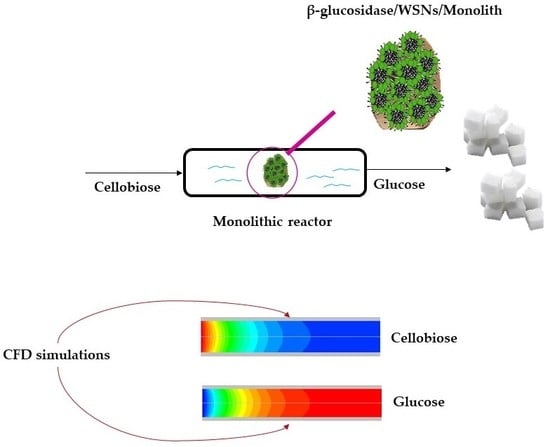
CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme
Abstract
1. Introduction
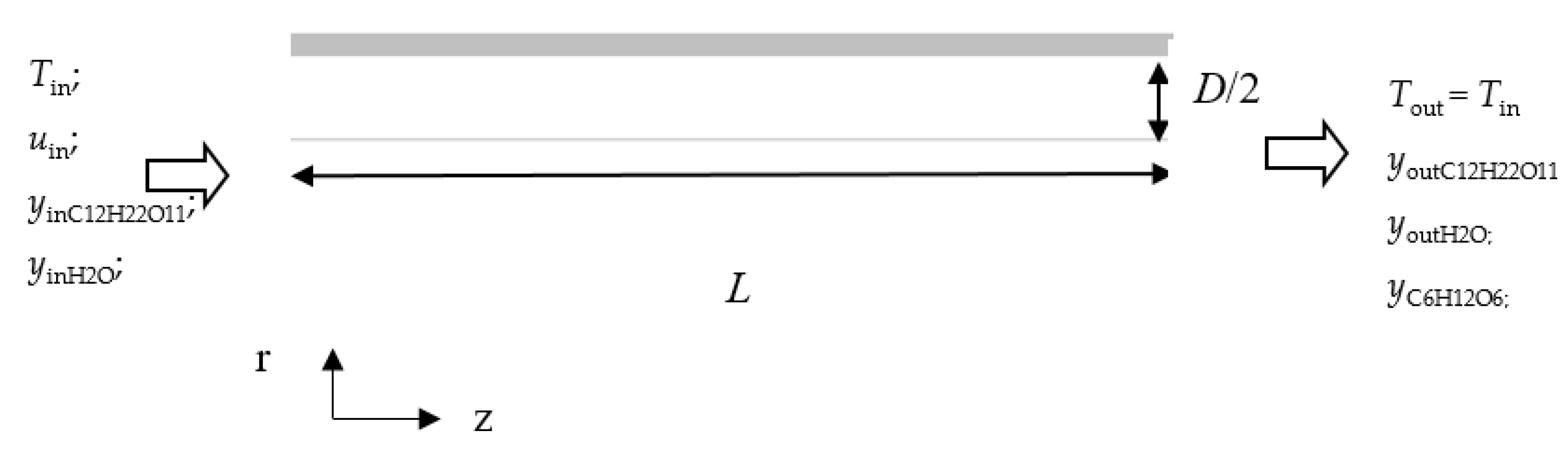
2. Model Description
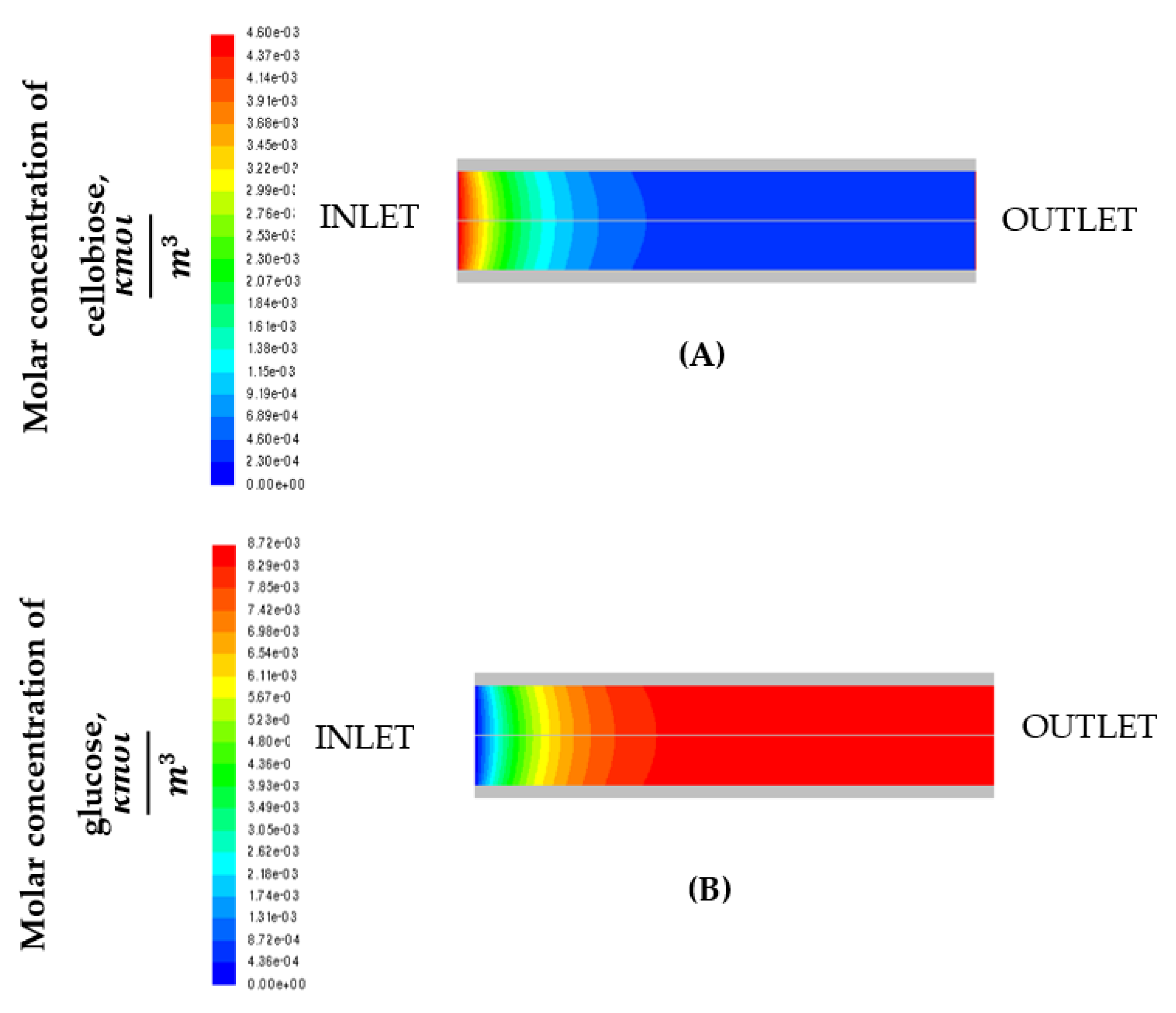
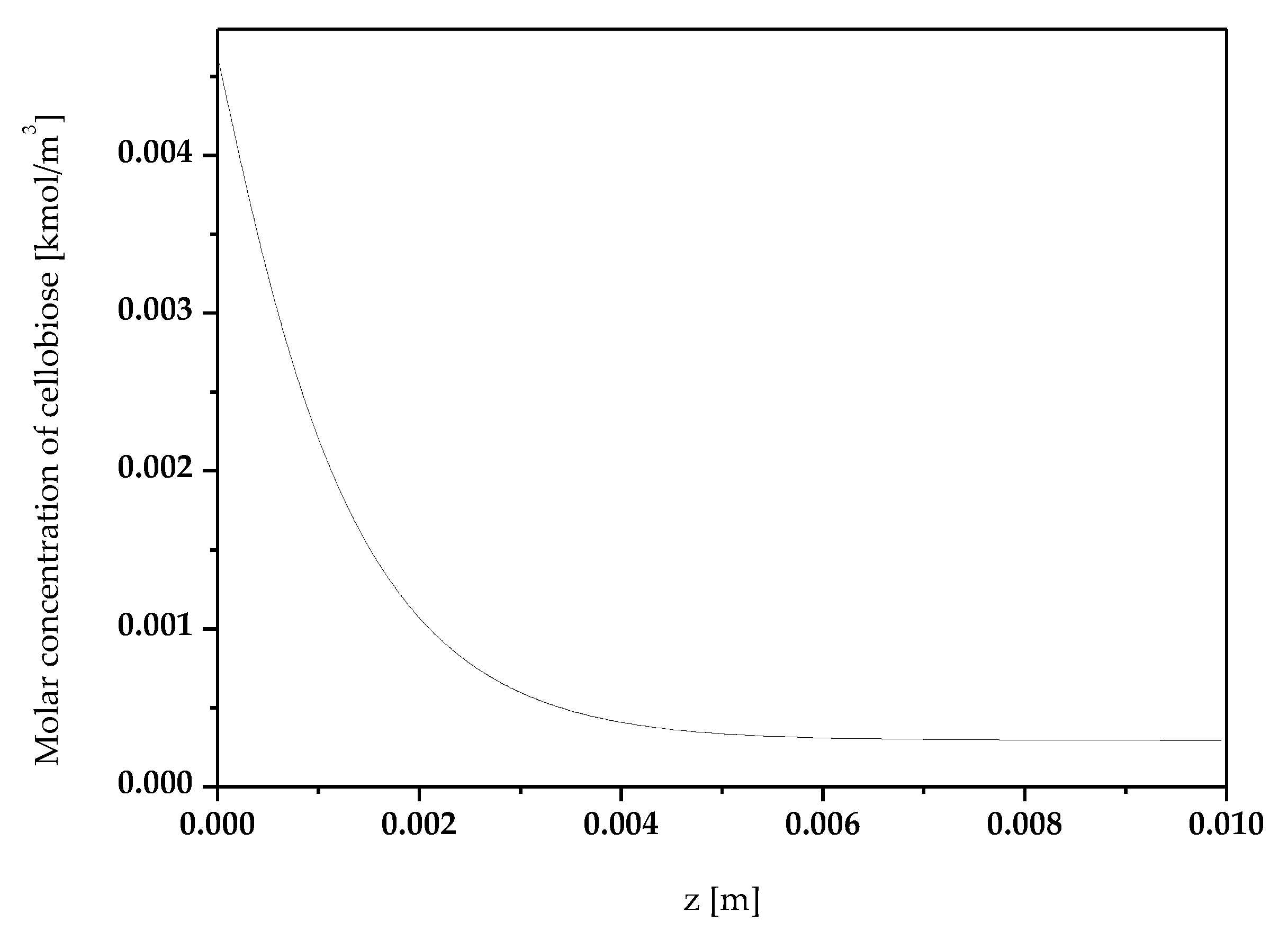
3. Results and Discussion
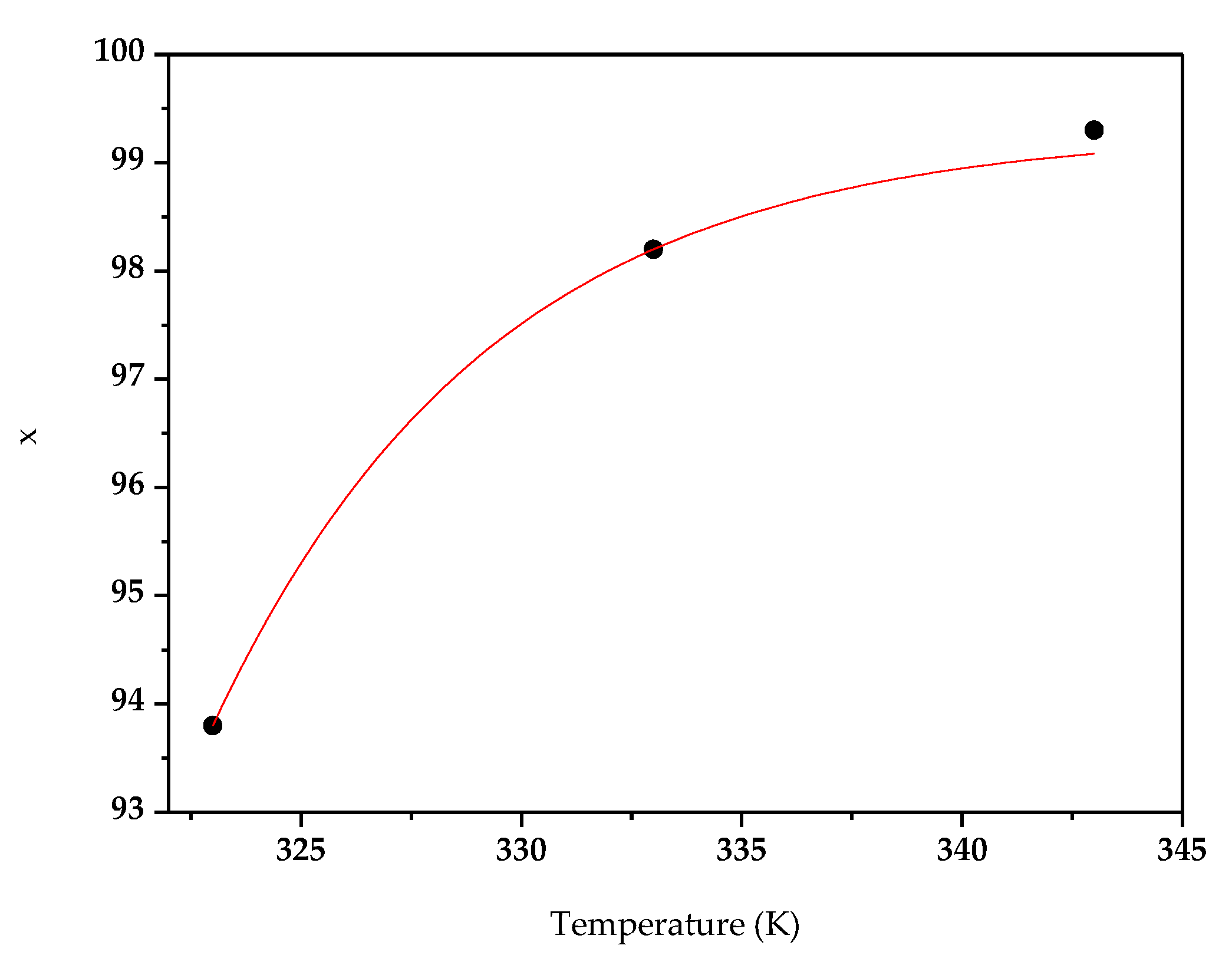
3.1. Effect of Temperature
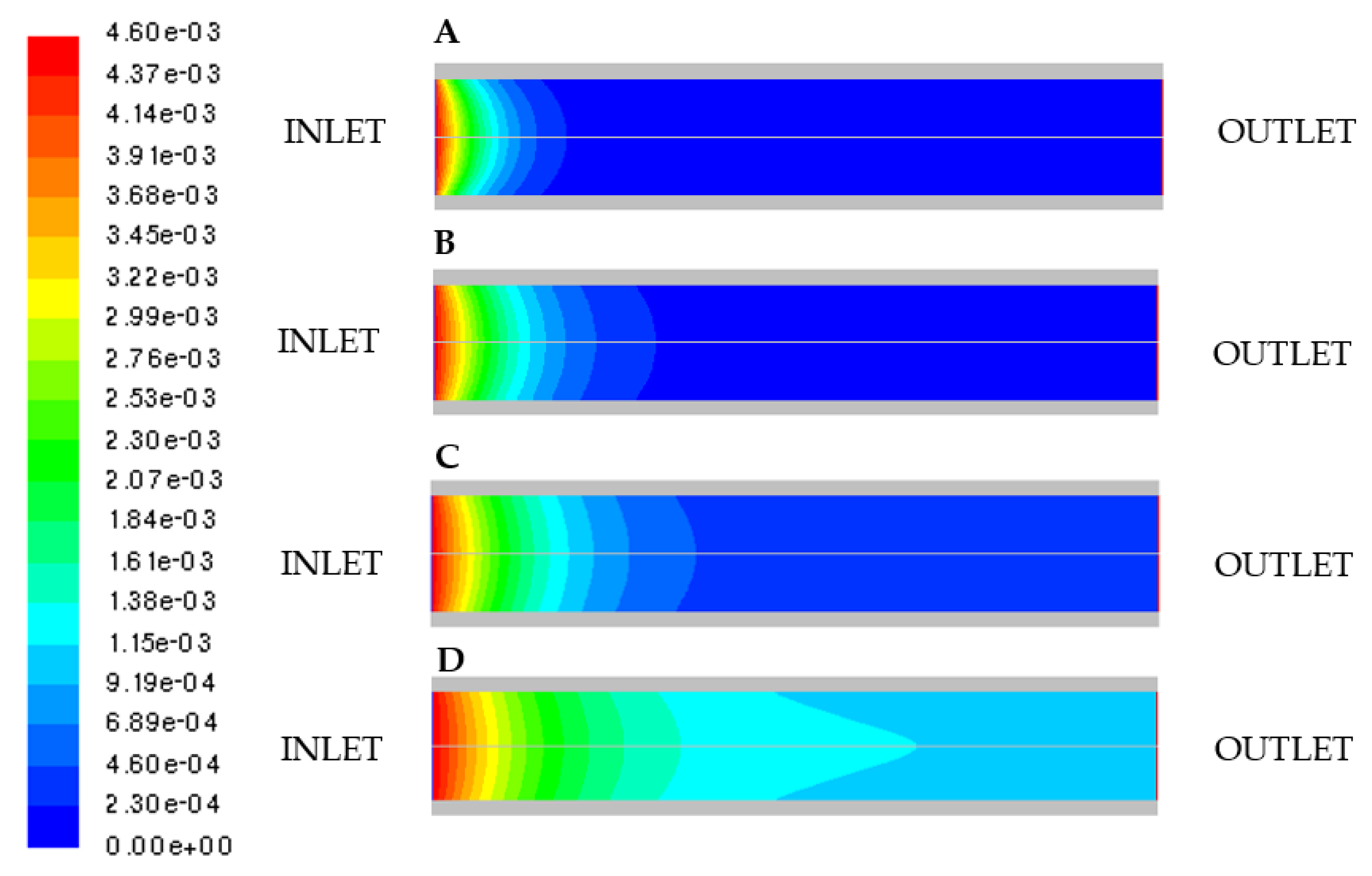
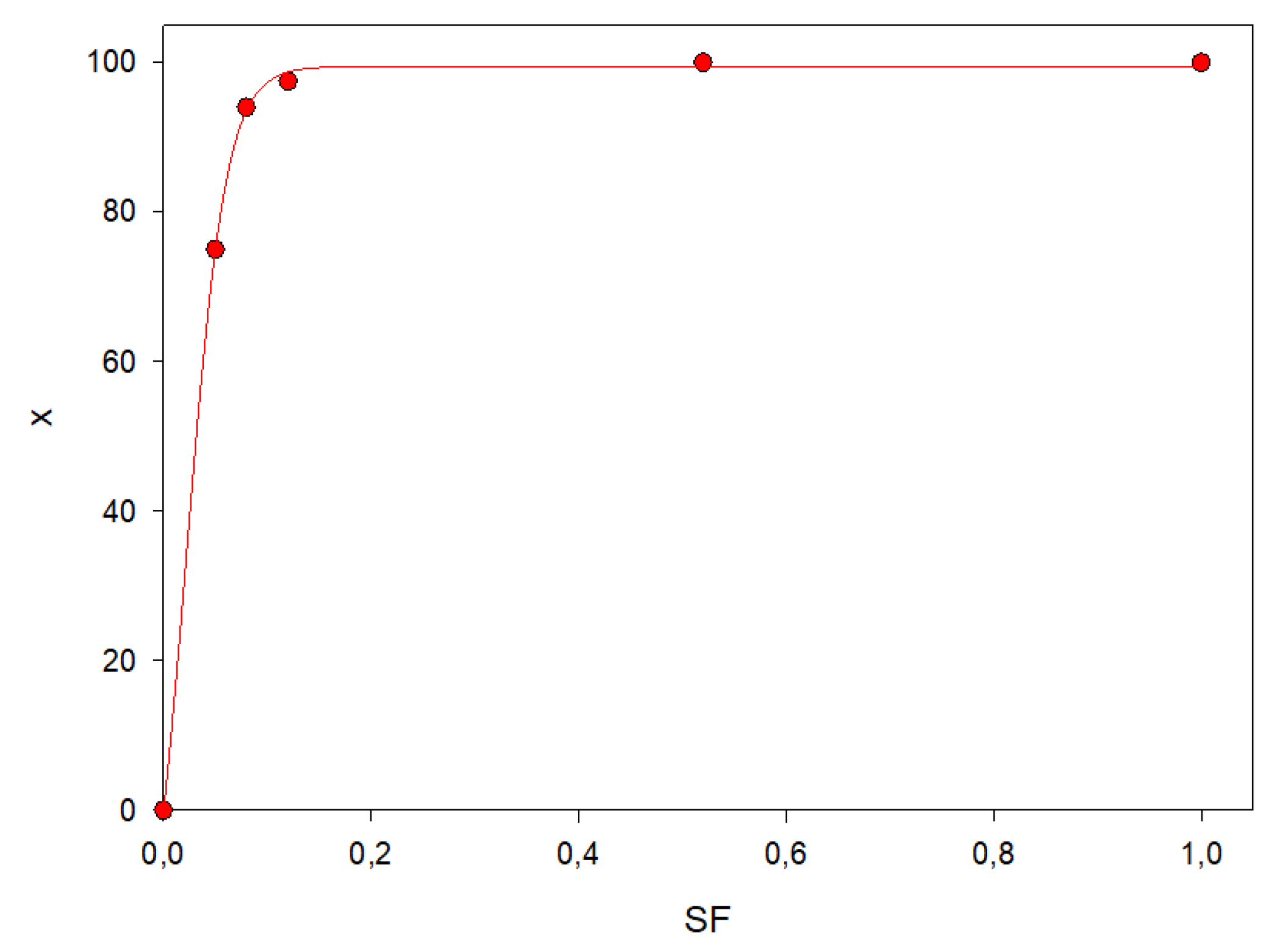
3.2. Effect of Enzyme Immobilization Yield
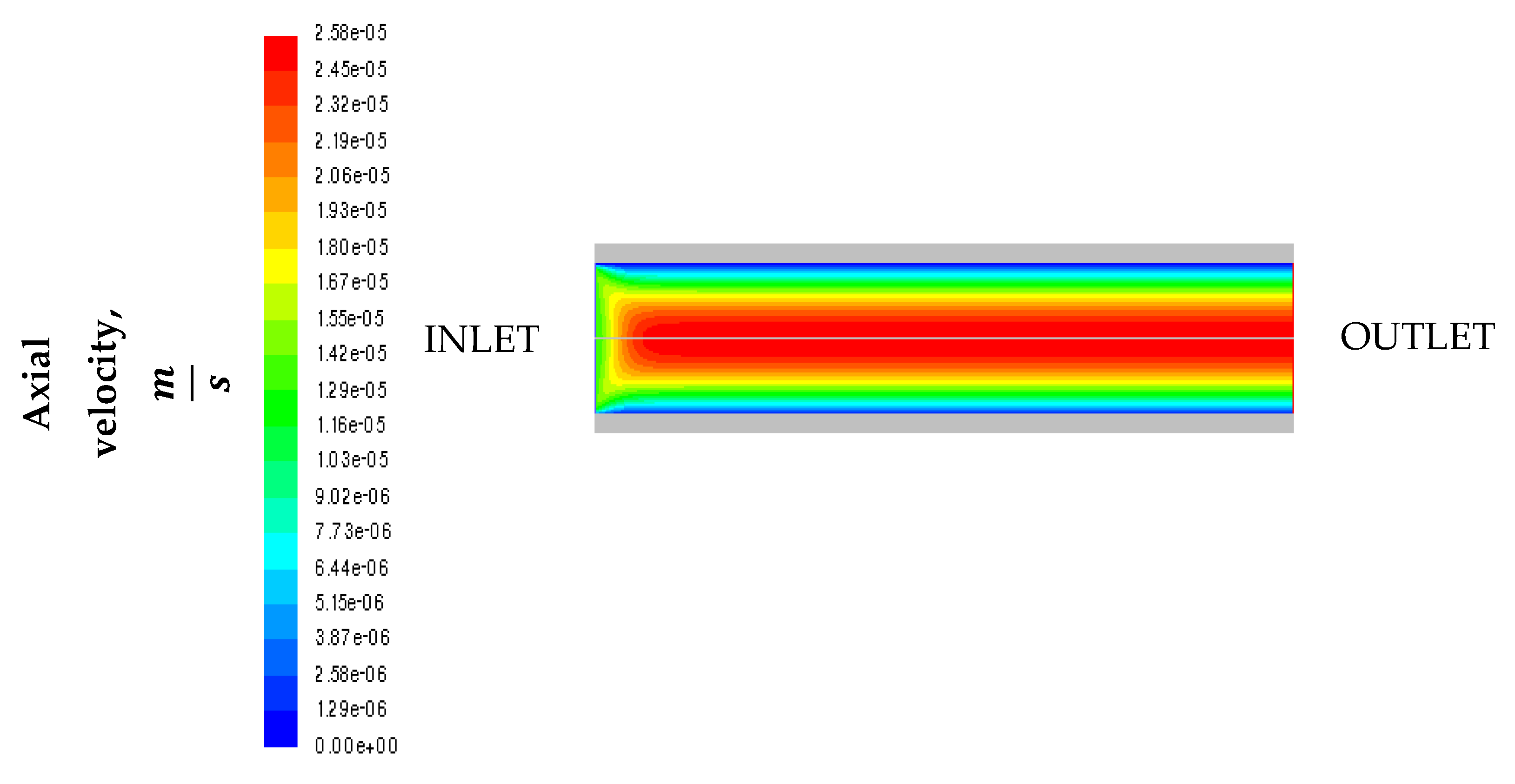
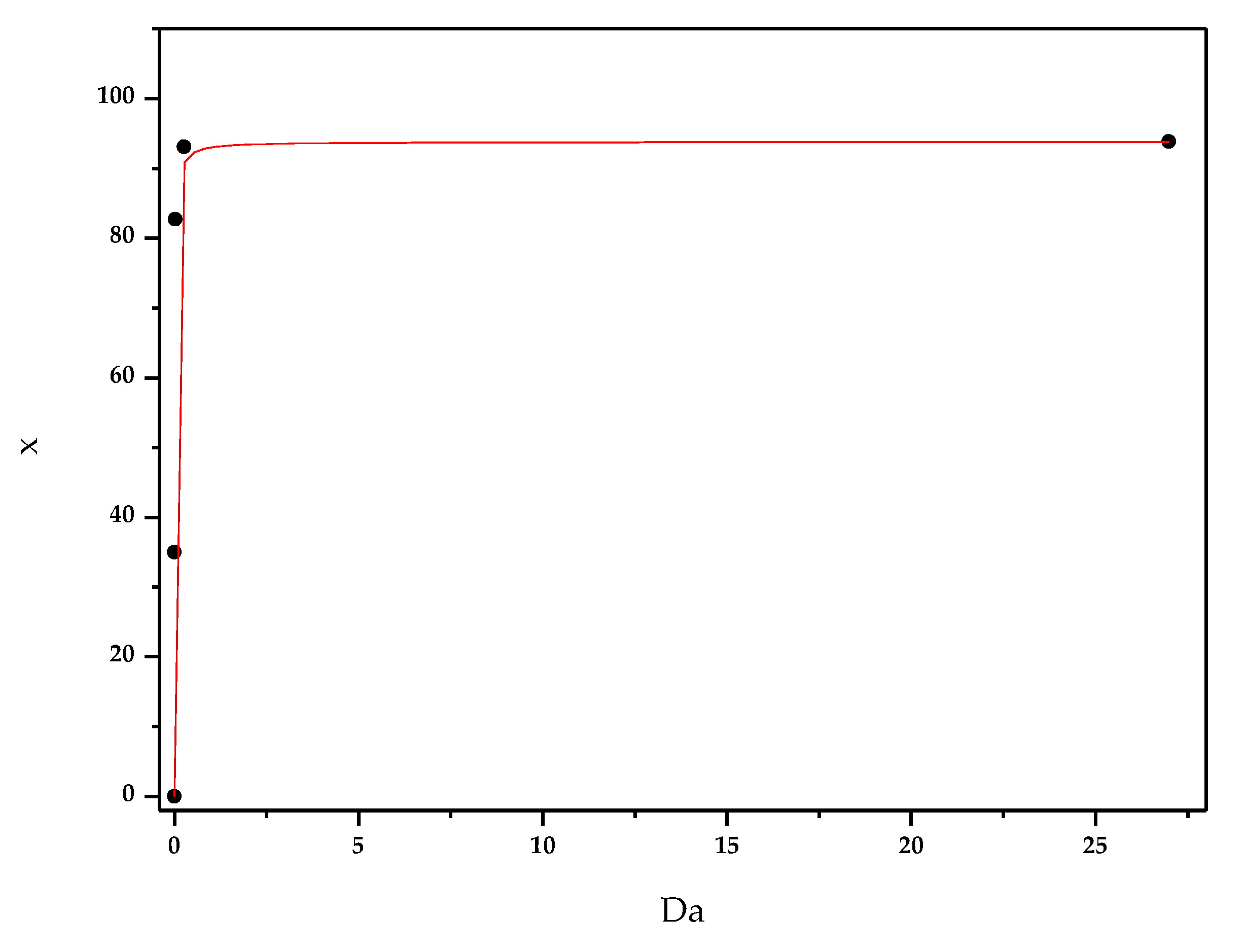
3.3. Effect of Mixture Inlet Velocity
4. Conclusions
- The temperature of the reaction;
- The catalyst loading by evaluating the SF factor;
- The mixture inlet velocity.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Gao, K.; Zhang, C.; Lang, W. Alternative Fuels for IC Engines and Jet Engines and Comparison of Their Gaseous and Particulate Matter Emissions; Woodhead Publishing: Sarston, UK, 2019; ISBN 9780081027912. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Woodward, J. Immobilized cellulases for cellulose utilization. J. Biotechnol. 1989, 11, 299–311. [Google Scholar] [CrossRef]
- Venezia, V.; Sannino, F.; Costantini, A.; Silvestri, B.; Cimino, S.; Califano, V. Mesoporous silica nanoparticles for β-glucosidase immobilization by templating with a green material: Tannic acid. Microporous Mesoporous Mater. 2020, 302. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled Silica Nanoparticles: Efficient Matrix for β-Glucosidase Immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Miyazaki, M.; Honda, T.; Yamaguchi, H.; Briones, M.P.P.; Maeda, H. Enzymatic processing in microfluidic reactors. Biotechnol. Genet. Eng. Rev. 2008, 25, 405–428. [Google Scholar] [CrossRef]
- Seo, T.Y.; Eum, K.W.; Han, S.O.; Kim, S.W.; Kim, J.H.; Song, K.H.; Choe, J. Immobilized cell microchannel bioreactor for evaluating fermentation characteristics of mixed substrate consumption and product formation. Process Biochem. 2012, 47, 1011–1015. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Nidetzky, B. Coated-wall microreactor for continuous biocatalytic transformations using immobilized enzymes. Biotechnol. J. 2009, 4, 98–107. [Google Scholar] [CrossRef]
- Barsan, M.M.; David, M.; Florescu, M.; Ţugulea, L.; Brett, C.M.A. A new self-assembled layer-by-layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 2014, 99, 46–52. [Google Scholar] [CrossRef]
- Kecskemeti, A.; Gaspar, A. Preparation and characterization of a packed bead immobilized trypsin reactor integrated into a PDMS microfluidic chip for rapid protein digestion. Talanta 2017, 166, 275–283. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. Structuring CuO/CeO2 Catalyst as Option to Improve Performance Towards CO-PROX. Top. Catal. 2016, 59, 1371–1382. [Google Scholar] [CrossRef]
