A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacture Procedure of Interdigitated Chain-Shaped Electrode (ICE)
2.3. Biosensor Construction
2.4. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
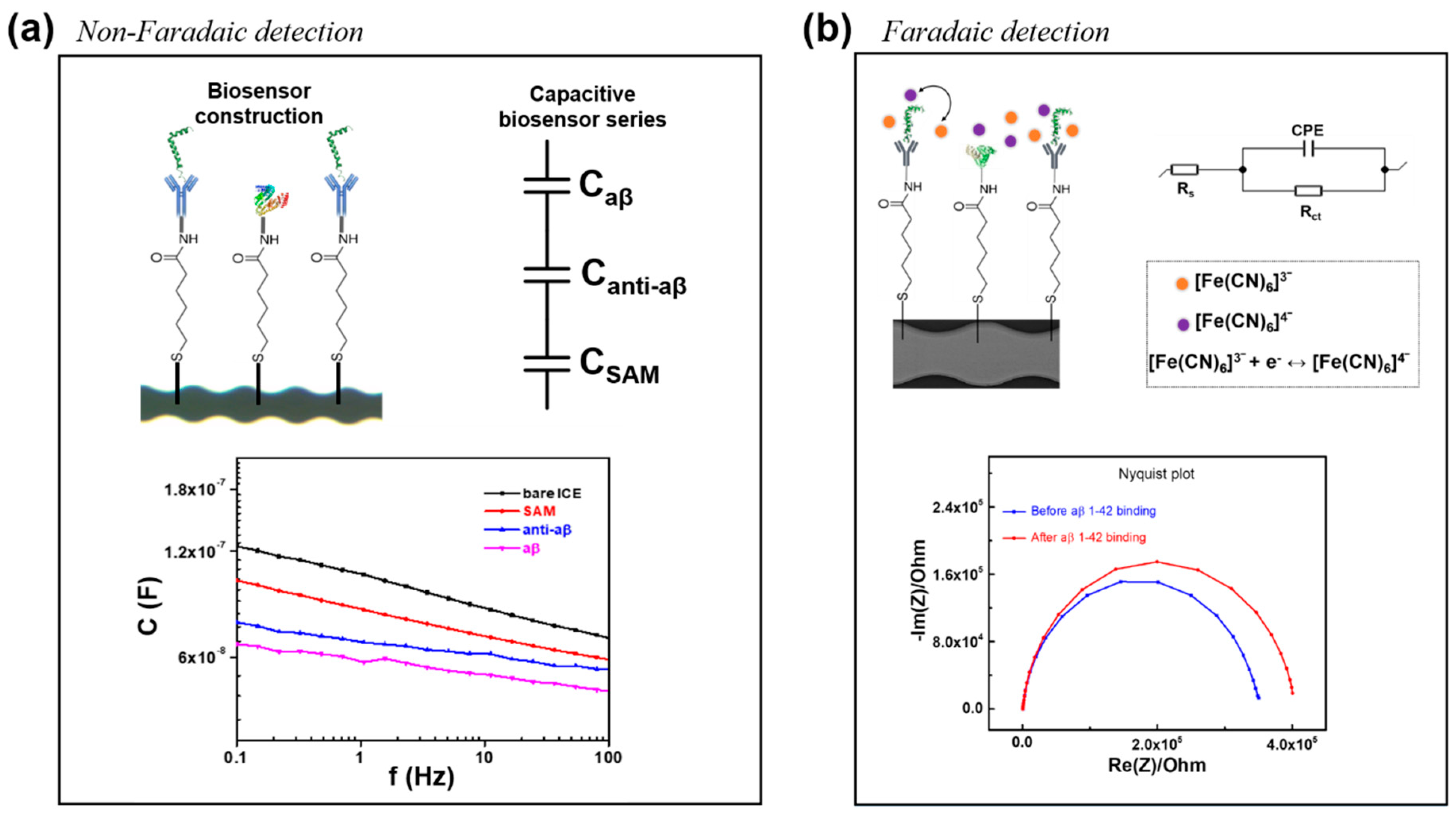
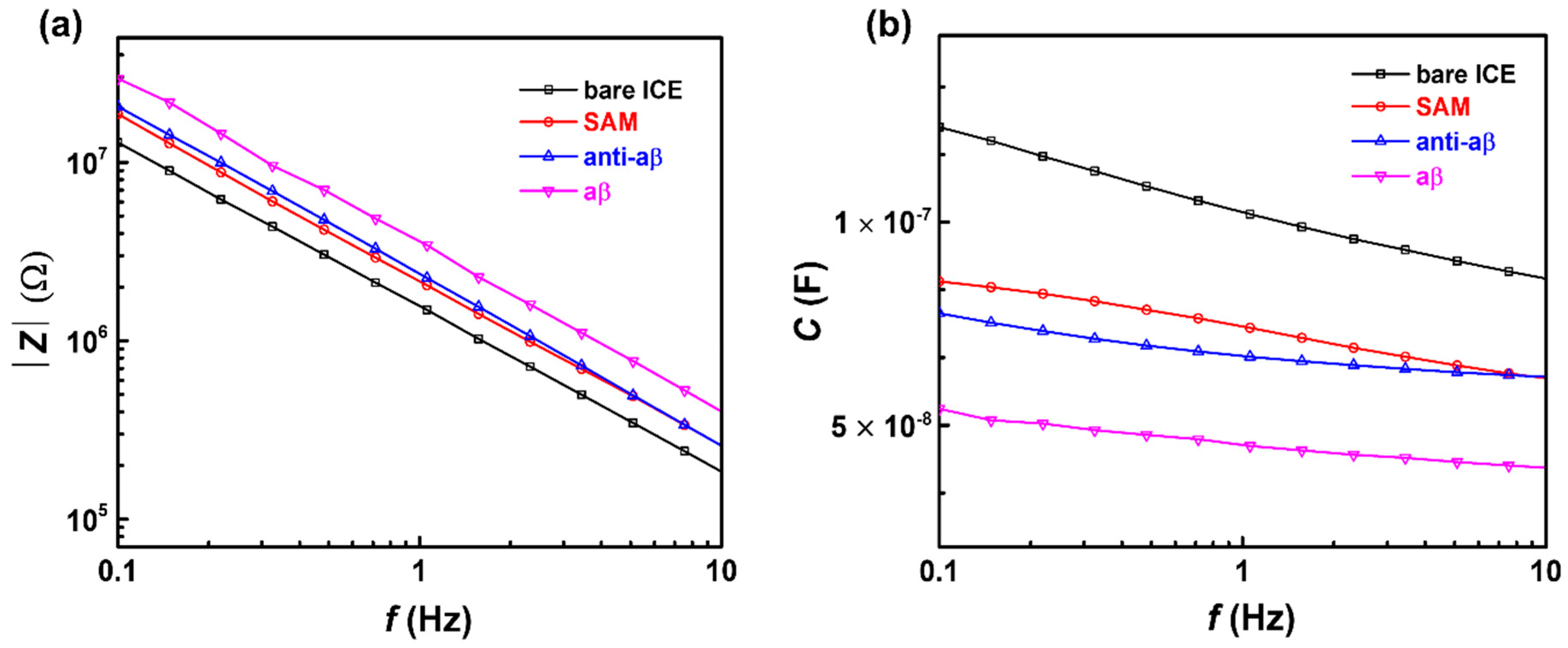
3.1. Electrochemical Characterization of the Anti-aβ Antibody onto a Self-Assembled Monolayer Functionalized Interdigitated Chain-Shaped Electrode (Anti-aβ/SAM/ICE) Biosensor
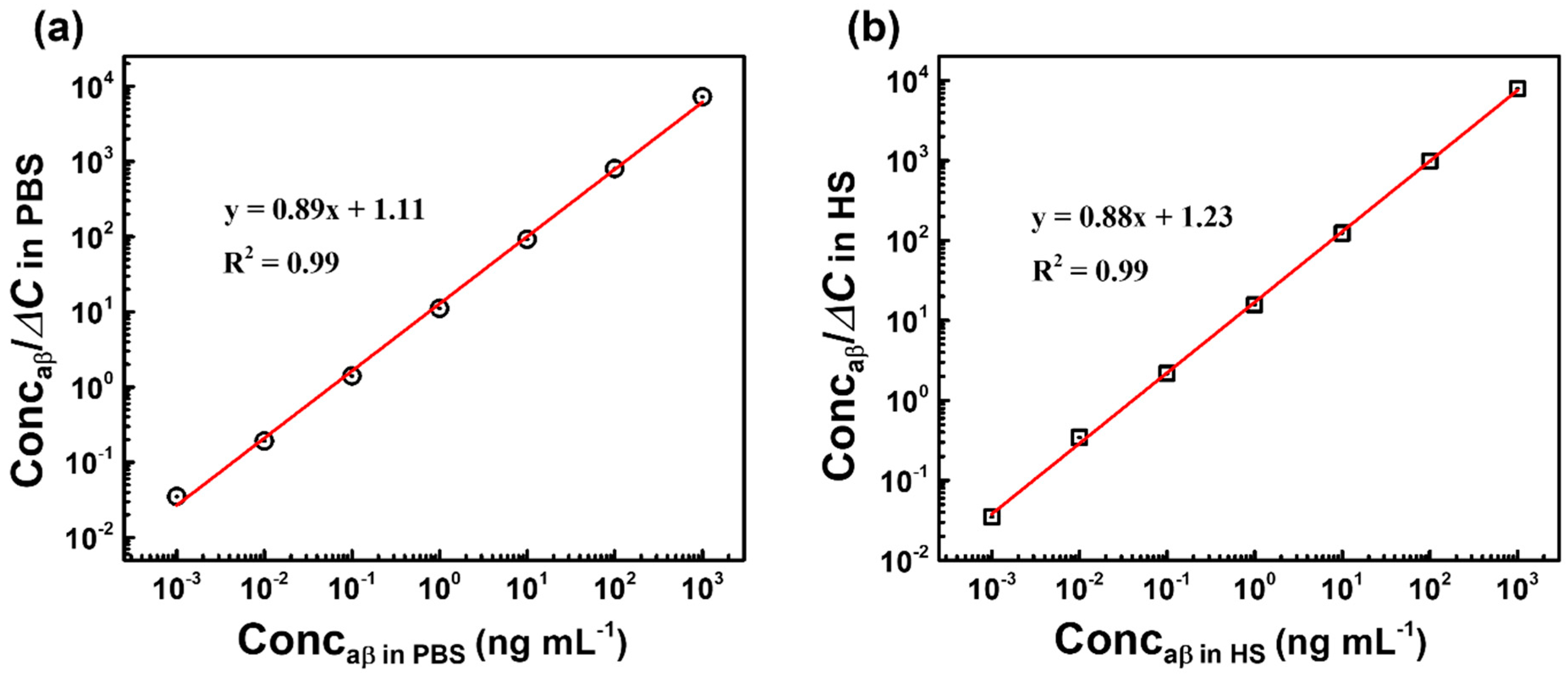
3.2. Capacitive Detection of aβ in PBS by the Biosensor
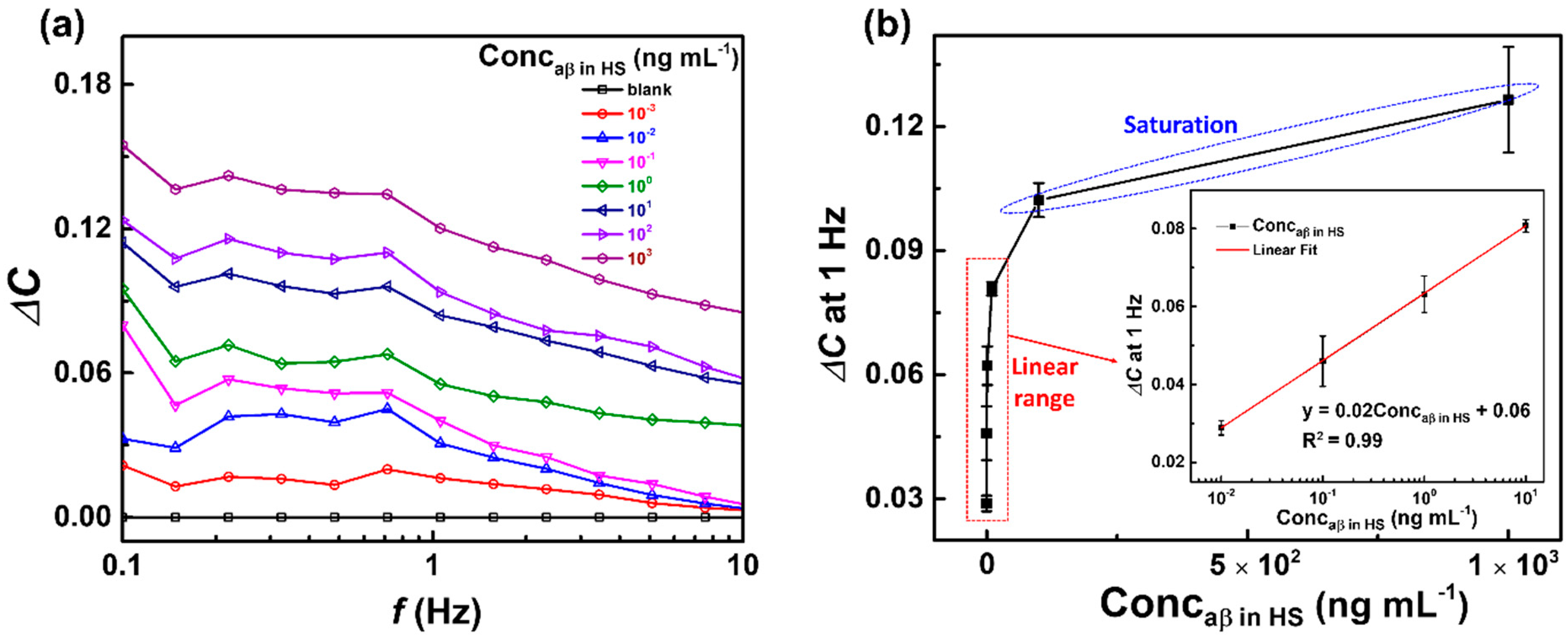
3.3. Capacitive Detection of aβ in Human Serum (HS) by the Biosensor
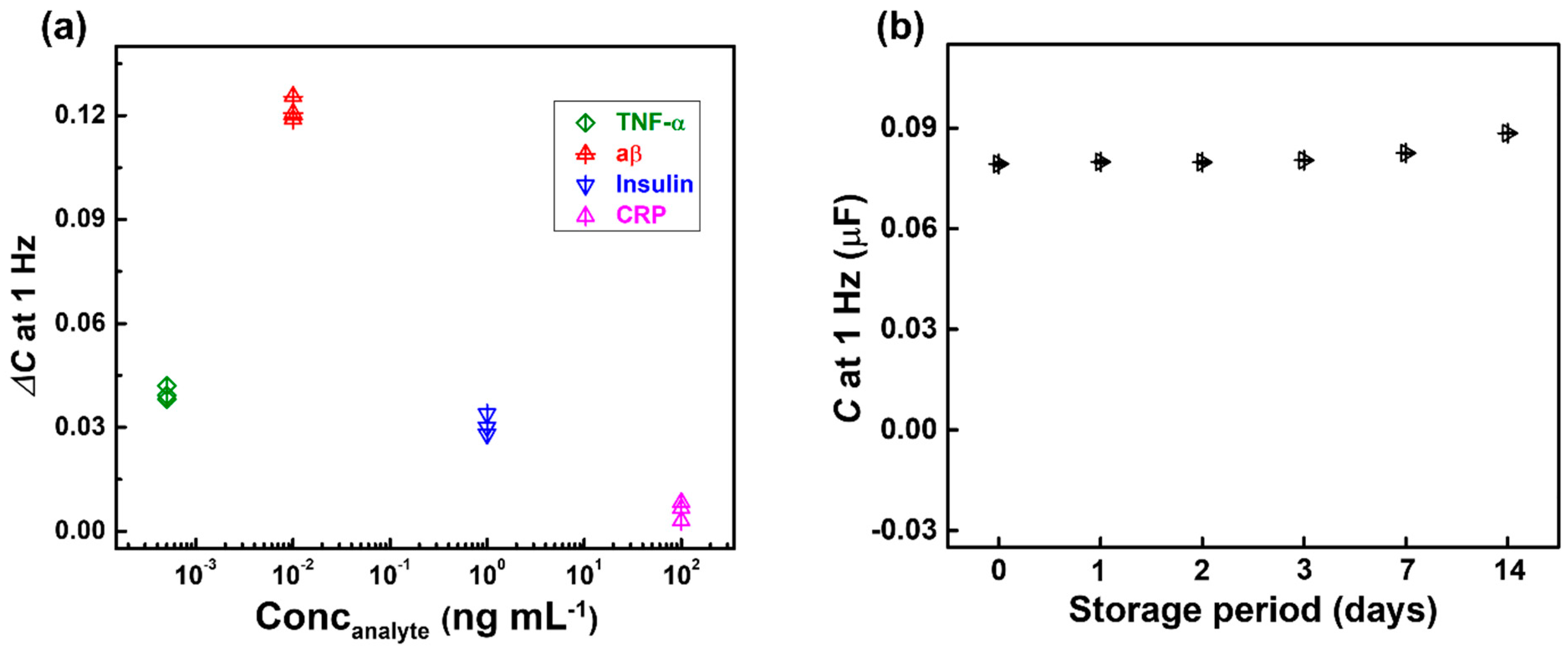
3.4. Selectivity and Stability
3.5. Binding Affinity and Dissociation Constant Kd of Anti-aβ Antibody—aβ 1-42 Peptide Interaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoo, Y.K.; Lee, J.W.; Kim, H.S.; Hwang, K.S.; Yoon, D.S.; Lee, J.H. Toward Exosome-Based Neuronal Diagnostic Devices. Micromachines 2018, 9, 634. [Google Scholar] [CrossRef]
- Awasthi, M.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Alzheimer’s disease: An overview of amyloid beta dependent pathogenesis and its therapeutic implications along with in silicon approaches emphasizing the role of natural products. J. Neurol. Sci. 2016, 201, 256–271. [Google Scholar] [CrossRef]
