Modeling a Microtubule Filaments Mesh Structure from Confocal Microscopy Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Artificial Muscle
2.2. Imaging with Confocal Microscopy
2.3. Building the Microtuble Filament Model from Volume Data
2.4. Rigid Body Dynamics Calculation
3. Results
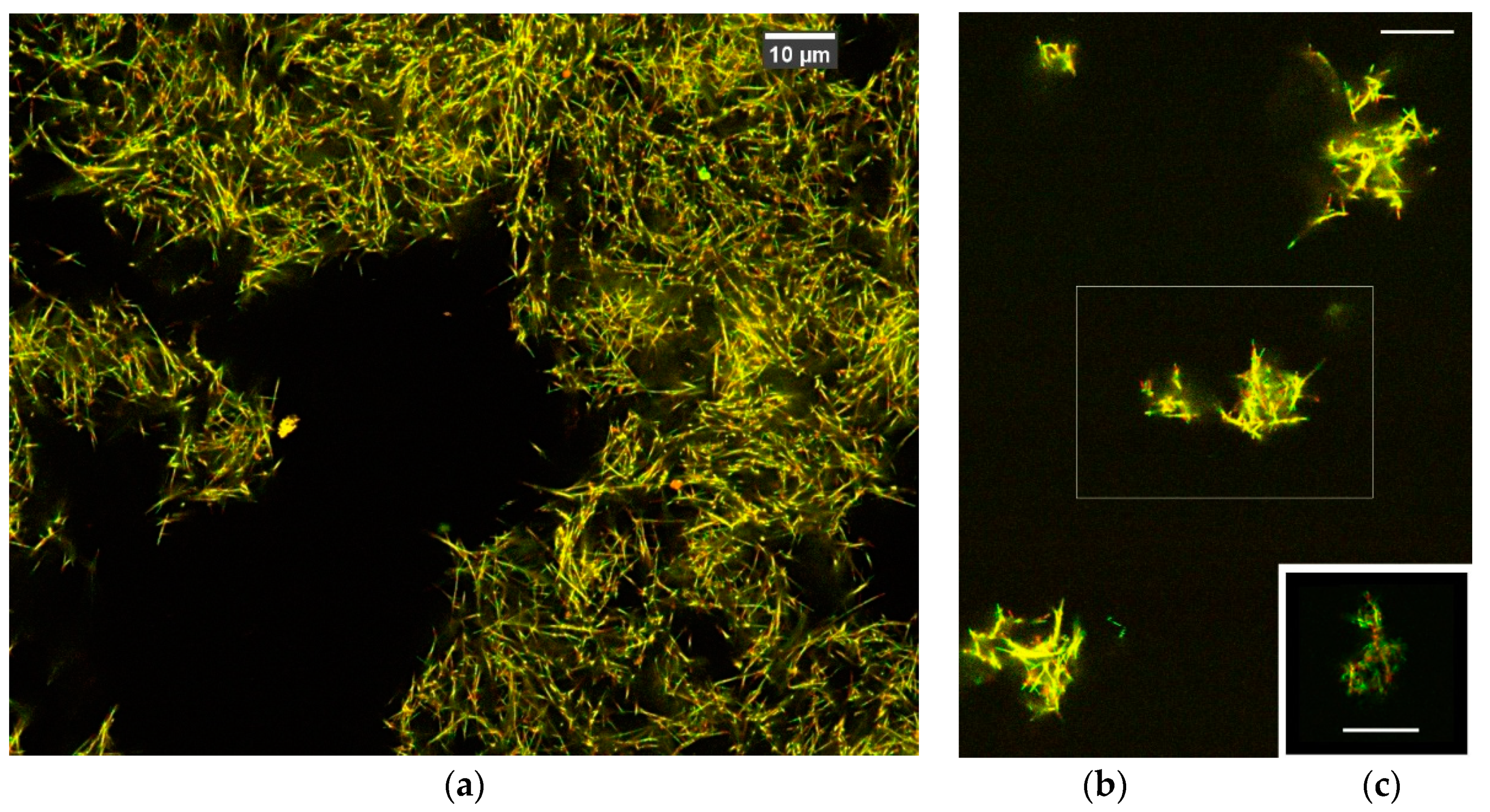
3.1. 3D Imaging of Microtubule Network
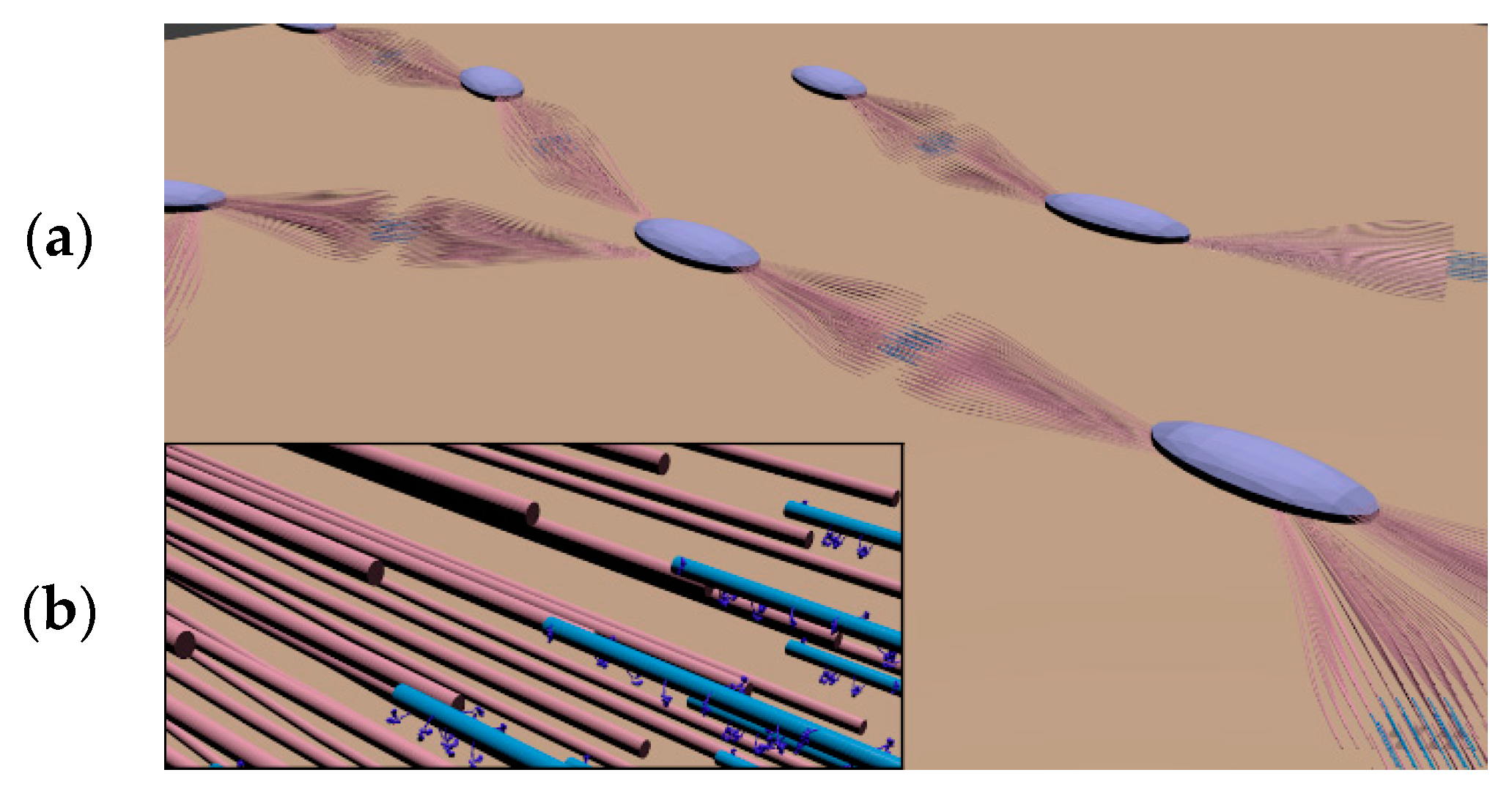
3.2. Fitting Microtubules into the 3D Volume
3.3. Dynamics of Microtubule Filaments
4. Discussion
4.1. 3D Imaging of Microtubule Filaments
4.2. Undestanding the Dynamics of Microtubule Filaments
4.3. Model Building with 3D Modeling Software
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hagiya, M.; Konagaya, A.; Kobayashi, S.; Saito, H.; Murata, S. Molecular Robots with Sensors and Intelligence. Acc. Chem. Res. 2014, 47, 1681–1690. [Google Scholar] [CrossRef]
- Foster, P.J.; Fürthauer, S.; Shelley, M.J.; Needleman, D.J. Active contraction of microtubule networks. eLife 2015, 4, e10837. [Google Scholar] [CrossRef]
