1. Introduction
In recent years, micro-electro mechanical systems (MEMS)-related technologies have been widely used to fabricate miniature safe systems and micro-initiators [
1,
2,
3,
4]. The currently used lead-based primary explosives can hardly meet the requirements of the micro-initiator for energy due to the miniaturization of the ignition device. There is an urgent need to find an environmentally friendly substitute with higher safety and energy density [
5,
6,
7,
8,
9,
10]. Energetic films can be integrated directly on the ignitor without manipulating dangerous reactants [
11,
12], which makes the process safe and attractive for commercial products. Copper azide (Cu(N
3)
2) is a promising alternative to lead-containing primary explosives. Compared with lead azide and lead styphnate, the reaction product of copper azide has little harm to the environment. Hence, it is a green and environmentally friendly primary explosive [
13,
14,
15]. In addition, copper azide has excellent initiation performance, and its limit charge of initiating pentaerythritol tetranitrate (PETN) is just 1/6 of that of lead azide [
16,
17]. In MEMS pyrotechnics, using copper azide as the initial charge can reduce the volume occupied by energetic charges while ensuring detonation performance, and is in line with the trend of miniaturization of modern pyrotechnics. However, the high electrostatic sensitivity of copper azide restricts its practical application [
18,
19].
Carbon nanotubes (CNTs) as a new type of one-dimensional nanomaterial with excellent electrical conductivity, heat transfer properties and mechanical properties have shown important application values in the field of energetic materials [
20,
21]. Rubtsov et al. [
22] added a small amount of carbon nanotubes to high-energy explosives such as trinitrotoluene, pentaerythritol tetranitrate and benzotrifuran nitrogen oxides (TNT, PETN, BTF) through recrystallization, reducing the electrostatic sensitivity of explosives. Compared with pure octogen (HMX), the impact and friction sensitivities of the carbon nanotubes/HMX nanocomposites synthesized by Li et al. [
23] have been reduced by 73% and 29%, respectively. Doping of carbon materials can effectively reduce the electrostatic sensitivity of copper azide. Pelletier et al. [
24] filled copper oxide particles into oriented carbon nanotubes with open ends prepared using a self-supporting alumina film as a template to reduce copper oxide to copper in a hydrogen atmosphere. Then, copper was azidated by hydrogen azide gas. In order to simplify the preparation of Cu(N
3)
2@CNTs/Al
2O
3 composites, Wang [
25] and Zhang [
26] deposited copper in oriented carbon nanotubes by electrochemical deposition directly. At present, the research of carbon nanotube composite energetic materials mainly focuses on mixing carbon nanotubes with energetic materials mechanically. The composite energetic material obtained in this way cannot give full play to the advantage of the unique structure of carbon nanotubes. The work of filling energetic materials into carbon nanotubes is still rare, and the templates for preparing carbon nanotubes are all self-supporting alumina films. The nano-energetic materials prepared in this way and the micro energetic devices prepared on the basis cannot be compatible with MEMS-related technologies.
In recent years, materials such as silicon and silicon carbide which have excellent properties of corrosion resistance, high-temperature and high-pressure resistance have been widely and maturely applied in MEMS-related technology [
27]. In order to apply Cu(N
3)
2 to the energetic charge of MEMS pyrotechnic products, a method for preparing silicon-based Cu(N
3)
2@CNTs composite energetic films with higher electrostatic safety and compatibility with MEMS technology was developed in this work. The micromorphology, crystal structure, composition and thermochemical properties of the material were studied. In the process of preparing silicon-based Cu(N
3)
2@CNTs composite energetic films, it is very important to prepare porous alumina films with a large pore size, straight and orderly channels and no barrier layer on the silicon substrate. It is related to the success of the subsequent deposition of aligned carbon nanotube arrays in the template. In addition, the processes for depositing Cu nanoparticles in aligned carbon nanotubes by electrochemical deposition are also key factors that determine the density of copper azide in carbon nanotubes.
2. Materials and Methods
Materials: phosphoric acid, oxalic acid, copper sulfate pentahydrate, boric acid, sodium hydroxide, sodium azide, nitric acid, absolute ethanol and thiourea. The reagents above are all analytical reagents. Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China); pure water: homemade. Nanjing Yipuyida Technology Development Co., Ltd. (Nanjing, China); hydrogen, argon and acetylene. The purity is 99.97%, 99.99% and 99.9%, respectively, Shanghai Pujiang Special Gas Co., Ltd. (Shanghai, China).
Warning of the hazards: Sodium azide is highly toxic and explosive. When it reacts with acid, it will produce hydrogen azide gas, which is also explosive. In this work, copper nanoparticles were reacted with hydrogen azide gas to produce copper azide with high energy density as the primary explosive. These azides are very dangerous, so they must be dealt with carefully during the experiment.
The silicon wafer was placed in acetone, isopropano and ultrapure water sequentially for ultrasonic cleaning and dried with nitrogen. The electron beam evaporation method was used to deposit Ti and Al layers on the silicon wafer. First, we adjusted the current to stabilize the filament current to 4.5 A and the voltage to 9 kV. During this process, the pressure raised slightly, indicating that the electron beam hit the evaporation material and the film began to deposit. The Ti layer served as a transition layer to increase the strength of the adhesion between the Al layer and the silicon substrate.
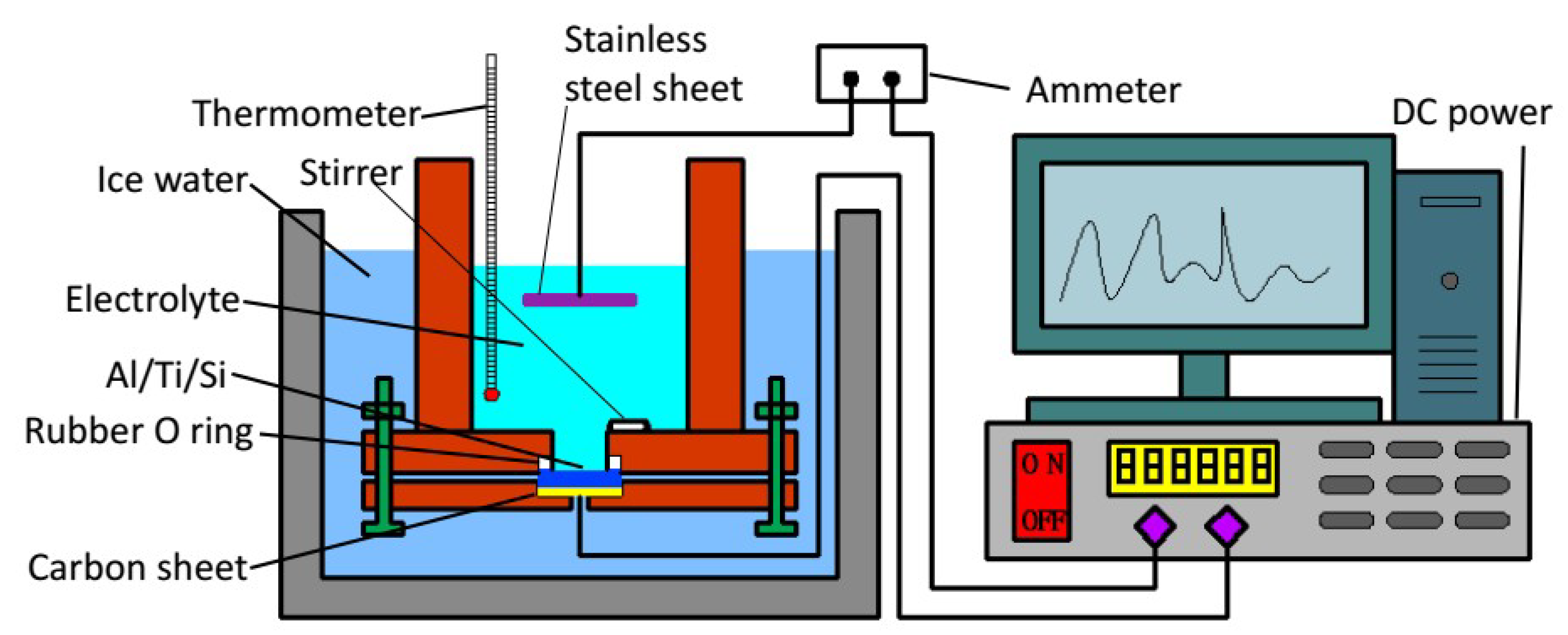
An improved two-step anodic oxidation method [
28] was used to prepare the silicon-based porous alumina film, the device is shown in
Figure 1. The preparation process can mainly be divided into three steps:
First, 0.3 mol/L oxalic acid was used as the electrolyte, and ethanol/water (v/v = 1:1) as the electrolyte solvent; we kept the temperature of the electrolyte at 1–3 °C, and increased the voltage from 0 to 100 V; the speed of magnetic stirring was 430 r·min−1, and the time of the first step of anodizing the silicon-based aluminum layer was 40 min;
Next, the sample was immersed in a mixed solution of 1.8% CrO3 and 6% phosphoric acid at 65 °C for 3 h to remove the alumina film formed, and then the second step of anodizing was performed, the electrolyte was 0.5% phosphoric acid solution;
Finally, the sample was immersed in a 5% phosphoric acid solution for 40 min at 30 °C. The purpose of this step was to enlarge the diameter of the pores.