- Křenková, J.; Foret, F. Immobilized microfluidic enzymatic reactors. Electrophoresis 2004, 25, 3550–3563. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, Y.; Zhuang, W.; Li, G.; Jiang, M.; Zhang, H. Improving the performance of immobilized β-glucosidase using a microreactor. J. Biosci. Bioeng. 2018, 125, 377–384. [Google Scholar] [CrossRef]
- Lévesque, F.; Seeberger, P.H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 2012, 51, 1706–1709. [Google Scholar] [CrossRef]
- Venezia, V.; Costantini, A.; Landi, G.; Di Benedetto, A.; Sannino, F.; Califano, V. Immobilization of β-glucosidase over structured cordierite monoliths washcoated with wrinkled silica nanoparticles. Catalysts 2020, 10, 889. [Google Scholar]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol Gel Sci. Technol. 2014, 73, 54–61. [Google Scholar] [CrossRef]
- Díaz, J.F.; Balkus, K.J., Jr. Enzyme immobilization in MCM-41 molecular sieve. J. Mol. Catal. B Enzym. 1996, 2, 115–126. [Google Scholar] [CrossRef]
- Hartono, S.B.; Qiao, S.Z.; Liu, J.; Jack, K.; Ladewig, B.P.; Hao, Z.; Lu, G.Q.M. Functionalized mesoporous silica with very large pores for cellulase immobilization. J. Phys. Chem. C 2010, 114, 8353–8362. [Google Scholar] [CrossRef]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the Eumelanin-Silica Interface in Chemically Engineered Bulk Hybrid Nanoparticles for Targeted Subcellular Antioxidant Protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A.; Silvestri, B.; Venezia, V.; Cimino, S.; Sannino, F. The effect of pore morphology on the catalytic performance of β-glucosidase immobilized into mesoporous silica. Pure Appl. Chem. 2019. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent immobilization of β-glucosidase into mesoporous silica nanoparticles from anhydrous acetone enhances its catalytic performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef]
- Moon, D.-S.; Lee, J.-K. Tunable synthesis of hierarchical mesoporous silica nanoparticles with radial wrinkle structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.; Chen, Y. Highly Active Monolith Catalysts of LaKCoO3 Perovskite-type