- Bullicha, S.; Seibylb, J.; Catafaua, A.M.; Jovalekica, A.; Koglina, N.; Barthelc, H.; Sabric, O.; Santi, S.D. Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. Neuroimage Clin. 2017, 15, 325–332. [Google Scholar] [CrossRef]
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic accuracy of 18F amyloid PET tracers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385. [Google Scholar] [CrossRef]
- Madhavan, A.; Whitwell, J.L.; Weigand, S.D.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Tosakulwong, N.; Senjem, M.L.; Gunter, J.L.; Lowe, V.J.; et al. FDG PET and MRI in Logopenic Primary Progressive Aphasia versus Dementia of the Alzheimer’s Type. PLoS ONE 2013, 8, e62471. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Bakkour, A.; Salat, D.H.; Feczko, E.; Pacheco, J.; Greve, D.N.; Grodstein, F.; Wright, C.I.; Blacker, D.; Rosas, H.D.; et al. The Cortical Signature of Alzheimer’s Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cereb Cortex. 2009, 19, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Shui, B.Q.; Tao, D.; Florea, A.; Cheng, J.; Zhao, Q.; Gu, Y.; Li, W.; Jaffrezic-Renault, N.; Mei, Y.; Guo, Z. Biosensors for Alzheimer’s disease biomarker detection: A review. Biochimie 2018, 147, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K. A Review of Fluid Biomarkers for Alzheimer’s Disease: Moving from CSF to Blood. Neurol. Ther. 2017, 6, 15–24. [Google Scholar] [CrossRef]
- Sperling, R.A.; Karlawish, J.; Johnson, K.A. Preclinical Alzheimer disease—The challenges ahead. Nat. Rev. Neurol. 2013, 9, 54–58. [Google Scholar] [CrossRef]