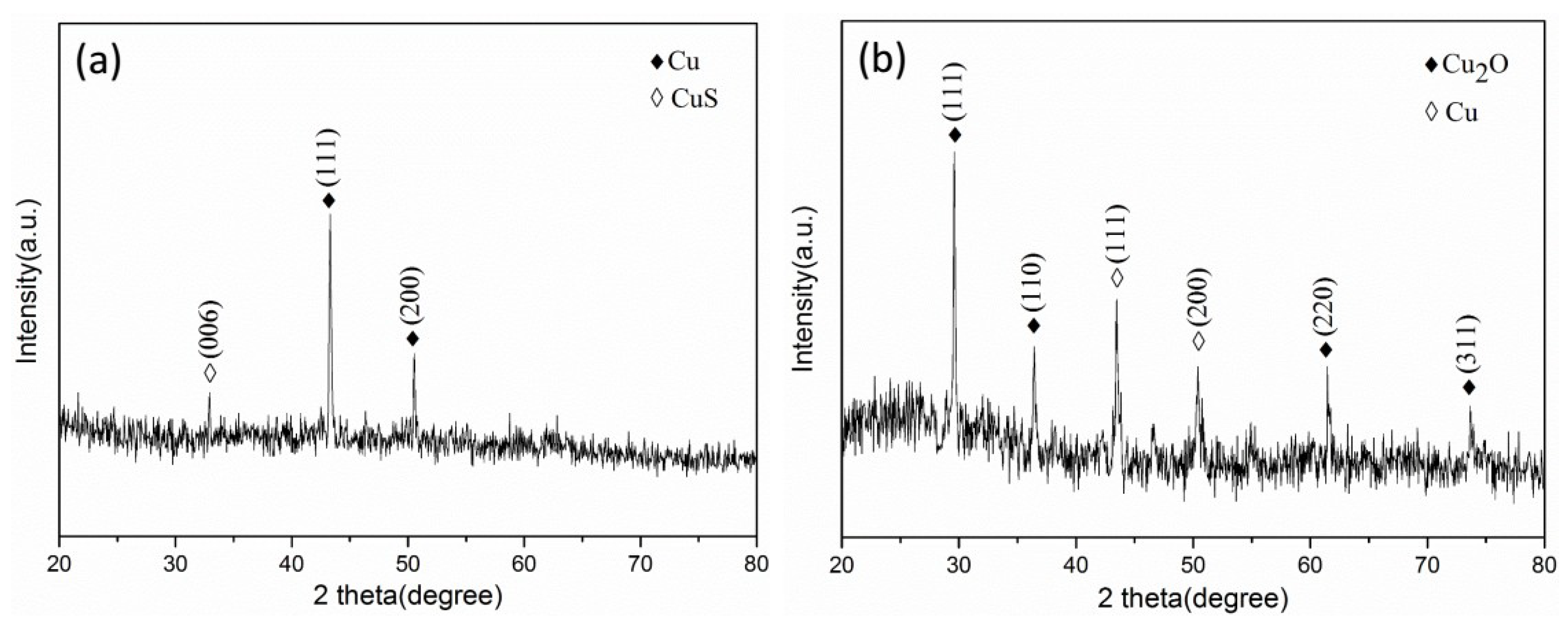
An aligned carbon nanotube array was prepared in a silicon-based porous alumina film channel using a chemical vapor deposition method. The silicon-based porous alumina film was placed in a tube furnace, and the temperature was raised to 700 °C under vacuum conditions, while a mixed gas of acetylene, hydrogen and argon was passed in. The vacuum degree was kept at 0.05 MPa, and the deposition time was 90 min. Then, the copper nanoparticles were deposited in the aligned carbon nanotubes by an electrochemical deposition method. The composite film was the working electrode and the platinum electrode was the counter electrode. The electrolytic solution was 0.08 mol/L copper sulfate pentahydrate and 0.4 mol/L boric acid, then thiourea or polyvinylpyrrolidone (PVP) were added as an additive and the current density was 0.1 mA·cm−2. The effects of different additives on the deposition of copper nanoparticles in the carbon nanotubes were explored. Finally, the sample was immersed in a 0.5 mol/L sodium hydroxide solution for 1 h to remove the aluminum oxide film. We left it in the air for 24 h, and then the oxidation of the sample was characterized.
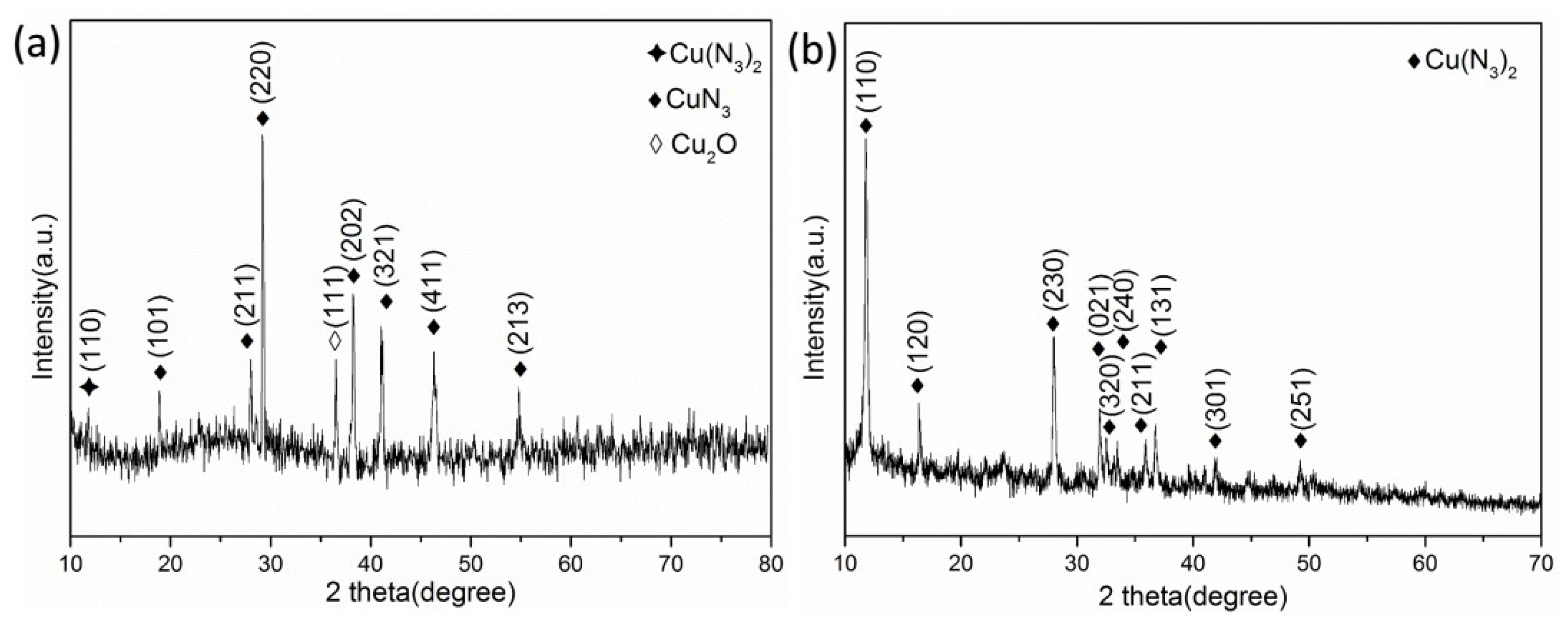
At normal temperature, nitric acid reacts with sodium azide to produce hydrogen azide gas. The gas–solid reaction of copper with hydrogen azide can produce copper azide. The reaction equation involved in the above process is as follows:
The silicon-based Cu2O@CNTs composite film and Cu@CNTs composite film were placed in a closed container. We passed N2 into it to remove the air and added 4 g·mL−1 of nitric acid and 2 g·mL−1 of sodium azide solution. After 72 h of reaction, a silicon-based Cu(N3)2@CNTs composite energetic film was obtained.
