Biological Aging Modulates Cell Migration via Lamin A/C-Dependent Nuclear Motion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunofluorescence Microscopy
2.3. Morphometric and Motility Analysis
2.4. Laser Ablation and Actin Retraction Measurement
2.5. Quantitative Image Analysis
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
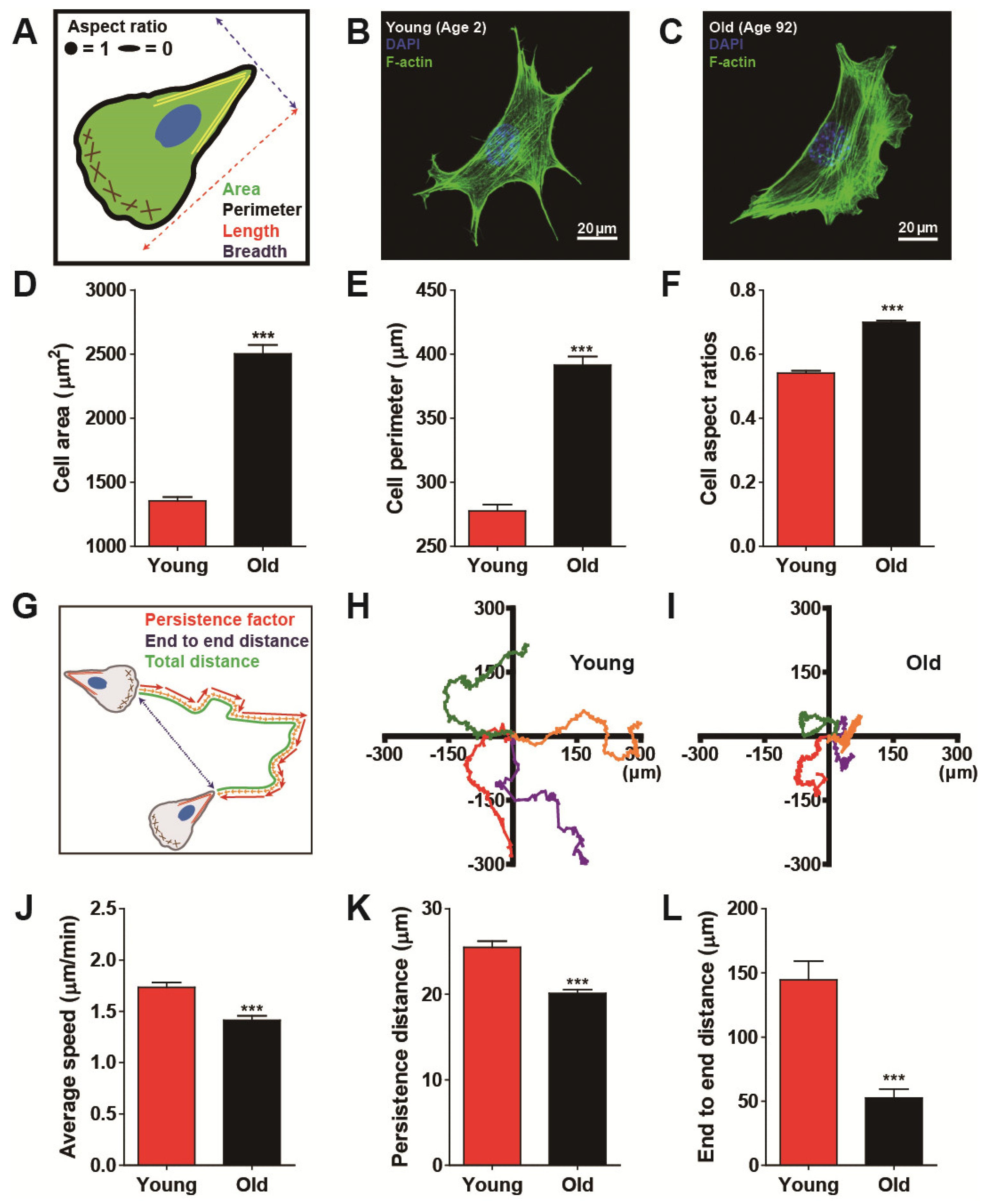
3.1. Biological Aging Declines Cell Migration
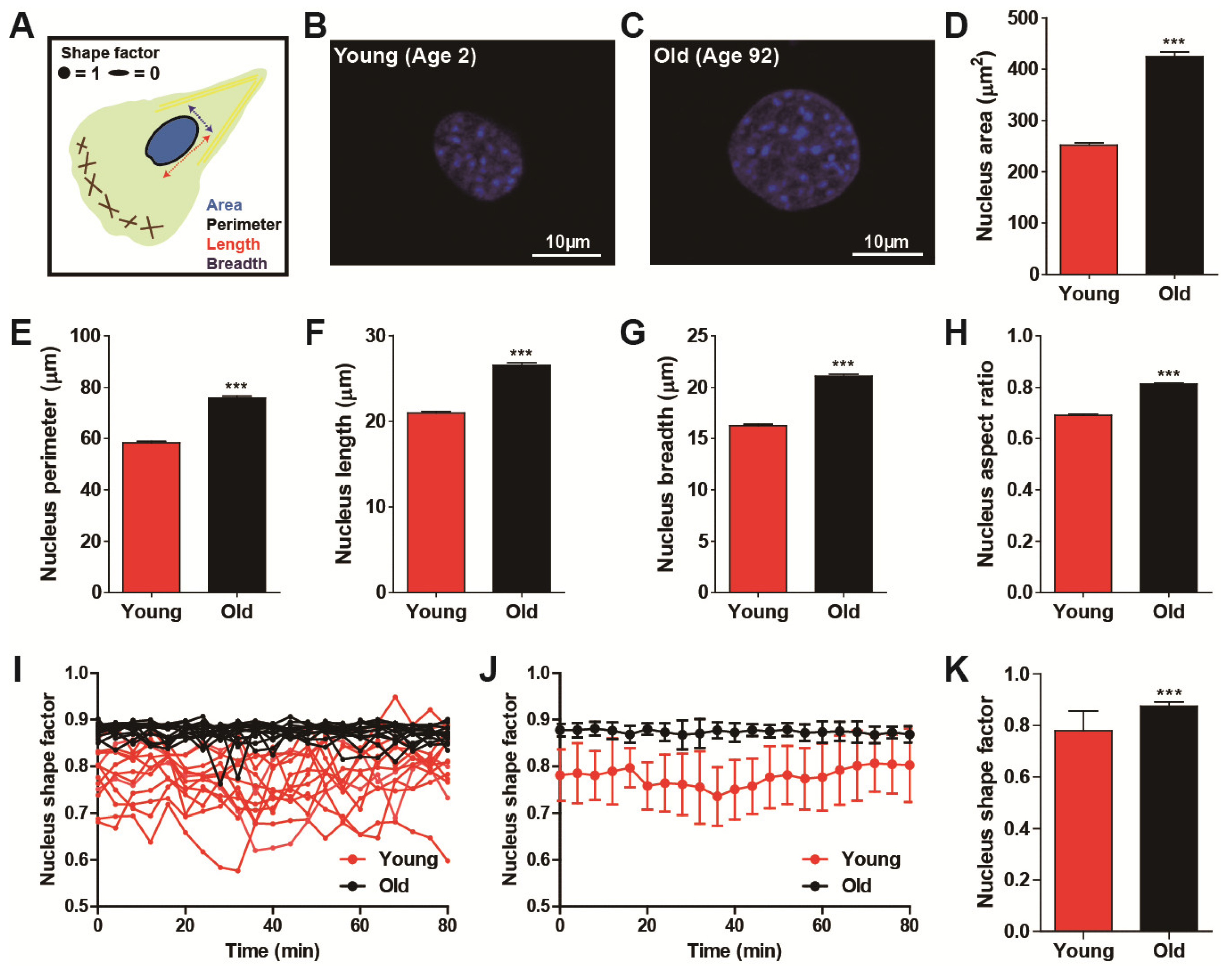
3.2. Age-Dependent Nuclear Morphology Distinguishes Cell Migration
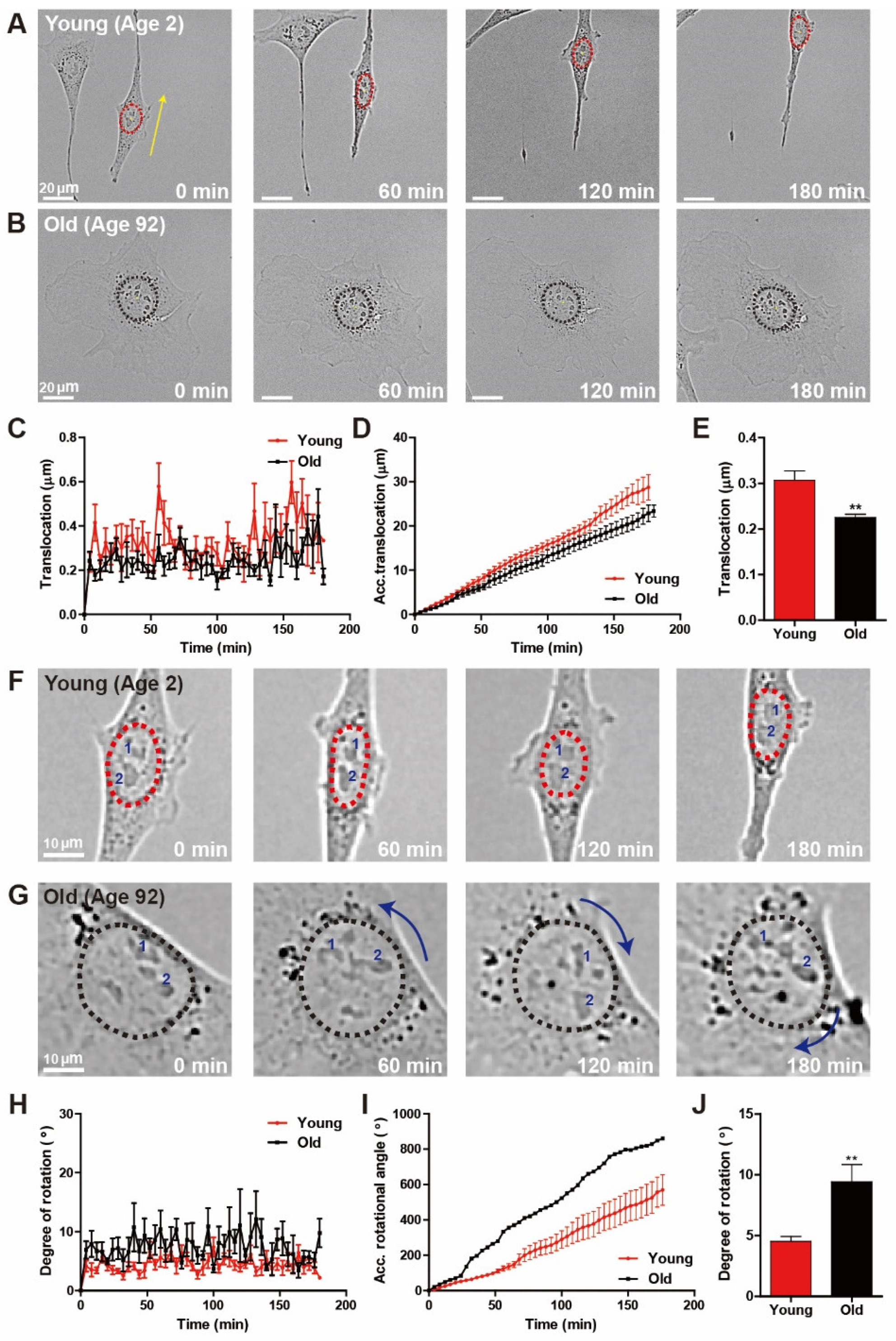
3.3. Distinct Nuclear Motility Synchronizes with Biological Ages
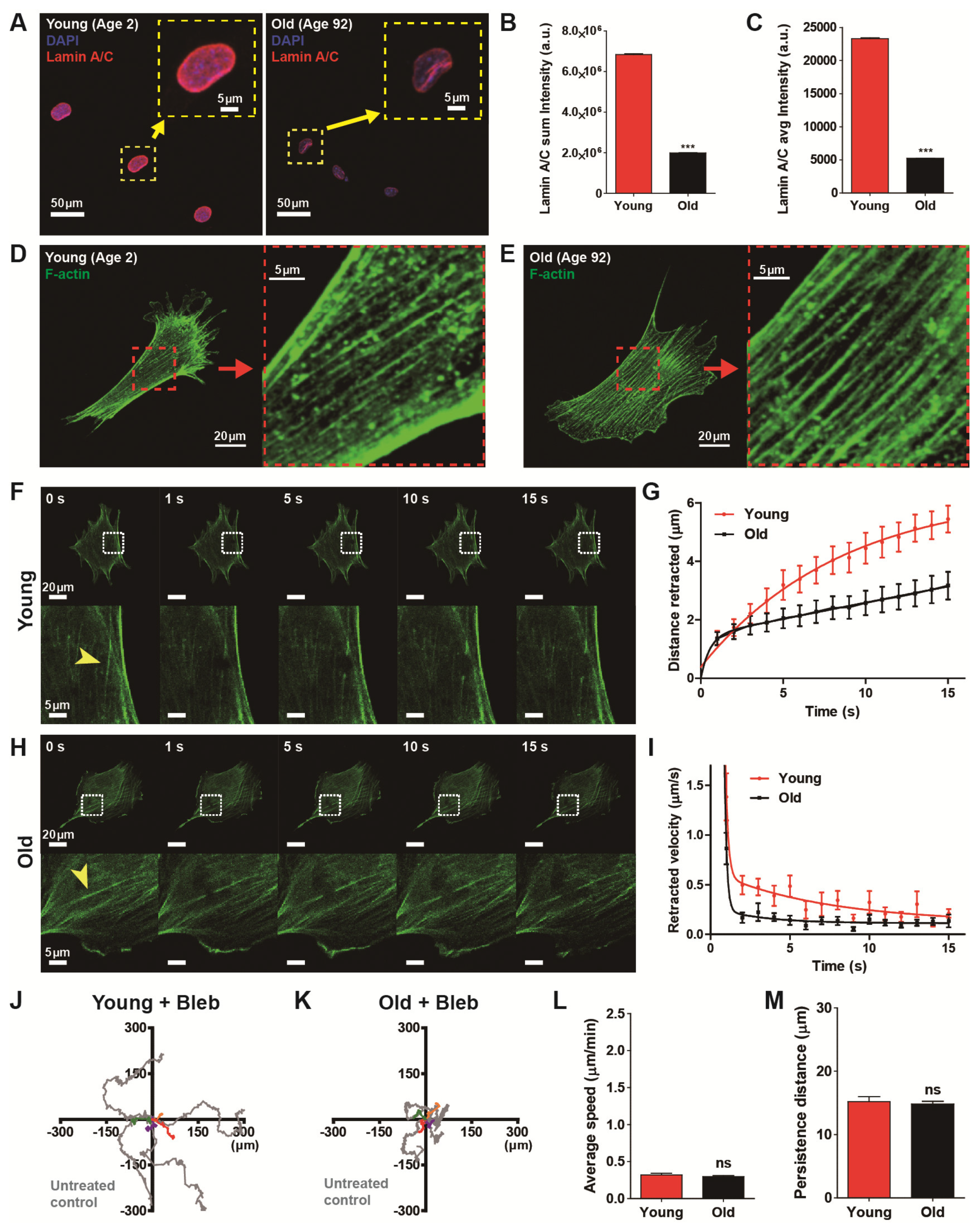
3.4. Nulcear Lamin A/C Mediated Actomyosin Contractility Determines Age-Dependent Cell Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The molecular inflammatory process in aging. Antioxid. Redox Signal. 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Houghton, D.; Jones, T.W.; Cassidy, S.; Siervo, M.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. The effect of age on the relationship between cardiac and vascular function. Mech. Ageing Dev. 2016, 153, 1–6. [Google Scholar] [CrossRef]