- Schuppler, M.; Keber, F.C.; Kröger, M.; Bausch, A.R. Boundaries steer the contraction of active gels. Nat. Commun. 2016, 7, 13120. [Google Scholar] [CrossRef]
- Matsuda, K.; Kabir, A.M.R.; Akamatsu, N.; Saito, A.; Ishikawa, K.; Matsuyama, T.; Ditzer, O.; Islam, M.S.; Ohya, Y.; Sada, K.; et al. Artificial Smooth Muscle Model Composed of Hierarchically Ordered Microtubule Asters Mediated by DNA Origami Nanostructures. Nano Lett. 2019, 19, 3933–3938. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, A.; Komiyama, M. DNA origami: Fold, stick, and beyond. Nanoscale 2010, 2, 310. [Google Scholar] [CrossRef] [PubMed]
- Pawley, J.B. Handbook of Biological Confocal Microscopy, 3rd ed.; Pawley, J., Ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Blender, Blender Foundation. Available online: http://www.blender.org (accessed on 24 July 2020).
- Peloquin, J.; Komarova, Y.; Borisy, G. Conjugation of fluorophores to tubulin. Nat. Methods. 2005, 2, 299–303. [Google Scholar] [CrossRef]
- Heintzmann, R.; Sarafis, V.; Munroe, P.; Nailon, J.; Hanley, Q.S.; Jovin, T.M. Resolution enhancement by subtraction of confocal signals taken at different pinhole sizes. Micron 2003, 34, 293–300. [Google Scholar] [CrossRef]
- Schermelleh, L.; Heintzmann, R.; Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 2010, 190, 165–175. [Google Scholar] [CrossRef]
- Blender for Molecular Biology. Available online: http://staff.aist.go.jp/yutaka.ueno/blend/ (accessed on 24 July 2020).
- Johnson, G.T.; Autin, L.; Al-Alusi, M.; Goodsell, D.S.; Sanner, M.F.; Olson, A.J. cellPACK: A virtual mesoscope to model and visualize structural systems biology. Nat. Methods. 2015, 12, 85–91. [Google Scholar] [CrossRef]
- Andrei, R.M.; Callieri, M.; Zini, M.F.; Loni, T.; Maraziti, G.; Pan, M.K.; Zoppe, M. Intuitive representation of surface properties of biomolecules using BioBlender. BMC Bioinform. 2012, 13, S16. [Google Scholar] [CrossRef] [PubMed]
- Bullet Real Time Physics Simulation. Available online: http://www.bulletphysics.org/ (accessed on 24 July 2020).
- Blender for Mesoscale Protein Modeling. Available online: https://github.com/uenoyt/abam/ (accessed on 24 July 2020).
