A Novel Electroporation System for Living Cell Staining and Membrane Dynamics Interrogation
Abstract
1. Introduction
1.1. Electroporation
1.2. Focal Adhesion
1.3. Cell Motility
1.4. Membrane Resealing
1.5. Electroporation Chip
2. Material and Method
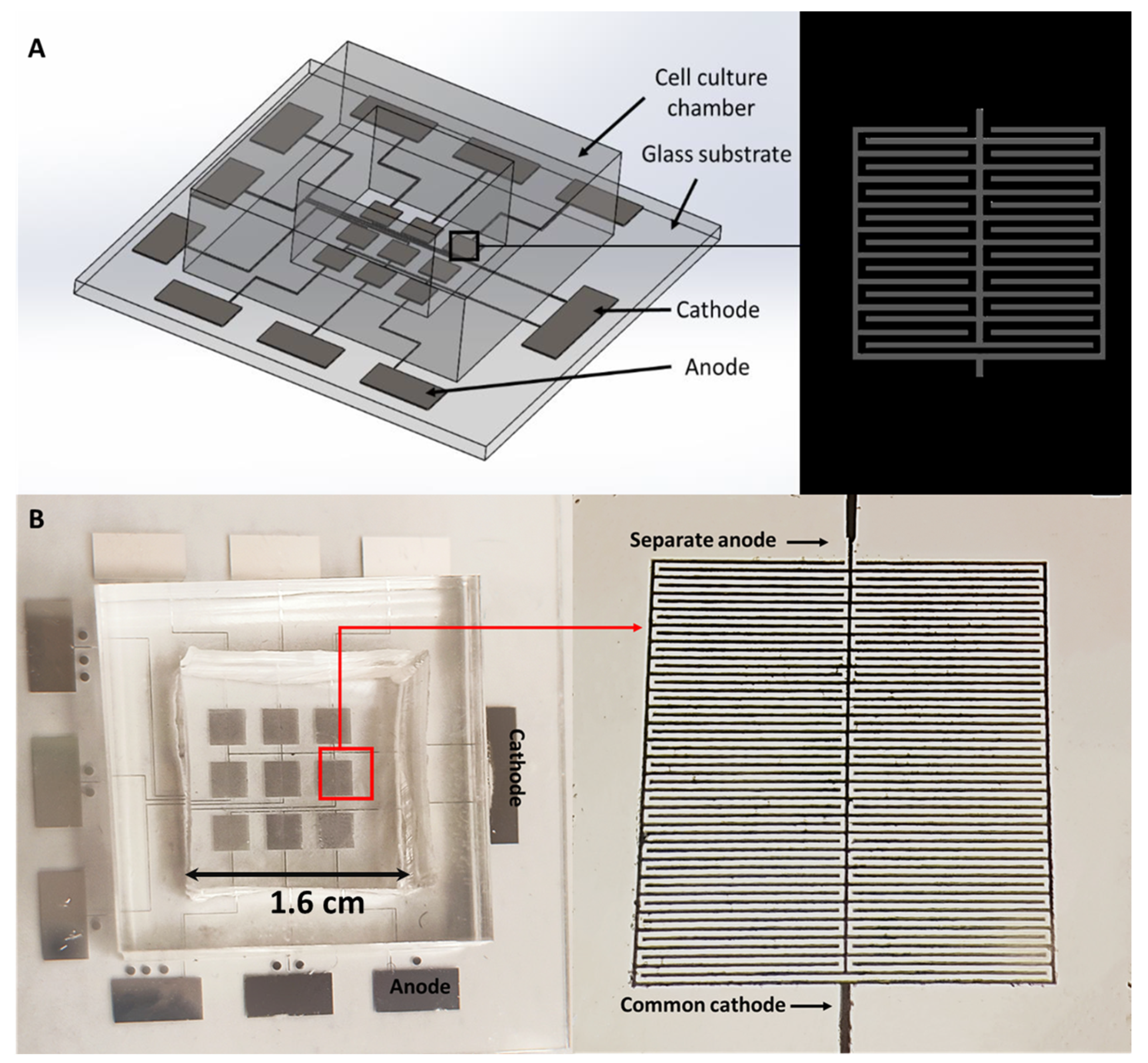
2.1. Fabrication of the Microelectrodes on a Glass Substrate
2.2. Program-Controlled Pulse Generator
2.3. Cell Culture
2.4. Plasmid Extraction
3. Results and Discussion
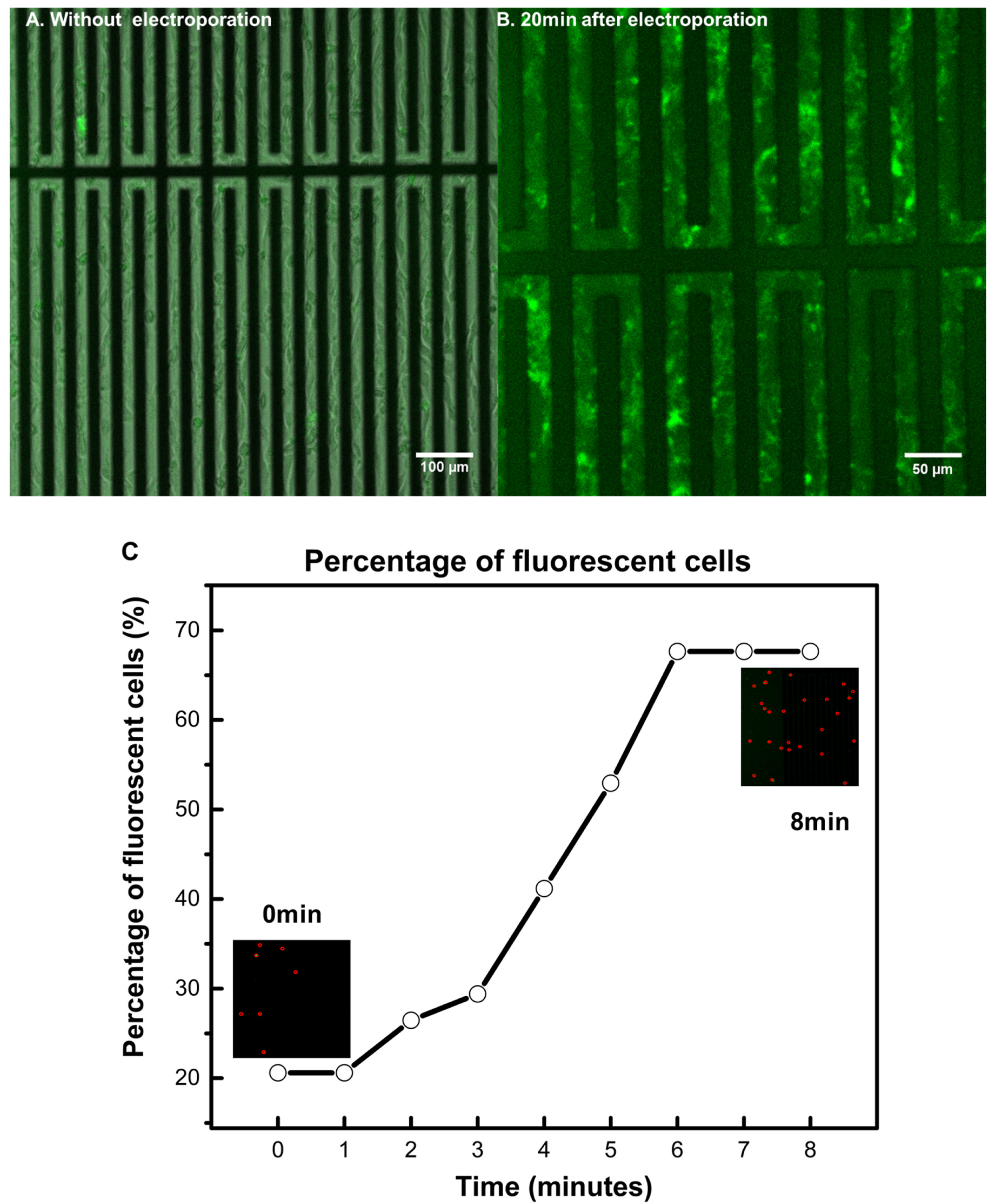
3.1. Characterizing the Resealing of Membrane Pores Induced by Electroporation
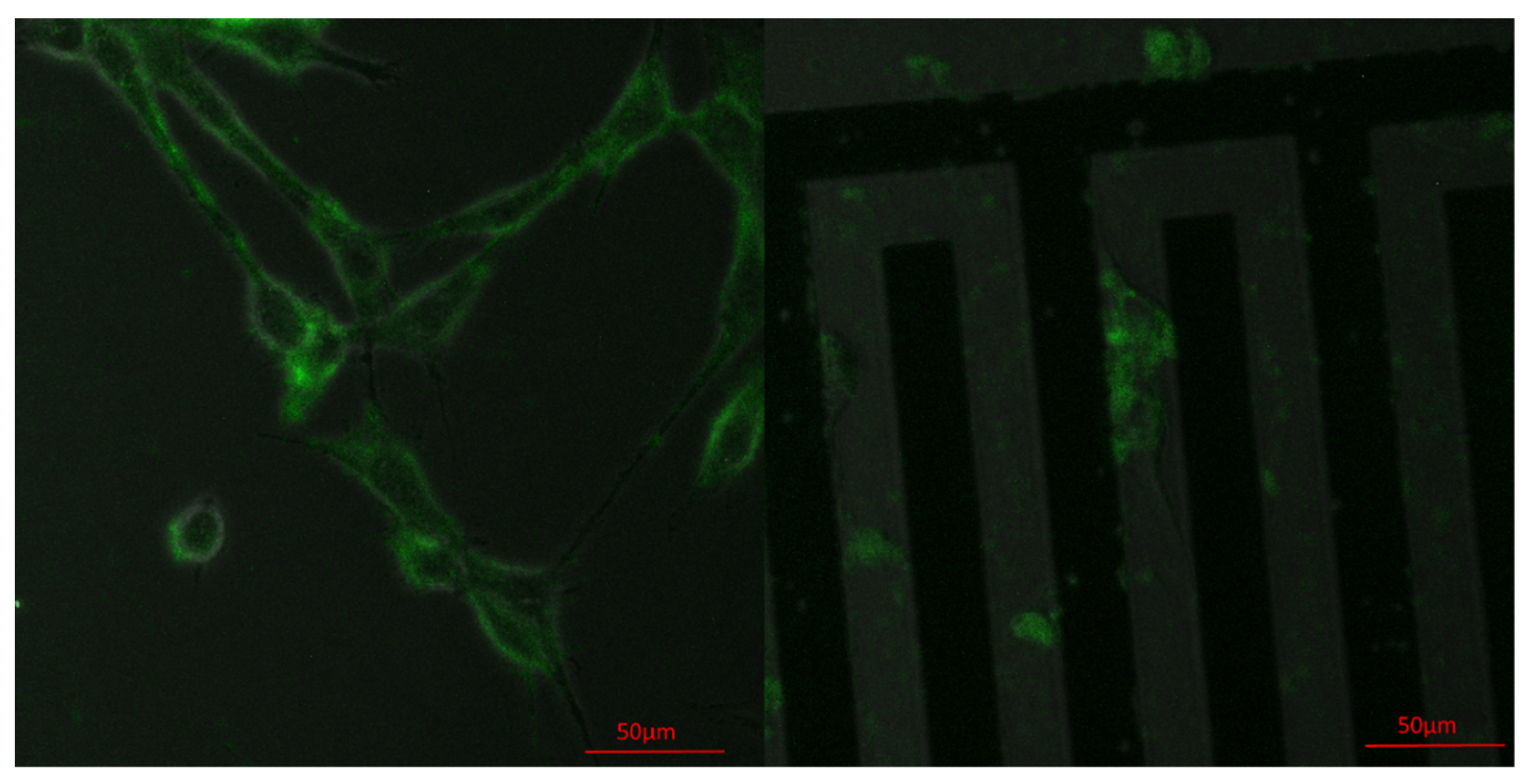
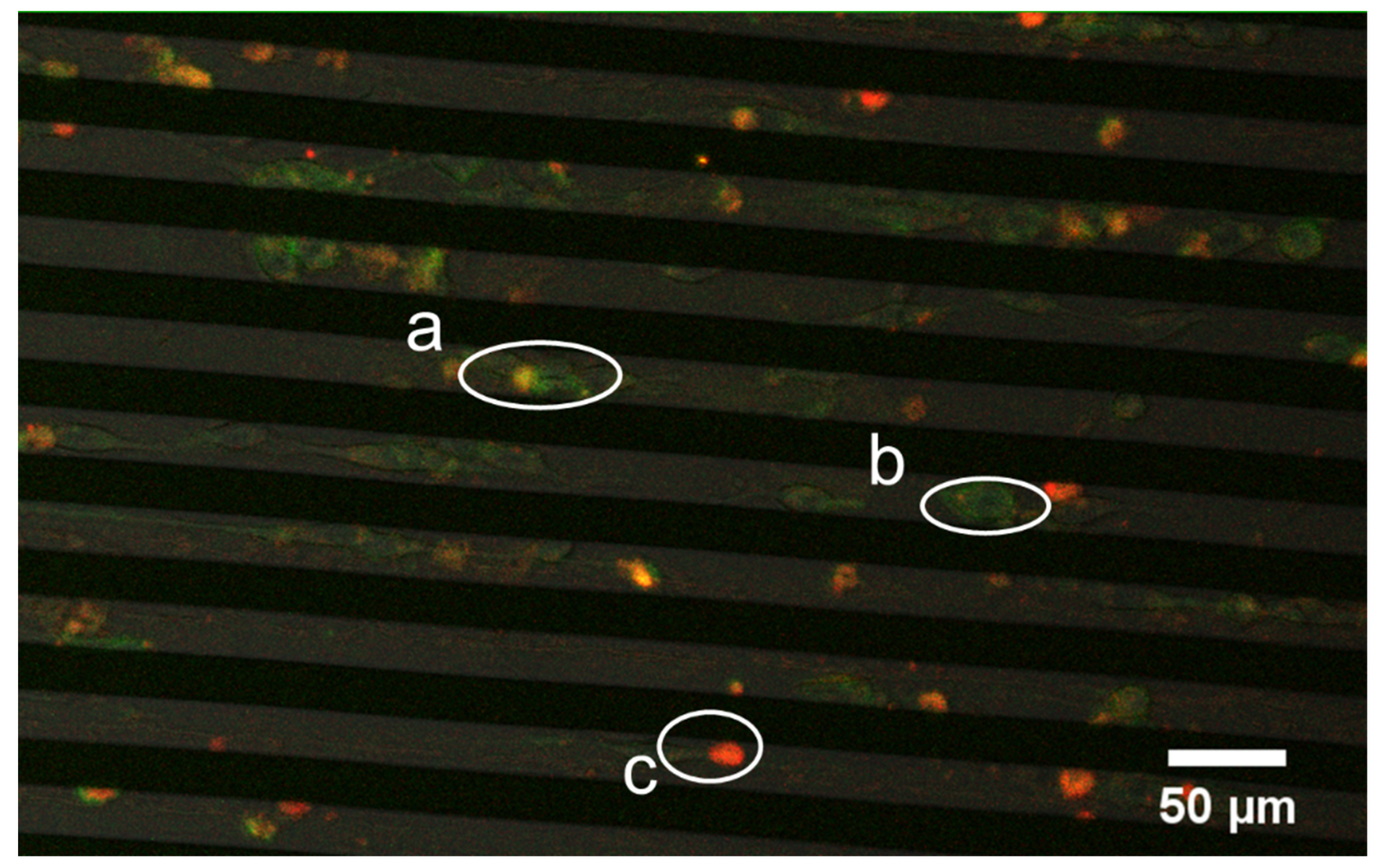
3.2. Achieving Live Cell F-Actin Staining
3.3. Achieving Fast Electrotransfection
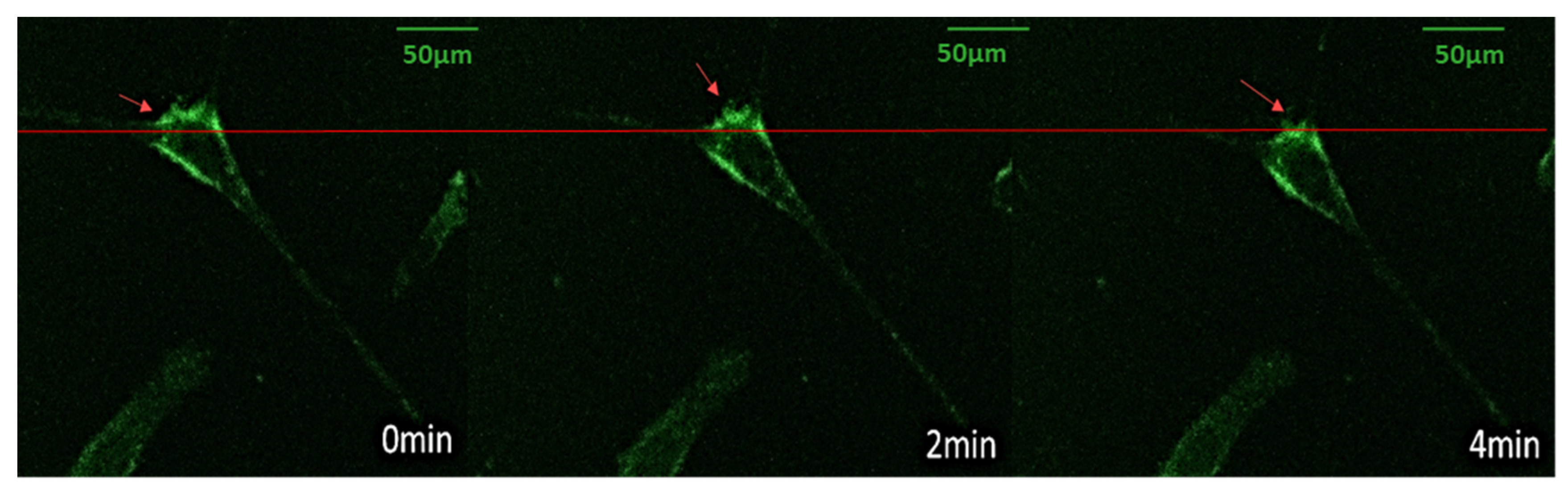
3.4. Vinculin Dynamics during Cell Migration
3.5. Successive Labelling of Vinculin and F-Actin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible electroporation: A new ablation modality—Clinical implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Dermol-Černe, J.; Batista Napotnik, T.; Reberšek, M.; Miklavčič, D. Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field. Sci. Rep. 2020, 10, 9149. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Sersa, G.; Kotnik, T.; Miklavcic, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Ann. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Heller, L.C. Gene electrotransfer clinical trials. Adv. Genet. 2015, 89, 235–262. [Google Scholar] [PubMed]
- Kotnik, T.; Frey, W.; Sack, M.; Meglic, S.H.; Peterka, M.; Miklavcic, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Hui, T.H.; Zhou, Z.L.; Fong, H.W.; Ngan, R.K.C.; Lee, T.Y.; Au, J.S.K.; Ngan, A.H.W.; Yip, T.T.C.; Lin, Y. Characterizing the malignancy and drug resistance of cancer cells from their membrane resealing response. Sci. Rep. 2016, 6, 26692. [Google Scholar] [CrossRef]
- Yan, Z.; Hui, T.H.; Fong, H.W.; Shao, X.; Cho, W.C.; Ngan, K.C.; Yip, T.C.; Lin, Y. An electroporation platform for Erlotinib resistance screening in living non-small cell lung cancer (NSCLC) cells. Biomed. Phys. Eng. Express 2018, 4, 6. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Li, S.; Wang, K.; Ma, F.; Tang, B. Potential application development of Sr/HCOOH metal organic framework in osteoarthritis. Microporous Mesoporous Mater. 2020, 294, 109835. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, R. Controllable In-Situ cell electroporation with cell positioning and impedance monitoring using micro electrode array. Sci. Rep. 2016, 6, 31392. [Google Scholar] [CrossRef]
- Cheng, Z.; Kuru, E.; Sachdeva, A.; Vendrell, M. Fluorescent amino acids as versatile building blocks for chemical biology. Nat. Rev. Chem. 2020, 4, 275–290. [Google Scholar] [CrossRef]
- Wu, R.G.; Yang, C.S.; Cheing, C.C.; Tseng, F.G. Nanocapillary electrophoretic electrochemical chip: Towards analysis of biochemicals released by single cells. Interface Focus 2011, 1, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fu, A.; Yossifon, G. Active particles as mobile microelectrodes for selective bacteria electroporation and transport. Sci. Adv. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D. Chapter One—The Dynamic Actin Cytoskeleton in Smooth Muscle. In Advances in Pharmacology; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 81, pp. 1–38. [Google Scholar]
- Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Chapter Five—Mechanical Cues Direct Focal Adhesion Dynamics. In Progress in Molecular Biology and Translational Science; Engler, A.J., Kumar, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 126, pp. 103–134. [Google Scholar]
- Plotnikov, V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 2012, 115, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Case, L.B.; Baird, M.A.; Shtengel, G.; Campbell, S.L.; Hess, H.F.; Davidson, M.W.; Waterman, C.M. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat. Cell Biol. 2015, 17, 880–892. [Google Scholar] [CrossRef]
- Goldmann, W.H. Role of vinculin in cellular mechanotransduction. Cell Biol. Int. 2016, 40, 241–256. [Google Scholar] [CrossRef]
- Padhi, A.; Singh, K.; Franco-Barraza, J.; Marston, D.J.; Cukierman, E.; Hahn, K.M.; Kapania, R.K.; Nain, A.S. Force-exerting perpendicular lateral protrusions in fibroblastic cell contraction. Commun. Biol. 2020, 3, 390. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Yang, L.; Gong, Z.; Lin, Y.; Chinthapenta, V.; Li, Q.; Webster, T.J.; Sheldon, B.W. Disordered Topography Mediates Filopodial Extension and Morphology of Cells on Stiff Materials. Adv. Funct. Mater. 2017, 27, 1702689. [Google Scholar] [CrossRef]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, 14. [Google Scholar] [CrossRef]