- Gagni, P.; Sola, L.; Cretich, M.; Chiari, M. Development of a high-sensitivity immunoassay for amyloid-beta 1–42 using a silicon microarray platform. Biosens. Bioelectron. 2013, 47, 490–495. [Google Scholar] [CrossRef]
- Hampel, H.; Teipel, S.J.; Fuchsberger, T.; Andreasen, N.; Wiltfang, J.; Otto, M.; Shen, Y.; Dodel, R.; Du, Y.; Farlow, M. Value of CSF β-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol. Psychiatry 2004, 9, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C. Biomarkers of Alzheimer Disease in Plasma. NeuroRx 2004, 1, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ridler, C. Blood amyloid-β successfully signals AD. Nat. Rev. Neurol. 2018, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Steffen, E.S.; Thomas, A.B.; Claus, U.P. Endothelial LRP1 transports amyloid-β1–42 across the blood-brain barrier. J. Clin. Investig. 2016, 126, 123–136. [Google Scholar]
- Daene, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-β peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Doecke, J.D.; Laws, S.M.; Faux, N.G. Blood-Based Protein Biomarkers for Diagnosis of Alzheimer Disease. Arch. Neurol. 2012, 69, 1318–1325. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Hwang, K.S.; Avila, D.; Elashoff, D.; Kohannim, O.; Teng, E.; Sokolow, S.; Jack, C.R.; Jagust, W.J.; Shaw, L. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology 2015, 84, 729–737. [Google Scholar] [CrossRef]