Complex Oxide on Alumina-washcoated Diesel Particulate Filter and the Catalytic Performances for the Combustion of Soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- An, H.; Li, A.; Sasmito, A.P.; Kurnia, J.C.; Jangam, S.V.; Mujumdar, A.S. Computational fluid dynamics (CFD) analysis of micro-reactor performance: Effect of various configurations. Chem. Eng. Sci. 2012, 75, 85–95. [Google Scholar] [CrossRef]
- Chen, J.; Gao, X.; Yan, L.; Xu, D. Computational fluid dynamics modeling of the millisecond methane steam reforming in microchannel reactors for hydrogen production. Rsc. Adv. 2018, 8, 25183–25200. [Google Scholar] [CrossRef]
- Zhai, X.; Ding, S.; Chang, Y.; Jin, Y.; Cheng, Y. CFD simulation with detailed chemistry of steam reforming of methane for hydrogen production in an integrated micro-reactor. Int. J. Hydrog. Energy 2010, 35, 5383–5392. [Google Scholar] [CrossRef]
- Gómez, L.E.; Sollier, B.M.; Lacoste, A.M.; Miró, E.E.; Boix, A.V. Hydrogen purification for fuel cells through CO preferential oxidation using PtCu/Al2O3 structured catalysts. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Alftrén, J.; Hobley, T.J. Covalent immobilization of β-Glucosidase on Magnetic particles for Lignocellulose Hydrolysis. Appl. Biochem. Biotechnol. 2013, 169, 2076–2087. [Google Scholar] [CrossRef]

| ΔHi, kJ/mol | νi | c, mol/m3 | cp, (kJ/(kg*K) | ρ, kg/m3 | |
|---|---|---|---|---|---|
| Cellobiose | −5401.50 | −1 | 4.67 | ||
| Water | −285.85 | −1 | |||
| Glucose | −1273.30 | 2 | |||
| Mixture | 4.186 | 1000 |
| Species | Stoichiometric Coefficients | Rate Exponent |
|---|---|---|
| Cellobiose | 1 | 1 |
| H2O | 1 | 0 |
| Glucose | 2 | 0 |
| Mesh Parameters | Number |
|---|---|
| Cells | 12,006 |
| Faces | 30,029 |
| Nodes | 12,692 |
| Partitions | 1 |
| Parameter | Value |
|---|---|
| Mixture inlet temperature, Tin (K) | 323–343 |
| Mixture inlet velocity, uin (m s−1) | 0.13; 0.013; 1.3 × 10−3; 1.3 × 10−5 |
| Inlet C12H22O11 molar fraction | 8.4 × 10−5 |
| Surface area washcoat factor | 0.0375–0.075–0.15–0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venezia, V.; Califano, V.; Pota, G.; Costantini, A.; Landi, G.; Di Benedetto, A. CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme. Micromachines 2020, 11, 790. https://doi.org/10.3390/mi11090790
Venezia V, Califano V, Pota G, Costantini A, Landi G, Di Benedetto A. CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme. Micromachines. 2020; 11(9):790. https://doi.org/10.3390/mi11090790
Chicago/Turabian StyleVenezia, Virginia, Valeria Califano, Giulio Pota, Aniello Costantini, Gianluca Landi, and Almerinda Di Benedetto. 2020. "CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme" Micromachines 11, no. 9: 790. https://doi.org/10.3390/mi11090790
APA StyleVenezia, V., Califano, V., Pota, G., Costantini, A., Landi, G., & Di Benedetto, A. (2020). CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme. Micromachines, 11(9), 790. https://doi.org/10.3390/mi11090790