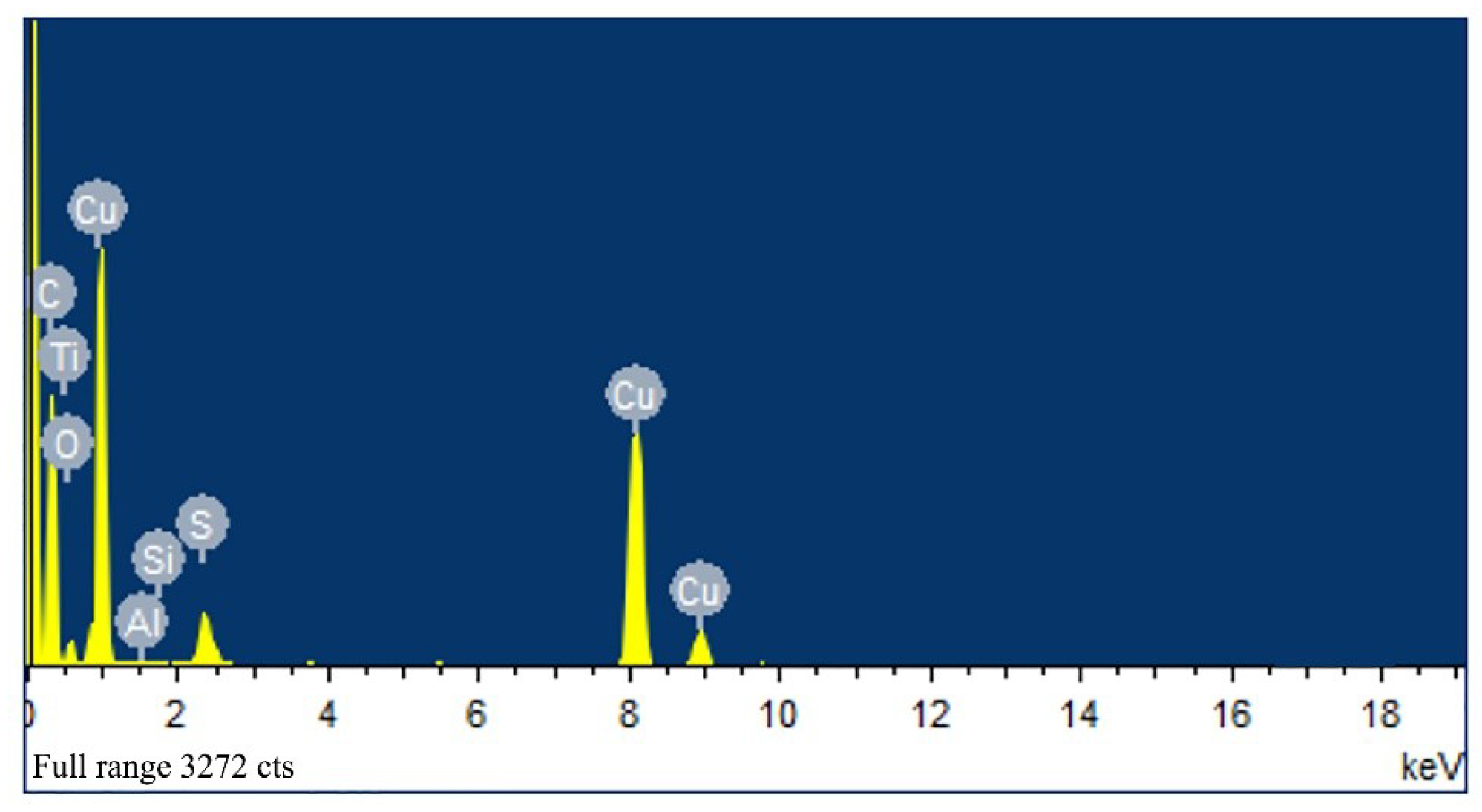
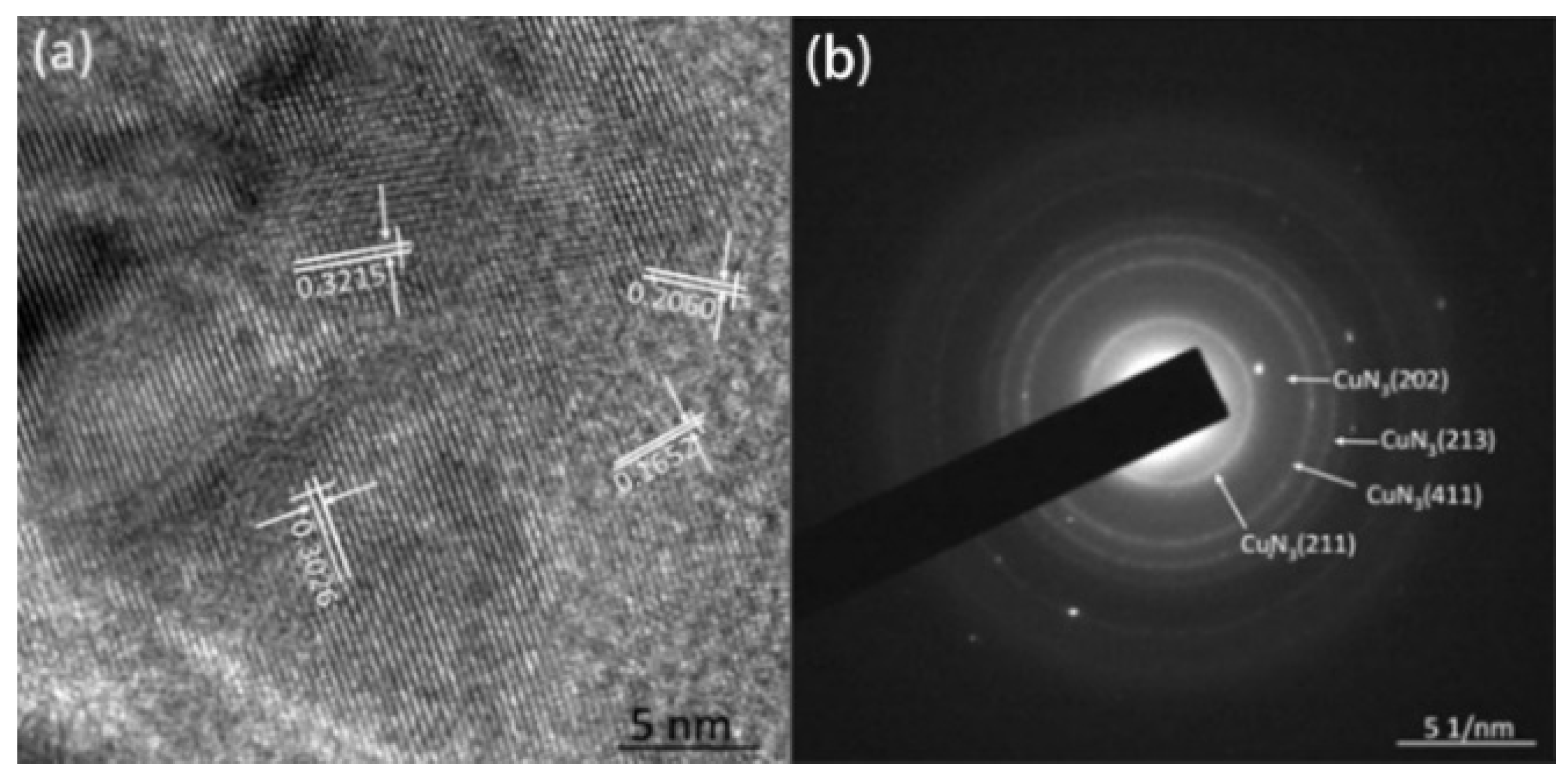
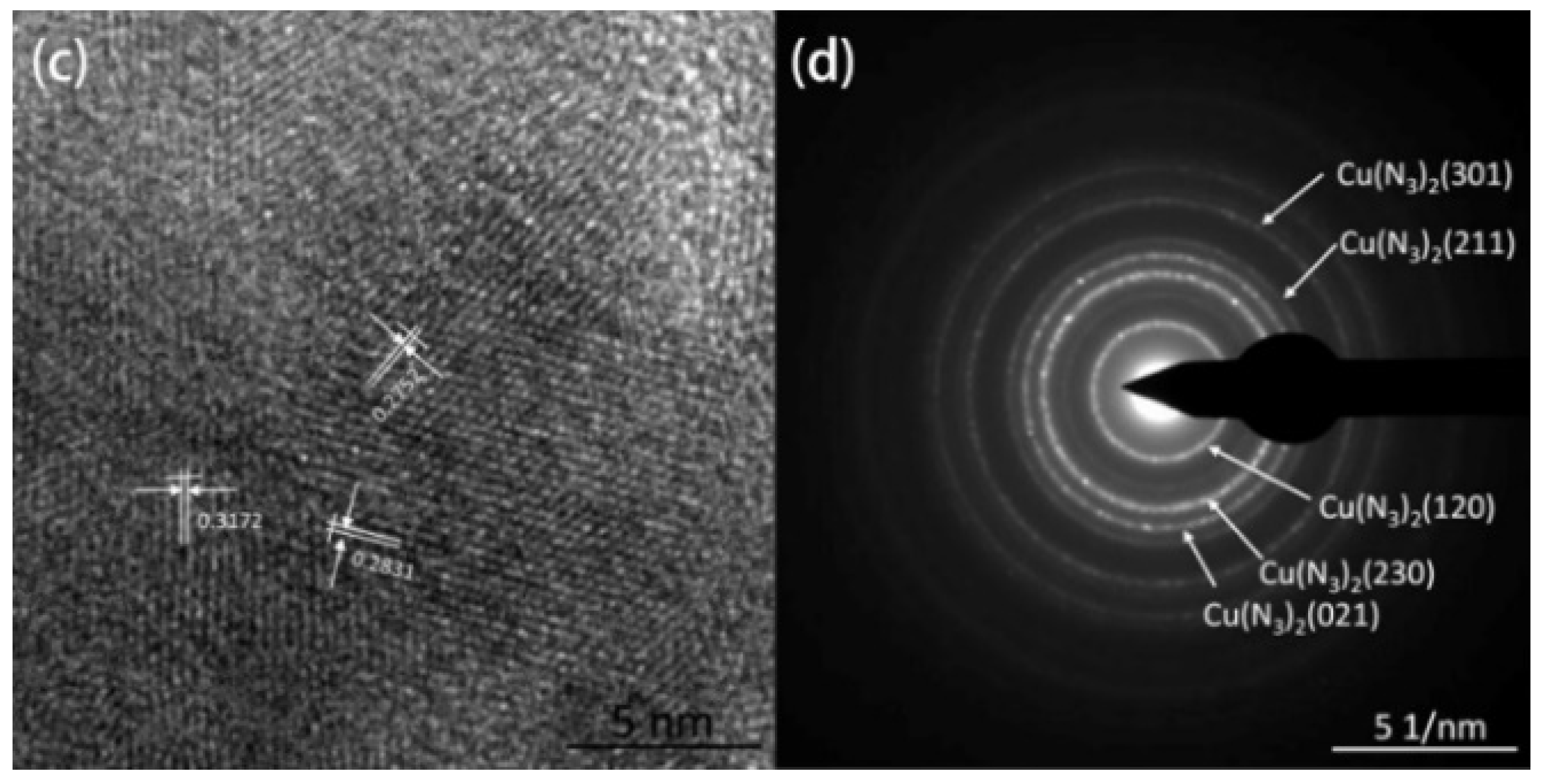
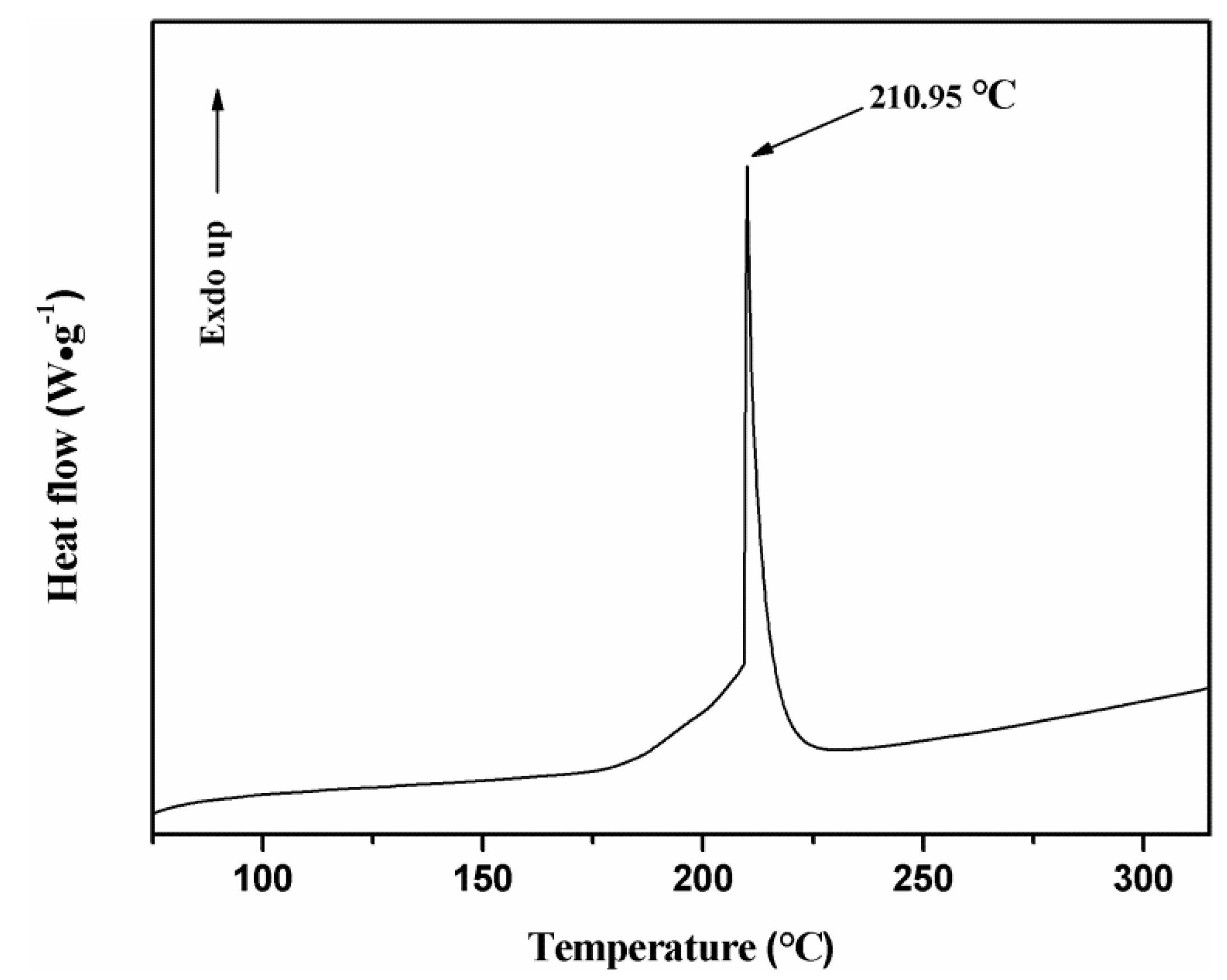
Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) were used to characterize the micro-morphology of the samples and X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), selective electron diffraction (SAED) and energy dispersive X-ray spectroscopy (EDS) were used to characterize the crystal structure and composition. The thermal decomposition kinetics of the Cu(N3)2@CNTs composite energetic film were studied by differential scanning calorimetry (DSC).