- Truong, T.N.L.; Takamura, Y.; Tamiya, E.; Vestergaard, M.C. Modified screen printed electrode for development of a highly sensitive label-free impedimetric immunosensor to detect amyloid beta peptides. Anal. Chim. Acta 2015, 892, 69–76. [Google Scholar]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Wu, X.; Ye, Z.; Li, G. Peptide-based electrochemical biosensor for amyloid β 1–42 soluble oligomer assay. Talanta 2012, 93, 358–363. [Google Scholar] [CrossRef]
- Chikae, M.; Fukuda, T.; Kerman, K.; Idegami, K.; Miura, Y.; Tamiya, E. Amyloid-β detection with saccharide immobilized gold nanoparticle on carbon electrode. Bioelectrochemistry 2008, 74, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Cheng, H.Y. Recent Developments of Flexible and Stretchable Electrochemical Biosensors. Micromachines 2020, 11, 243. [Google Scholar] [CrossRef]
- Pungetmongkol, P.P.; Yamamoto, T. Single-Molecule Detection of DNA in a Nanochannel by High-Field Strength-Assisted Electrical Impedance Spectroscopy. Micromachines 2019, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Chinnadayyala, S.R.; Park, J.; Kim, Y.H.; Choi, S.H.; Lee, S.M.; Cho, W.W.; Lee, G.Y.; Pyun, J.C.; Cho, S. Electrochemical Detection of C-Reactive Protein in Human Serum Based on Self-Assembled Monolayer-Modified Interdigitated Wave-Shaped Electrode. Sensors 2019, 19, 5560. [Google Scholar] [CrossRef] [PubMed]