- Lin, Y.; Shenoy, V.B.; Hu, B.; Bai, L. A microscopic formulation for the actin-driven motion of listeria in curved paths. Biophys. J. 2010, 99, 1043–1052. [Google Scholar] [CrossRef]
- Aranjuez, G.; Burtscher, A.; Sawant, K.; Majumder, P.; McDonald, J.A. Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue. Mol. Biol. Cell 2016, 27, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017, 11, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y. A model of cell motility leading to biphasic dependence of transport speed on adhesive strength. J. Mech. Phys. Solids 2010, 58, 502–514. [Google Scholar] [CrossRef]
- Blazek, A.D.; Paleo, B.J.; Weisleder, N. Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis. Physiology 2015, 30, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Li, X.L.; Li, G.H.; Fu, J.; Fu, Y.W.; Zhang, L.; Chen, W.; Arakaki, C.; Zhang, J.P.; Wen, W.; Zhao, M.; et al. Highly efficient genome editing via CRISPR-Cas9 in human pluripotent stem cells is achieved by transient BCL-XL overexpression. Nucleic Acids Res. 2018, 46, 10195–10215. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Torkzadeh-Mahanai, M.; Pardakhty, A.; Ebrahimi Meimand, H.A.; Asadikaram, G. Ternary complex of plasmid DNA with NLS-Mu-Mu protein and cationic niosome for biocompatible and efficient gene delivery: A comparative study with protamine and lipofectamine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1781–1791. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, Z.; Xia, X.; Lin, Y. A Novel Electroporation System for Living Cell Staining and Membrane Dynamics Interrogation. Micromachines 2020, 11, 767. https://doi.org/10.3390/mi11080767
Zhang Y, Yan Z, Xia X, Lin Y. A Novel Electroporation System for Living Cell Staining and Membrane Dynamics Interrogation. Micromachines. 2020; 11(8):767. https://doi.org/10.3390/mi11080767
Chicago/Turabian StyleZhang, Yuanjun, Zishen Yan, Xingyu Xia, and Yuan Lin. 2020. "A Novel Electroporation System for Living Cell Staining and Membrane Dynamics Interrogation" Micromachines 11, no. 8: 767. https://doi.org/10.3390/mi11080767
APA StyleZhang, Y., Yan, Z., Xia, X., & Lin, Y. (2020). A Novel Electroporation System for Living Cell Staining and Membrane Dynamics Interrogation. Micromachines, 11(8), 767. https://doi.org/10.3390/mi11080767




