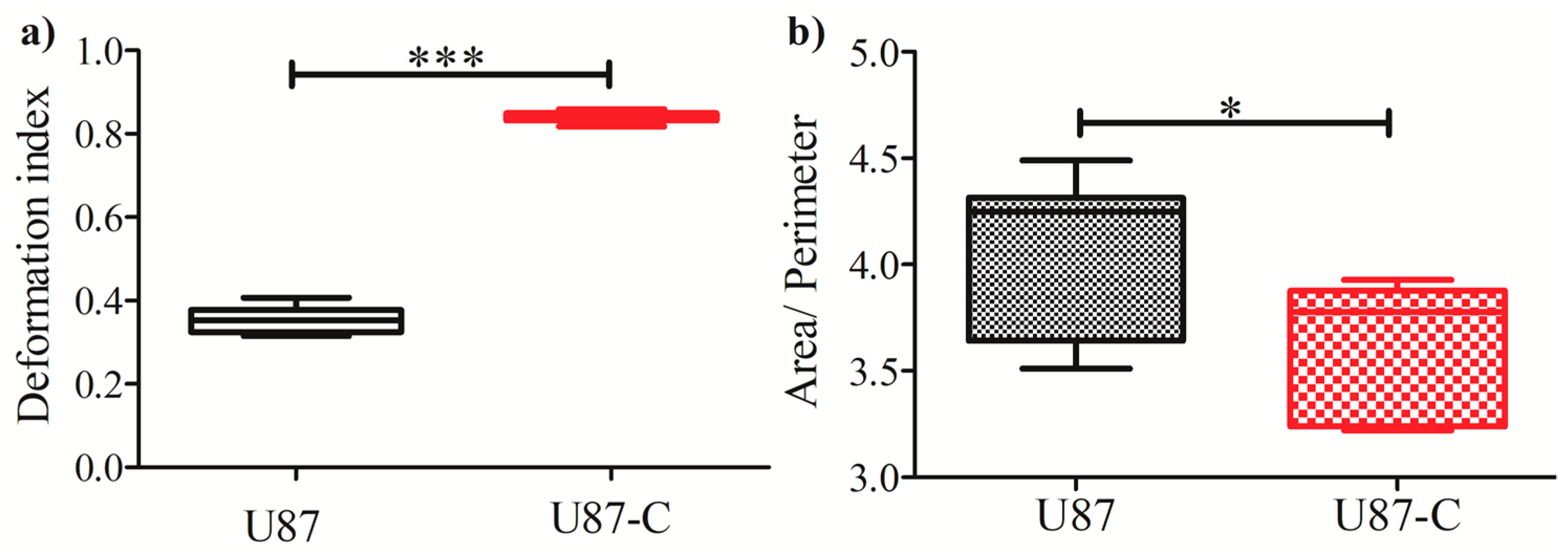
3.3. Influence of Conditional Medium on U87 Cell Migration Using a Microfluidic Device
3.4. Influence of Conditional Medium on U87 Cell Migration Using a Microfluidic Device
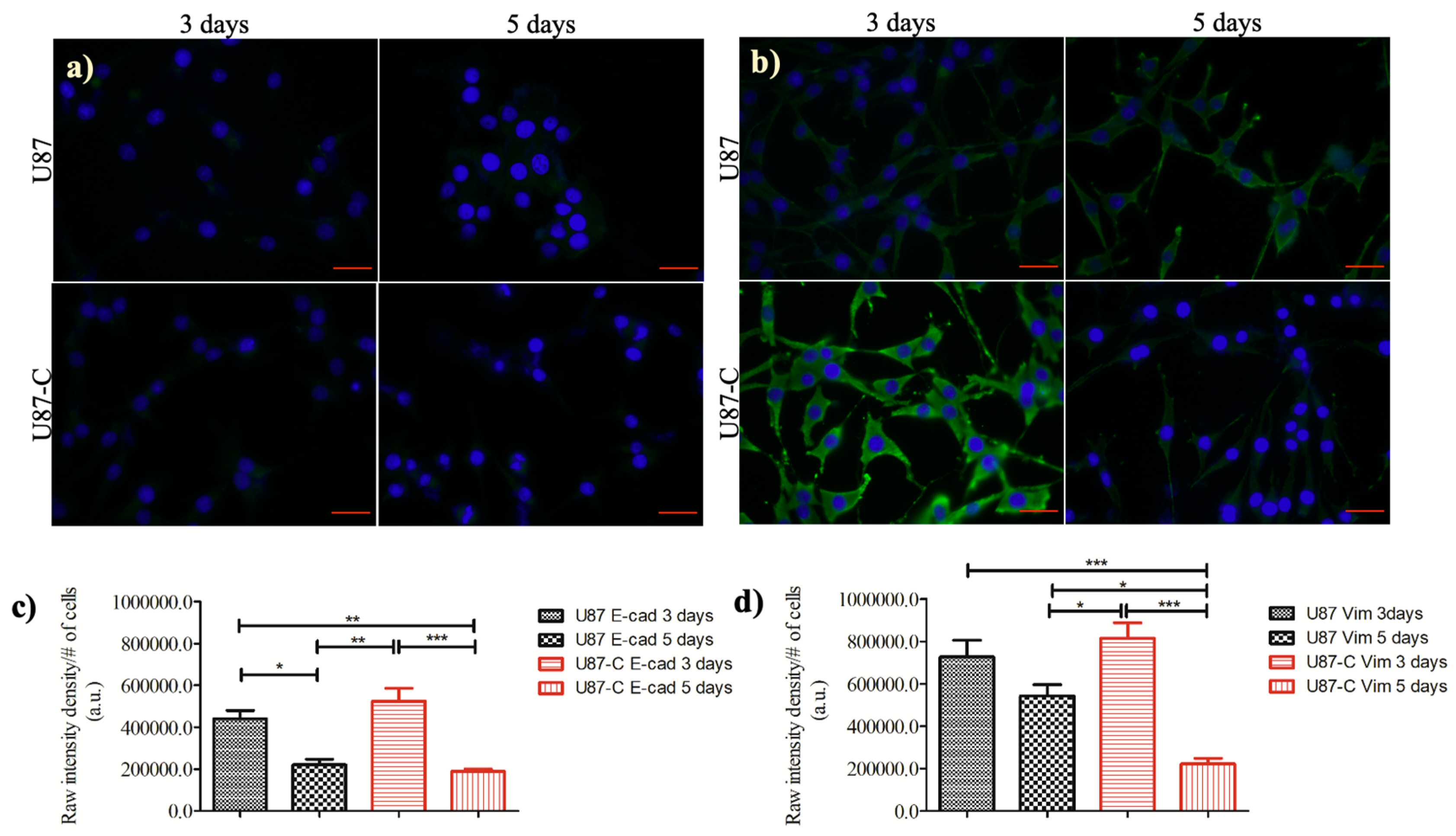
3.5. Influence of Conditional Medium on the Expression of E-cadherin and Vimentin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-Oncol. 2014, 16 (Suppl. S4), iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol. Med.-Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef]
- Holland, E.C. Glioblastoma Multiforme: The Terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Gimple, R.C.; Kidwell, R.L.; Kim, L.J.; Sun, T.; Gromovsky, A.D.; Wu, Q.; Wolf, M.; Lv, D.; Bhargava, S.; Jiang, L.; et al. Glioma Stem Cell–Specific Superenhancer Promotes Polyunsaturated Fatty-Acid Synthesis to Support EGFR Signaling. Cancer Discov. 2019, 9, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and Niche Concept. Cancers 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Kawaguchi, A.; Yamanaka, R. Promising Prognosis Marker Candidates on the Status of Epithelial–Mesenchymal Transition and Glioma Stem Cells in Glioblastoma. Cells 2019, 8, 1312. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular Subclasses of High-Grade Glioma Predict Prognosis, Delineate a Pattern of Disease Progression, and Resemble Stages in Neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Guardia, G.D.A.; Correa, B.R.; Araujo, P.R.; Qiao, M.; Burns, S.; Penalva, L.O.F.; Galante, P.A.F. Proneural and Mesenchymal Glioma Stem Cells Display Major Differences in Splicing and LncRNA Profiles. npj Genom. Med. 2020, 5. [Google Scholar] [CrossRef]
- Scherer, H.J. A Critical Review: The Pathology of Cerebral Gliomas. J. Neurol. Neurosur. Psychiatry 1940, 3, 147–177. [Google Scholar] [CrossRef]
- Kozminsky, M.; Sohn, L.L. The Promise of Single-Cell Mechanophenotyping for Clinical Applications. Biomicrofluidics 2020, 14, 031301. [Google Scholar] [CrossRef]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A Comparison of Methods to Assess Cell Mechanical Properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The Physics of Cancer: The Role of Physical Interactions and Mechanical Forces in Metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.T.; Gosset, R.D.; Moon, Y.S.; Masaeli, M.; Sohsman, M.; Ying, Y.; Mislick, K.; Adams, P.; Rao, J.; Di Carlo, D. Quantitative Diagnosis of Malignant Pleural Effusions by Single-Cell Mechanophenotyping. Sci. Transit. Med. 2013, 5, 212ra163. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, M.; Muñoz, H.E.; Bagnall, J.S.; Otto, O.; Manalis, S.R.; Carlo, D.D.; Guck, J. A Comparison of Microfluidic Methods for High-Throughput Cell Deformability Measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Eluru, G.; Srinivasan, R.; Gorthi, S.S. Deformability Measurement of Single-Cells at High-Throughput With Imaging Flow Cytometry. J. Lightwave. Technol. 2015, 33, 3475–3480. [Google Scholar] [CrossRef]
- Krutzik, P.O.; Nolan, G.P. Fluorescent Cell Barcoding in Flow Cytometry Allows High-Throughput Drug Screening and Signaling Profiling. Nat. Methods 2006, 3, 361–368. [Google Scholar] [CrossRef]
- Pelling, A.E.; Veraitch, F.S.; Chu, C.P.-K.; Mason, C.; Horton, M.A. Mechanical Dynamics of Single Cells during Early Apoptosis. Cell Motil. Cytoskeleton. 2009, 66, 409–422. [Google Scholar] [CrossRef]
- Radmacher, M. Studying the Mechanics of Cellular Processes by Atomic Force Microscopy. Methods Cell Biol. 2007, 347–372. [Google Scholar] [CrossRef]
- Puig-De-Morales, M.; Grabulosa, M.; Alcaraz, J.; Mullol, J.; Maksym, G.N.; Fredberg, J.J.; Navajas, D. Measurement of Cell Microrheology by Magnetic Twisting Cytometry with Frequency Domain Demodulation. J. Appl. Physiol. 2001, 91, 1152–1159. [Google Scholar] [CrossRef]
- Thoumine, O.; Ott, A.; Cardoso, O.; Meister, J.-J. Microplates: A New Tool for Manipulation and Mechanical Perturbation of Individual Cells. J. Biochem. Bioph. Meth. 1999, 39, 47–62. [Google Scholar] [CrossRef]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef]
- Huang, N.-T.; Zhang, H.-L.; Chung, M.-T.; Seo, J.H.; Kurabayashi, K. Recent Advancements in Optofluidics-Based Single-Cell Analysis: Optical on-Chip Cellular Manipulation, Treatment, and Property Detection. Lab. Chip. 2014, 14, 1230–1245. [Google Scholar] [CrossRef] [PubMed]