- Brosel-Oliu, S.; Abramova, N.; Uria, N.; Bratov, A. Impedimetric transducers based on interdigitated electrode arrays for bacterial detection—A review. Anal. Chim. Acta 2019, 1088, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Park, J.; Chinnadayyala, S.R.; Cho, S. Sensitive electrochemical detection of amyloid beta peptide in human serum using an interdigitated chain-shaped electrode. Biosens. Bioelectron. 2019, 144, 111694. [Google Scholar]
- Huang, S.Y.; Chou, C.M.; Chen, T.H.; Chiou, P.C.; Hsiao, V.K.S.; Ching, C.T.S.; Sun, T.P. Enhanced Sensitivity Using Microfluidic, Interdigitated Microelectrode Based Capacitance Glucose Sensor Measured at 4 MHz. J. Electrochem. Soc. 2014, 161, B102–B105. [Google Scholar] [CrossRef]
- Nair, N.G.; Perry, G.; Smith, M.A.; Reddy, V.P. NMR studies of zinc, copper, and iron binding to histidine, the principal metal ion complexing site of amyloid-beta peptide. J. Alzheimers Dis. 2010, 20, 57–66. [Google Scholar] [CrossRef]
- Lermyte, F.; Everett, J.; Lam, Y.P.; Wootton, C.A.; Brooks, J.; Barrow, M.P.; Telling, N.D.; Sadler, P.J.; O’Connor, P.B.; Collingwood, J.F. Metal Ion Binding to the Amyloid β Monomer Studied by Native Top-Down FTICR Mass Spectrometry. Am. Soc. Mass Spectrom. 2019, 30, 2123–2134. [Google Scholar] [CrossRef]
- Rushworth, J.V.; Ahmed, A.; Griffiths, H.H.; Pollock, N.M.; Hooper, N.M.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 2014, 56, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Kerman, K.; Saito, M.; Nagatani, N.; Takamura, Y.; Tamiya, E. A Rapid Label-Free Electrochemical Detection and Kinetic Study of Alzheimer’s Amyloid Beta Aggregation. J. Am. Chem. Soc. 2005, 127, 11892–11893. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Heli, H. An electrochemical peptide-based biosensor for the Alzheimer biomarker amyloid-β(1-42) using a microporous gold nanostructure. Microchim. Acta 2019, 186, 766. [Google Scholar] [CrossRef] [PubMed]
- Newton, L.; Slater, T.; Clark, N.; Vijayaraghavan, A. Self-assembled monolayers (SAMs) on metallic surfaces (gold and graphene) for electronic applications. J. Mater. Chem. C 2013, 1, 376–393. [Google Scholar] [CrossRef]
- Ahmad, N.; Souhir, S.; Rashad, A.G.; Viktor, K.; Muataz, A.A. Enhanced Fouling Resistance and Antibacterial Properties of Novel Graphene Oxide-Arabic Gum Polyethersulfone Membranes. Appl. Sci. 2019, 9, 513. [Google Scholar]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Lou, X.; Xu, M.; Freeman, C.; James, T.; Davis, J.J. Ultrasensitive Label Free Electrical Detection of Insulin in Neat Blood Serum. Anal. Chem. 2013, 85, 4129–4134. [Google Scholar]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Yin, H.; Melissa, C.B.; Ian, I.S. Impedance Biosensor for Peanut Protein Ara h1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar]
- Tao, Z.; Luitgard, N.G.; Dieter, W. Solution-Based Determination of Dissociation Constants for the Binding of Aβ42 to Antibodies. ChemistryOpen 2019, 8, 989–994. [Google Scholar]