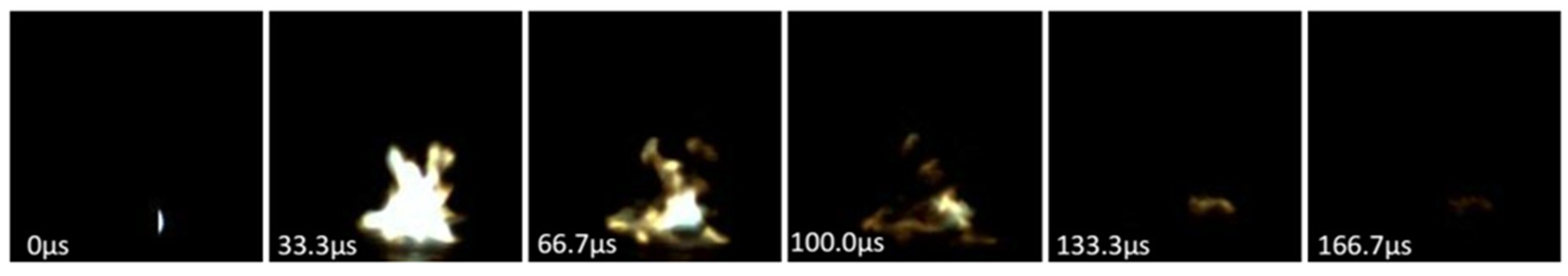
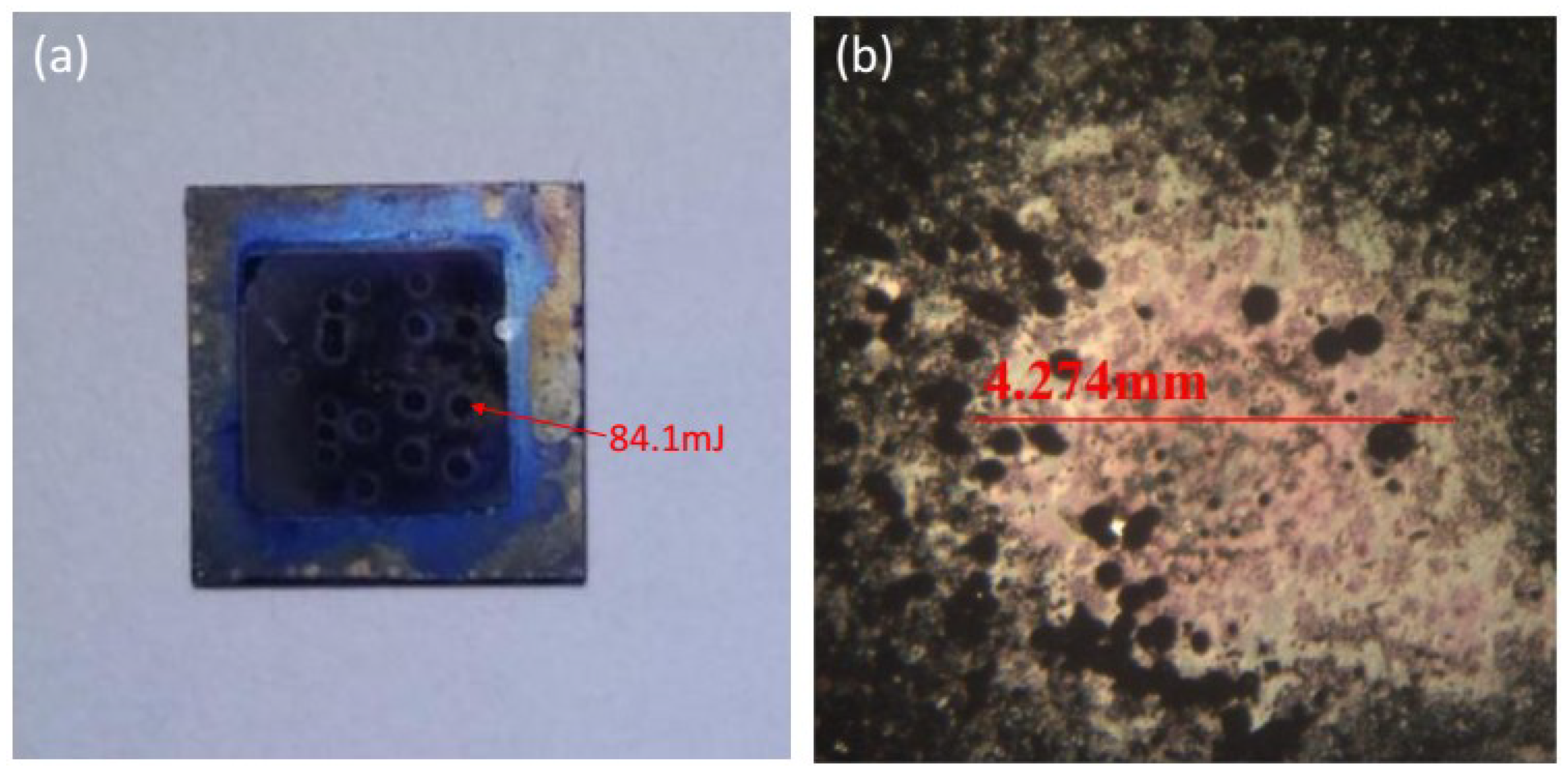
The performance of the energetic films was tested by laser ignition experiments, and high-speed photography was used to record the ignition process. By changing the output voltage of the laser, the output energy and the optimal detonation energy can be obtained (84.1 mJ). The model of the high-speed camera used in this experiment was APHJ-100, the frequency of high-speed camera was 30,000 fps and the laser is a Nd:YAG Dawa-200 single pulse laser with a wavelength of 1064 nm and pulse width of 6 ns.
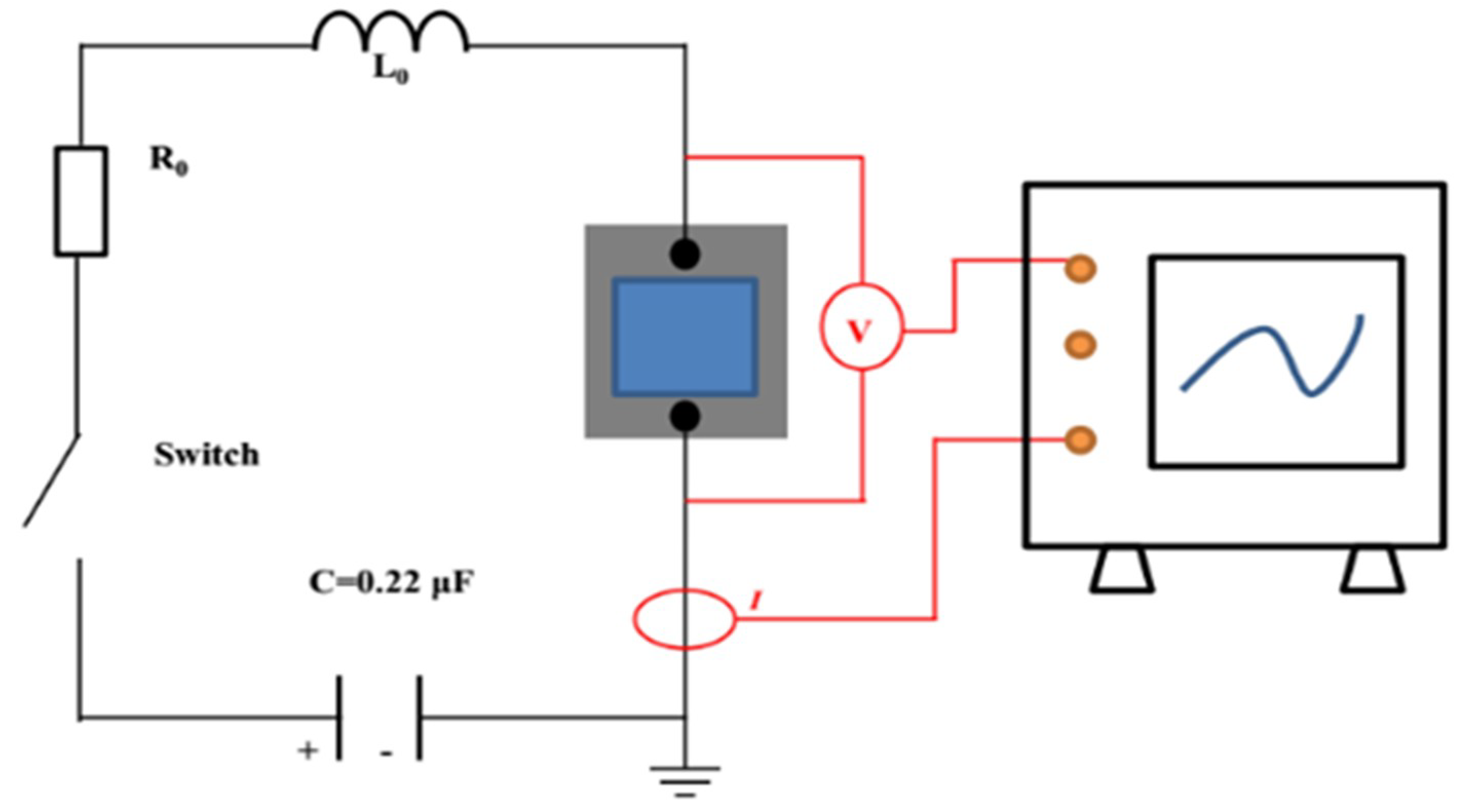
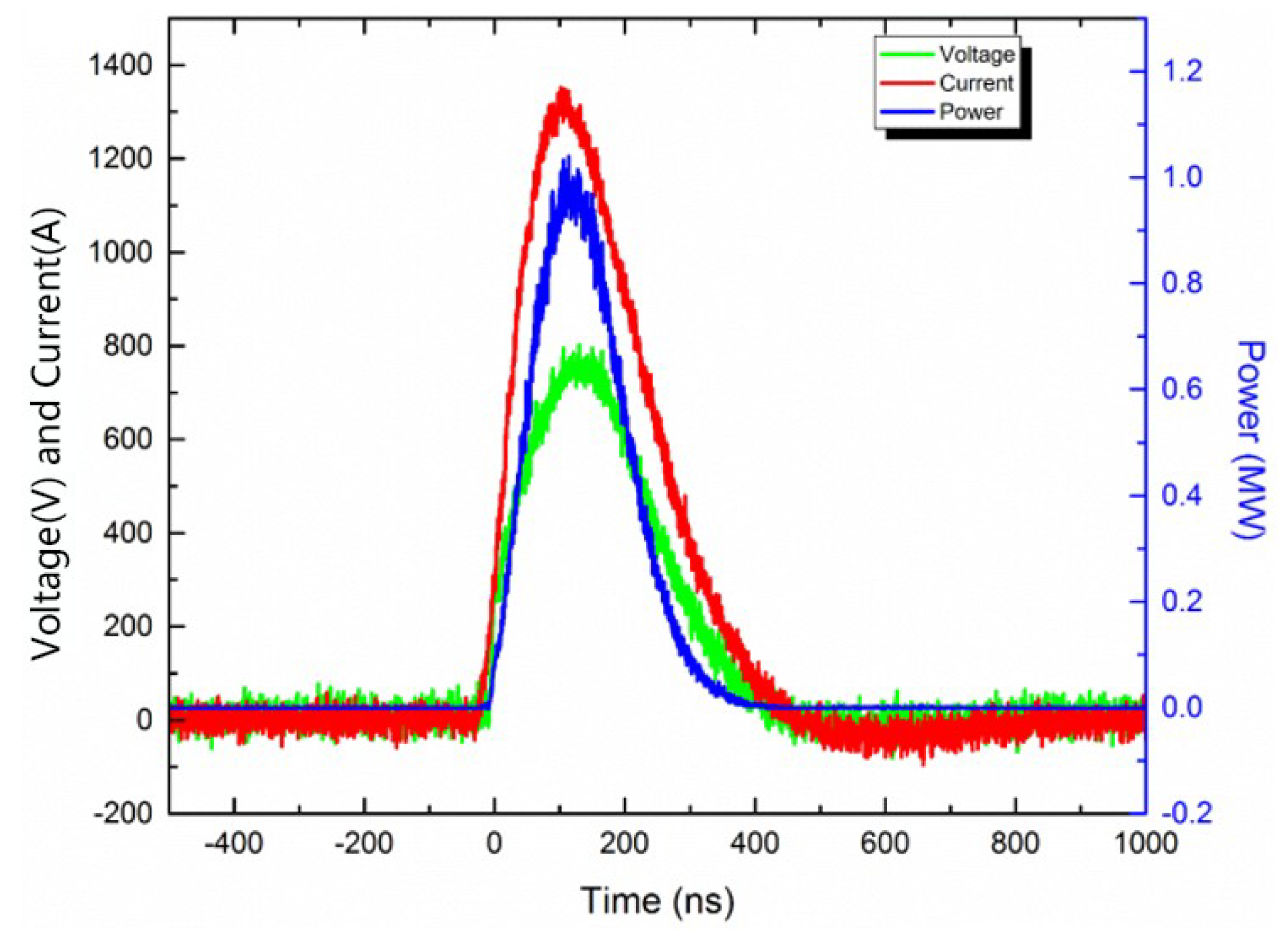
An electrical explosion performance test was performed on the energetic film. The schematic diagram of the electrical explosion experiment device is shown in
Figure 2. A high-voltage ceramic capacitor was used as the energy storage capacitor. The capacitance was 0.22 μF and the initial explosion voltage was 1500 V. The high-voltage probe and the Rogowski current loop were used to test the voltage and current changes in the energetic film during the ignition process and recorded with an oscilloscope. The electric explosion curve was analyzed.
The circuit was connected first, then the sample was soldered to the positive and negative terminals of the high-voltage output terminal of the printed circuit board (PCB), and the resistance was measured to be 0.4 Ω. The high voltage (1500 V) of the pulse power source acted on both ends of the high-pressure gas switch, and the trigger signal (3000 V) was output by the pulse power source, thereby turning on the gas switch, completing the high-voltage output and acting on both ends of the sample.