- Musielak, M. Red Blood Cell-Deformability Measurement: Review of Techniques. Clin. Hemorheol. Microcirc. 2009, 42, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Artmann, G. Microscopic Photometric Quantification of Stiffness and Relaxation Time of Red Blood Cells in a Flow Chamber. Biorheology 1995, 32, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.D.; Matthews, K.; Ma, H. Assessing the Vascular Deformability of Erythrocytes and Leukocytes: From Micropipettes to Microfluidics. In Current and Future Aspects of Nanomedicine; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhao, R.; Sider, K.L.; Simmons, C.A. Measurement of Layer-Specific Mechanical Properties in Multilayered Biomaterials by Micropipette Aspiration. Acta Biomater. 2011, 7, 1220–1227. [Google Scholar] [CrossRef]
- Lee, L.M.; Lee, J.W.; Chase, D.; Gebrezgiabhier, D.; Liu, A.P. Development of an Advanced Microfluidic Micropipette Aspiration Device for Single Cell Mechanics Studies. Biomicrofluidics 2016, 10, 054105. [Google Scholar] [CrossRef]
- Tee, S.-Y.; Bausch, A.R.; Janmey, P.A. The Mechanical Cell. Curr. Biol. 2009, 19. [Google Scholar] [CrossRef]
- Leggett, S.E.; Patel, M.; Valentin, T.M.; Gamboa, L.; Khoo, A.S.; Williams, E.K.; Franck, C.; Wong, I.Y. Mechanophenotyping of 3D Multicellular Clusters Using Displacement Arrays of Rendered Tractions. Proc. Natl. Acad. Sci. USA 2020, 117, 5655–5663. [Google Scholar] [CrossRef]
- Shah, M.K.; Garcia-Pak, I.H.; Darling, E.M. Influence of Inherent Mechanophenotype on Competitive Cellular Adherence. Ann. Biomed. Eng. 2017, 45, 2036–2047. [Google Scholar] [CrossRef]
- Diao, W.; Tong, X.; Yang, C.; Zhang, F.; Bao, C.; Chen, H.; Liu, L.; Li, M.; Ye, F.; Fan, Q.; et al. Behaviors of Glioblastoma Cells in in Vitro Microenvironments. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, L.; Bourkoula, E.; Migliorini, E.; Palma, A.; Pucer, A.; Skrap, M.; Scoles, G.; Beltrami, A.P.; Cesselli, D.; Lazzarino, M. Investigation of Adhesion and Mechanical Properties of Human Glioma Cells by Single Cell Force Spectroscopy and Atomic Force Microscopy. PLoS ONE 2014, 9, e112582. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Brangwynne, C.; Kasza, K.; Filippidi, E.; Gordon, V.; Deisboeck, T.; Weitz, D. Glioma Expansion in Collagen I Matrices: Analyzing Collagen Concentration-Dependent Growth and Motility Patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, T.A.; Pardo, E.M.D.J.; Kumar, S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Memmel, S.; Sukhorukov, V.L.; Höring, M.; Westerling, K.; Fiedler, V.; Katzer, A.; Krohne, G.; Flentje, M.; Djuzenova, C.S. Cell Surface Area and Membrane Folding in Glioblastoma Cell Lines Differing in PTEN and p53 Status. PLoS ONE 2014, 9, e87052. [Google Scholar] [CrossRef]
- Texier, B.D.; Laurent, P.; Stoukatch, S.; Dorbolo, S. Wicking through a confined micropillary array. Microfluid. Nanofluid. 2016, 20, 53. [Google Scholar] [CrossRef]
- Elitas, M.; Sadeghi, S.; Karamahmutoglu, H.; Gozuacik, D.; Turhal, N.S. Microfabricated platforms to quantitatively investigate cellular behavior under the influence of chemical gradients. Biomed. Phys. Eng. Express 2017, 3, 03023. [Google Scholar] [CrossRef]
- Nawas, A.A.; Ubanska, M.; Herbig, M.; Nötzel, M.; Kräter, M.; Rosendahk, P.; Herold, C.; Toepfner, N.; Kubánková, M.; Goswami, R.; et al. Intelligent Image-based Deformation Assisted Cell Sorting with Molecular Specificity. Nat. Methods 2020, 17, 595–599. [Google Scholar] [CrossRef]
- Elitas, M.; Sengul, E. Quantifying Heterogeneity According to Deformation of the U937 Monocytes and U937-Differentiated Macrophages Using 3D Carbon Dielectrophoresis in Microfluidics. Micromachines 2020, 11, 576. [Google Scholar] [CrossRef]
- Shankar, J.; Nabi, I.R. Actin Cytoskeleton Regulation of Epithelial Mesenchymal Transition in Metastatic Cancer Cells. PLoS ONE 2015, 10, e0119954. [Google Scholar] [CrossRef]
- Paguirigan, A.L.; Beebe, D.J. Microfluidics Meet Cell Biology: Bridging the Gap by Validation and Application of Microscale Techniques for Cell Biological Assays. BioEssays 2008, 30, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, T.A.; Tentori, A.M.; Herr, A.E. Microfluidics: Reframing Biological Enquiry. Nat. Rev. Mol. Cell Biol. 2015, 16, 5540567. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.T.; deMello, A.J.; Di Carlo, D.; Doyle, P.S.; Hansen, C.; Maceiczyk, R.M.; Wooton, R.C.R. Small but Perfectly Formed? Successes, Challenges, and Opportunities for Microfluidics in the Chemical and Biological Sciences. Chem 2017, 2, 201–223. [Google Scholar] [CrossRef]