- Guanglei, L.; Yang, S.; Wubin, Z.; Jiajia, Y.; Chunxia, L.; Jun, L. Fluorescence Detection and Dissociation of Amyloid-β Species for the Treatment of Alzheimer’s Disease. Adv. Ther. 2019, 2, 1900054. [Google Scholar]
- Wahlberg, E.; Rahman, M.M.; Lindberg, H.; Gunneriusson, E.; Schmuck, B.; Lendel, C.; Sandgren, M.; Löfblom, J.; Ståhl, S.; Härd, T. Identifcation of proteins that specifcally recognize and bind protofbrillar aggregates of amyloid-β. Sci. Rep. 2017, 7, 5949. [Google Scholar]



| Transducer | Immobilization | Detection | Redox Probe/Electrolyte | LOD (pg mL−1) | LRD (pg mL−1) | Refs |
|---|---|---|---|---|---|---|
| Carbon disposable electrochemical printed chip | SAM a-AuNPs b | Electrochemical impedance spectroscopy | K3[Fe(CN)]6/K4[Fe(CN)]6 in KCl | 2.6 × 103 ## | 45–4.5 × 105 | [18] |
| Gold electrode | Peptide probe (11-mercaptou-ndecanoic acid + Peptide chain + Ferrocene)/9-mercapto-1-nonanol | Square-Wave Voltammetry | 0.1 M KCl containing 5 mM Fe(CN)63−/4− | 1.1 × 103 # | 2.2 × 103–5.4 × 104 | [20] |
| Carbon electrode | AuNPs b/S-AM a formation of the acetylenyl group/cycloaddition reaction of an azide-terminated sialic acid | Differential Pulse Voltammetry | K3[Fe(CN)6] in PBS (pH 7.4) | 4.5 × 106 # | 2.3 × 106–4.5 × 107 | [21] |
| Interdigitate-d chain-shaped electrode | SAM a | Faradaic detection | Fe(CN)63−/4− in PBS (pH 7.4) | 100 | 1–106 | [27] |