The electrostatic sensitivity test of the silicon-based Cu(N3)2@ CNTs composite energetic film was carried out by the Bruceton method. We tested the sample at a static ignition energy and ignition voltage to evaluate the electrostatic sensitivity of the sample prepared. The 50% ignition energy (E50%) of the sample was calculated by the critical ignition voltage (U50%).
4. Discussion
In this work, carbon nanotube arrays were prepared by chemical vapor deposition and template methods. Levin et al. [
29] analyzed the existence form of an anodic aluminum oxide (AAO) template at different temperature stages during heating. When the temperature is above 1200 °C, the existence form of AAO is stable α-Al
2O
3. When the temperature reaches 230–500 °C, it changes to η-Al
2O
3, and the existence form is mainly γ-Al
2O
3 when the temperature is 450–750 °C. The η-Al
2O
3 and γ-Al
2O
3 have higher porosity and a large specific surface area, so they have better catalytic activity. The commonly used carbon source gas can only be thermally decomposed at high temperatures (800 °C). When acetylene was used as the carbon source, the aligned carbon nanotube array can be prepared in the AAO template channel at about 600 °C. It shows that the AAO template plays a catalytic role in the thermal decomposition of acetylene, so that it can be decomposed at lower temperatures. Liu et al. [
30] studied that the catalytic process of the AAO template has two main steps. The first is the catalytic effect when the carbon source is decomposed, and the second is the catalytic effect during the growth of carbon nanotubes.