- Bangasser, B.L.; Shamsan, G.A.; Chan, C.E.; Opoku, J.N.; Tüzel, E.; Schlichtmann, B.W.; Kasim, J.A.; Fuller, B.J.; McCullough, B.R.; Rosenfeld, S.S.; et al. Shifting the Optimal Stiffness for Cell Migration. Nat. Commun. 2017, 8, 15313. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.-G.; Kim, P. The Mode and Dynamics of Glioblastoma Cell Invasion into a Decellularized Tissue-Derived Extracellular Matrix-Based Three-Dimensional Tumor Model. Sci. Rep. 2018, 8, 4608. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Bucki, R.; Byfield, F.J.; Cruz, K.; Lee, T.; Marcinkiewicz, C.; Janmey, P.A. Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells. Biomacromolecules 2017, 18, 3040–3051. [Google Scholar] [CrossRef] [PubMed]
- Manini, L.; Caponnetto, F.; Bartolini, A.; Ius, T.; Mariuzzi, L.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D. Role of Microenvironment in Glioma Invasivness: What We Learned from In Vitro Models. Int. J. Mol. Sci. 2018, 19, 147. [Google Scholar] [CrossRef]
- Fayzullin, A.; Sandberg, C.J.; Spreadbury, M.; Saberniak, B.M.; Grieg, Z.; Skaga, E.; Langmoen, I.A.; Vik-Mo, E.O. Phenotypic and Expressional Heterogeneity in the Invasive Glioma Cells. Transl. Oncol. 2019, 12, 122–133. [Google Scholar] [CrossRef]
- Parker, J.J.; Canoll, P.; Niswander, L.; Kleinschmidt-DeMasters, B.K.; Foshay, K.; Waziri, A. Intratumoral Heterogeneity of Endogenous Tumor Cell Invasive Behavior in Human Glioblastoma. Sci. Rep. 2018, 8, 18002. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengul, E.; Elitas, M. Single-Cell Mechanophenotyping in Microfluidics to Evaluate Behavior of U87 Glioma Cells. Micromachines 2020, 11, 845. https://doi.org/10.3390/mi11090845
Sengul E, Elitas M. Single-Cell Mechanophenotyping in Microfluidics to Evaluate Behavior of U87 Glioma Cells. Micromachines. 2020; 11(9):845. https://doi.org/10.3390/mi11090845
Chicago/Turabian StyleSengul, Esra, and Meltem Elitas. 2020. "Single-Cell Mechanophenotyping in Microfluidics to Evaluate Behavior of U87 Glioma Cells" Micromachines 11, no. 9: 845. https://doi.org/10.3390/mi11090845
APA StyleSengul, E., & Elitas, M. (2020). Single-Cell Mechanophenotyping in Microfluidics to Evaluate Behavior of U87 Glioma Cells. Micromachines, 11(9), 845. https://doi.org/10.3390/mi11090845