- Oldenbourg, R.; Salmon, E.D.; Tran, P.T. Birefringence of single and bundled microtubules. Biophys. J. 1998, 74, 645–654. [Google Scholar] [CrossRef]
- Tomishige, M.; Vale, R.D. Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change. J. Cell Biol. 2000, 151, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, A.; Ohya, Y. Nanomechanical Molecular Devices made of DNA Origami. Acc. Chem. Res. 2014, 47, 1742. [Google Scholar] [CrossRef]
- Chong, W.M.; Wang, W.J.; Lo, C.H.; Chou, T.Y.; Chang, T.J.; Riu, Y.P.; Tanos, B.; Mazo, G.; Tsou, M.F.B.; Jane, W.N.; et al. Super-resolution microscopy reveals coupling between mammalian centriole subdistal appendages and distal appendages. eLife 2020, 9, e53580. [Google Scholar] [CrossRef] [PubMed]
- Azuma, R.; Kish, S.; Gutmann, G.; Konagaya, A. All-atom molecular dynamics of film supported flat-shaped DNA origami in water. Chem-Bio Inform. J. 2018, 18, 96–118. [Google Scholar] [CrossRef]
- Gutmann, G.; Azuma, R.; Konagaya, A. A Virtual Reality Computational Platform Dedicated for the Emergence of Global Dynamics in a Massive Swarm of Objects. J. Imaging Soc. Jpn. 2018, 57, 647–653. [Google Scholar]
- Torisawa, T.; Taniguchi, D.; Ishihara, S.; Oiwa, K. Spontaneous Formation of a Globally Connected Contractile Network in a Microtubule-Motor. System. Biophys. J. 2016, 111, 373–385. [Google Scholar] [CrossRef]
- Alvarado, J.; Sheinman, M.; Sharma, A.; MacKintosh, F.C.; Koenderink, G.H. Molecular motors robustly drive active gels to a critically connected state. Nat. Phys. 2013, 9, 591–597. [Google Scholar] [CrossRef]
- Gunst, S.J.; Zhang, W. Actin cytoskeletal dynamics in smooth muscle: A new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 2008, 295, C576–C587. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sugimoto, Y.; Takezawa, Y.; Oshima, K.; Matsuo, T.; Ueno, Y.; Irving, T.C. Muscle Contraction Mechanisms: Use of Synchrotron X-ray Diffraction. eLS 2010. [Google Scholar] [CrossRef]
- Wang, L.; Geist, J.; Grogan, A.; Hu, L.-Y.R.; Kontrogianni-Konstantopoulos, A. Thick Filament Protein Network, Functions, and Disease Association. Compr. Physiol. 2018, 8, 631–709. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, Y.; Matsuda, K.; Katoh, K.; Kuzuya, A.; Kakugo, A.; Konagaya, A. Modeling a Microtubule Filaments Mesh Structure from Confocal Microscopy Imaging. Micromachines 2020, 11, 844. https://doi.org/10.3390/mi11090844
Ueno Y, Matsuda K, Katoh K, Kuzuya A, Kakugo A, Konagaya A. Modeling a Microtubule Filaments Mesh Structure from Confocal Microscopy Imaging. Micromachines. 2020; 11(9):844. https://doi.org/10.3390/mi11090844
Chicago/Turabian StyleUeno, Yutaka, Kento Matsuda, Kaoru Katoh, Akinori Kuzuya, Akira Kakugo, and Akihiko Konagaya. 2020. "Modeling a Microtubule Filaments Mesh Structure from Confocal Microscopy Imaging" Micromachines 11, no. 9: 844. https://doi.org/10.3390/mi11090844
APA StyleUeno, Y., Matsuda, K., Katoh, K., Kuzuya, A., Kakugo, A., & Konagaya, A. (2020). Modeling a Microtubule Filaments Mesh Structure from Confocal Microscopy Imaging. Micromachines, 11(9), 844. https://doi.org/10.3390/mi11090844