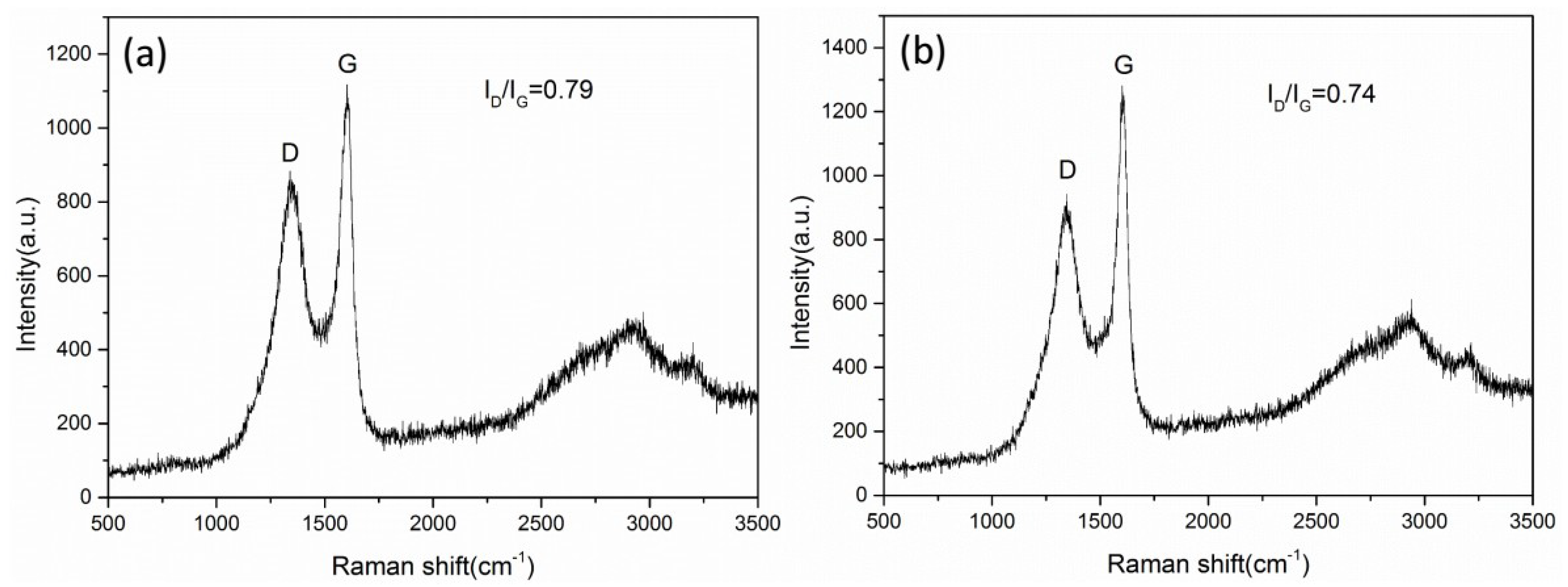
Yang et al. [
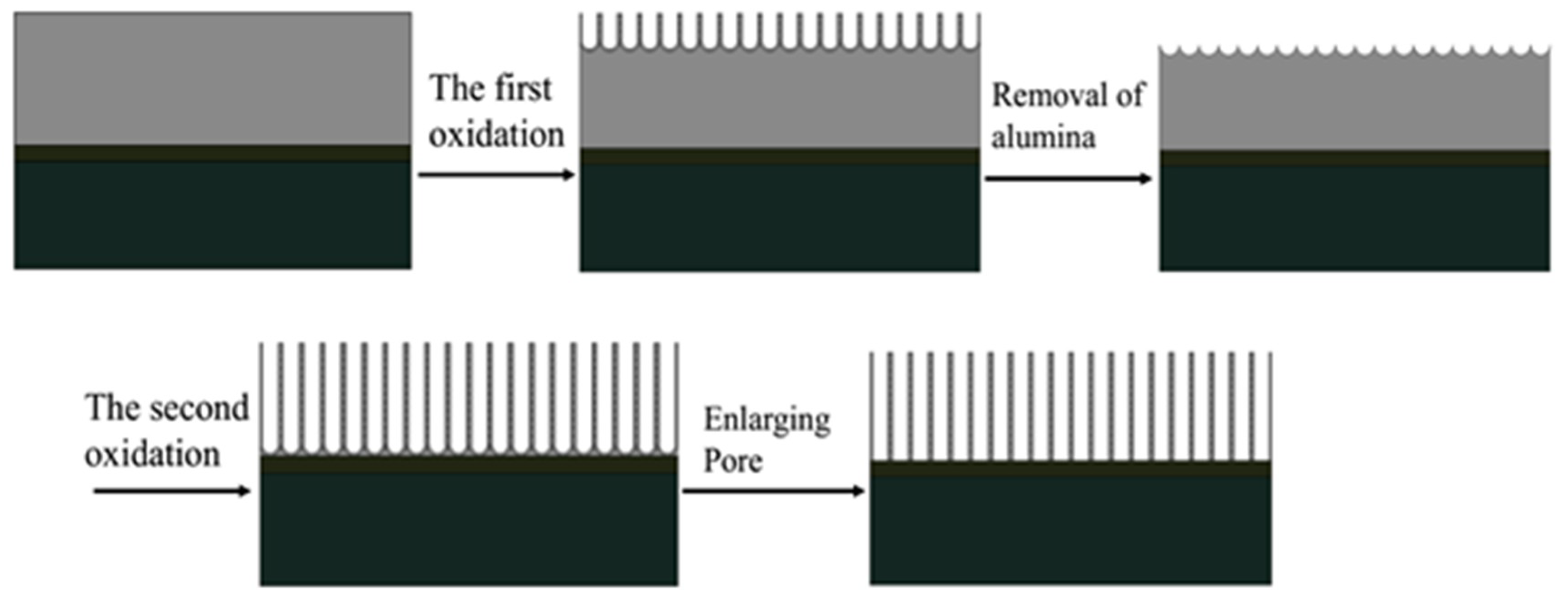
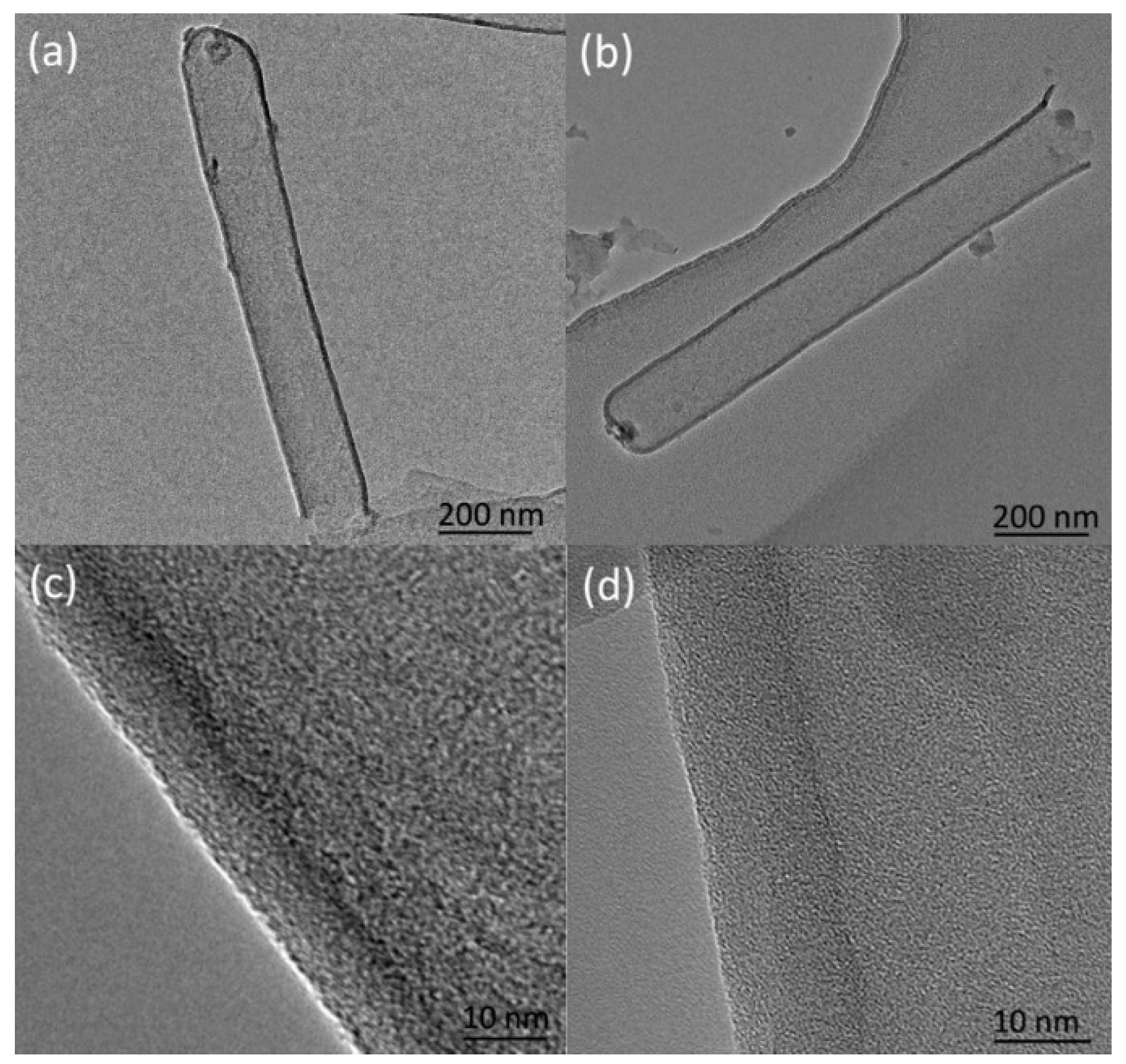
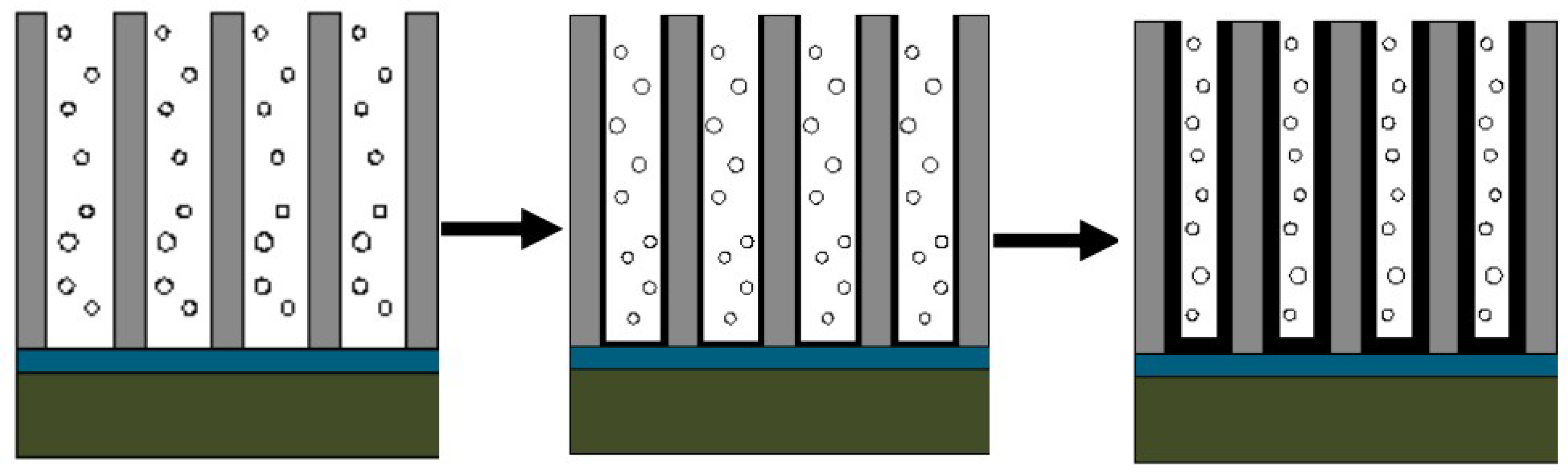
31] studied the growth mechanism of carbon nanotubes prepared by the AAO templates and found that carbon atoms were deposited on the walls of the alumina template and the channels of the AAO template were copied to obtain the carbon nanotube structure. The carbon nanotubes are named a-CNTs (amorphous carbon nanotubes). By analyzing the Raman spectrum and TEM, it can be known that the carbon nanotubes obtained in this work are also a-CNTs. With reference to Yang’s conclusion, we consider the mechanism of preparing carbon nanotube arrays through PAM, as shown in
Figure 18.
First, the carbon source is cracked to generate carbon atoms under the catalysis of the PAM. Carbon atoms are deposited in the pores of the PAM. Under the autocatalysis of the PAM, the carbon atoms replicate the pore structure of the PAM and grow into carbon nanotubes at a relatively lower temperature. The carbon atoms generated by the continuous decomposition of the carbon source continue to enter the hollow lumen of the carbon nanotubes, deposit on the inner wall of the formed carbon nanotubes and repeat the growth of the carbon nanotubes. With the increase in the reaction time, the thickness of the carbon nanotube wall also increases and even solid carbon nanorods may be obtained. Therefore, carbon nanotubes of different thicknesses can be obtained by controlling the reaction time.