| Glassy carbon electrode | Direct immobilization on the electrode surface | Square-Wave Voltammetry | 20 mM Tris/HCl buffer, pH 7.0 (TBS) | 7.0 × 105 | 2.8 × 106–1.6 × 107 | [32] |
| Au c electrode | Microporous Au c nanostructure/SAM a | Differential Pulse Voltammetry | Fe(CN)63−/4− in KCl | 0.2 | 3–7000 | [33] |
| Interdigitate-d chain-shaped electrode | SAM a | Non-Faradaic detection | No probe | 7.5 | 10–104 | This work |
| Method | inding Partner | Kd (nM) | Refs |
|---|---|---|---|
| AUC a | antibody 6E10 | 30.1; 13.2; 63.3 | [42] |
| MST b | antibody 4G8 | 11.3; 1.0; 45.5 | |
| SPR c | antibody 12F4 | 14.6; 4.7; 37.1 | |
| Fluorescent | NIAD-4 d | 10 | [43] |
| CRANAD-2 e | 38.7 | ||
| DANIR-2c f | 26.9 | ||
| SPR c | Affibody molecules | 1–3 | [44] |
| Capacitive biosensor | antibody MOAB-2 | 0.014; 0.016 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.T.N.; Park, J.; Cho, S. A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines 2020, 11, 791. https://doi.org/10.3390/mi11090791
Le HTN, Park J, Cho S. A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines. 2020; 11(9):791. https://doi.org/10.3390/mi11090791
Chicago/Turabian StyleLe, Hien T. Ngoc, Jinsoo Park, and Sungbo Cho. 2020. "A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode" Micromachines 11, no. 9: 791. https://doi.org/10.3390/mi11090791
APA StyleLe, H. T. N., Park, J., & Cho, S. (2020). A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines, 11(9), 791. https://doi.org/10.3390/mi11090791