- Calvanese, V.; Lara, E.; Kahn, A.; Fraga, M.F. The role of epigenetics in aging and age-related diseases. Ageing Res. Rev. 2009, 8, 268–276. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Han, S.B.; Lee, J.H.; Kim, H.W.; Kim, D.H. Cancer Mechanobiology: Microenvironmental Sensing and Metastasis. ACS Biomater. Sci. Eng. 2019, 5, 3735–3752. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, Y.J.; Ha, L.J.; Kim, D.H.; Kim, D.H. Unraveling the Mechanobiology of the Immune System. Adv. Healthc. Mater. 2019, 8, e1801332. [Google Scholar] [CrossRef]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 54. [Google Scholar] [CrossRef]
- Li, R.; Gundersen, G.G. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 2008, 9, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Leube, R.E.; Moch, M.; Windoffer, R. Intermediate filaments and the regulation of focal adhesion. Curr. Opin. Cell Biol. 2015, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gupton, S.L.; Waterman-Storer, C.M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006, 125, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Mejat, A.; Misteli, T. LINC complexes in health and disease. Nucleus 2010, 1, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.; Kim, D.H. Deciphering Nuclear Mechanobiology in Laminopathy. Cells 2019, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Razafsky, D.; Hodzic, D. Bringing KASH under the SUN: The many faces of nucleo-cytoskeletal connections. J. Cell Biol. 2009, 186, 461–472. [Google Scholar] [CrossRef]
- Dahl, K.N.; Kahn, S.M.; Wilson, K.L.; Discher, D.E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004, 117, 4779–4786. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lammerding, J. Lamins at a glance. J. Cell Sci. 2012, 125, 2087–2093. [Google Scholar] [CrossRef]
- Uhler, C.; Shivashankar, G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017, 18, 717–727. [Google Scholar] [CrossRef]
- Simon, D.N.; Wilson, K.L. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat. Rev. Mol. Cell Biol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; Hah, J.; Wirtz, D.; Kim, D.H. Recapitulation of molecular regulators of nuclear motion during cell migration. Cell Adh. Migr. 2019, 13, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.G.; Worman, H.J. Nuclear positioning. Cell 2013, 152, 1376–1389. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, S.; Wirtz, D. Tight coupling between nucleus and cell migration through the perinuclear actin cap. J. Cell Sci. 2014, 127, 2528–2541. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013, 27, 1351–1361. [Google Scholar] [CrossRef]
- Chambliss, A.B.; Wu, P.H.; Chen, W.C.; Sun, S.X.; Wirtz, D. Simultaneously defining cell phenotypes, cell cycle, and chromatin modifications at single-cell resolution. FASEB J. 2013, 27, 2667–2676. [Google Scholar] [CrossRef]
- Kim, D.H.; Khatau, S.B.; Feng, Y.; Walcott, S.; Sun, S.X.; Longmore, G.D.; Wirtz, D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci. Rep. 2012, 2, 555. [Google Scholar] [CrossRef]
- Makhija, E.; Jokhun, D.S.; Shivashankar, G.V. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2016, 113, E32–E40. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef]
- Kim, D.H.; Wirtz, D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 2015, 48, 161–172. [Google Scholar] [CrossRef]
- Kim, J.K.; Louhghalam, A.; Lee, G.; Schafer, B.W.; Wirtz, D.; Kim, D.H. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 2017, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Maxwell, I.Z.; Heisterkamp, A.; Polte, T.R.; Lele, T.P.; Salanga, M.; Mazur, E.; Ingber, D.E. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 2006, 90, 3762–3773. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Han, S.-B.; Hah, J.; Lee, G.; Kim, J.-K.; Kim, S.H.; Kim, D.-H. Biological Aging Modulates Cell Migration via Lamin A/C-Dependent Nuclear Motion. Micromachines 2020, 11, 801. https://doi.org/10.3390/mi11090801
Park J-W, Han S-B, Hah J, Lee G, Kim J-K, Kim SH, Kim D-H. Biological Aging Modulates Cell Migration via Lamin A/C-Dependent Nuclear Motion. Micromachines. 2020; 11(9):801. https://doi.org/10.3390/mi11090801
Chicago/Turabian StylePark, Jung-Won, Seong-Beom Han, Jungwon Hah, Geonhui Lee, Jeong-Ki Kim, Soo Hyun Kim, and Dong-Hwee Kim. 2020. "Biological Aging Modulates Cell Migration via Lamin A/C-Dependent Nuclear Motion" Micromachines 11, no. 9: 801. https://doi.org/10.3390/mi11090801
APA StylePark, J.-W., Han, S.-B., Hah, J., Lee, G., Kim, J.-K., Kim, S. H., & Kim, D.-H. (2020). Biological Aging Modulates Cell Migration via Lamin A/C-Dependent Nuclear Motion. Micromachines, 11(9), 801. https://doi.org/10.3390/mi11090801