During the stage of electrochemical deposition, the type of additive has a great influence on the filling effect of the copper nanoparticles. Additives can be generally classified into three categories: halide ions, supporting electrolytes and organic additives. Xu [
32] found that the addition of Cl
− in the electrolyte would form a “chlorine bridge” under enhanced polarization conditions, changing the charge transfer mechanism of the charged particles in the solution. The role of the supporting electrolyte is generally considered to increase the charge transfer rate and the conductivity of the electrolyte.
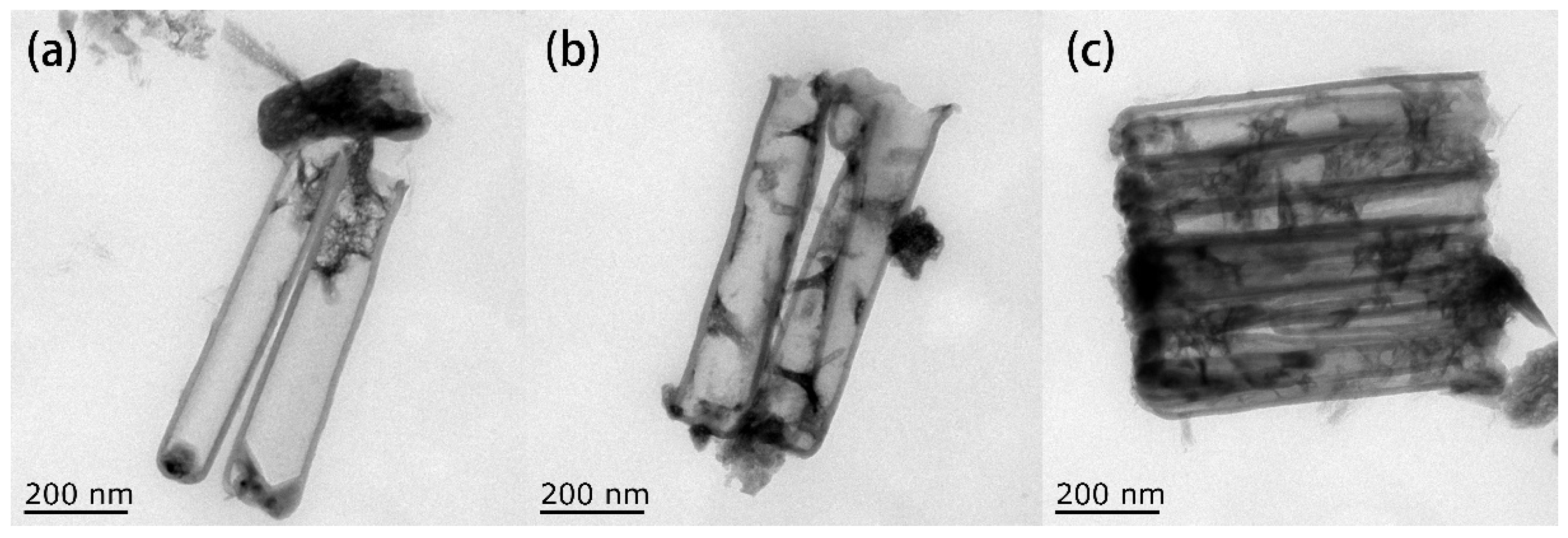
By comparing the different morphologies of copper deposited in CNTs, it is obvious that when no additives were added, the crystal size of copper grew too large, resulting in the inability of copper nanoparticles to be deposited in CNTs. When TU was added, the particle size of the copper nanoparticles decreased significantly. These results confirm that TU as an additive has the effect of inhibiting the growth of copper particles. First, TU adsorbs on the surface of the copper particles and reacts with Cu2+ to form compounds. These compounds become adsorption layers and cover the active sites of copper, preventing further reduction of Cu2+. Then TU and CuS generated by Cu2+ are also adsorbed on the surface of the copper particles, making the copper atoms unable to reach the active site and inhibiting the growth of grains. When TU and PVP were added together, the particle size of the copper nanoparticles became smaller, indicating that the inhibitory effect is more pronounced when PVP and TU work together.
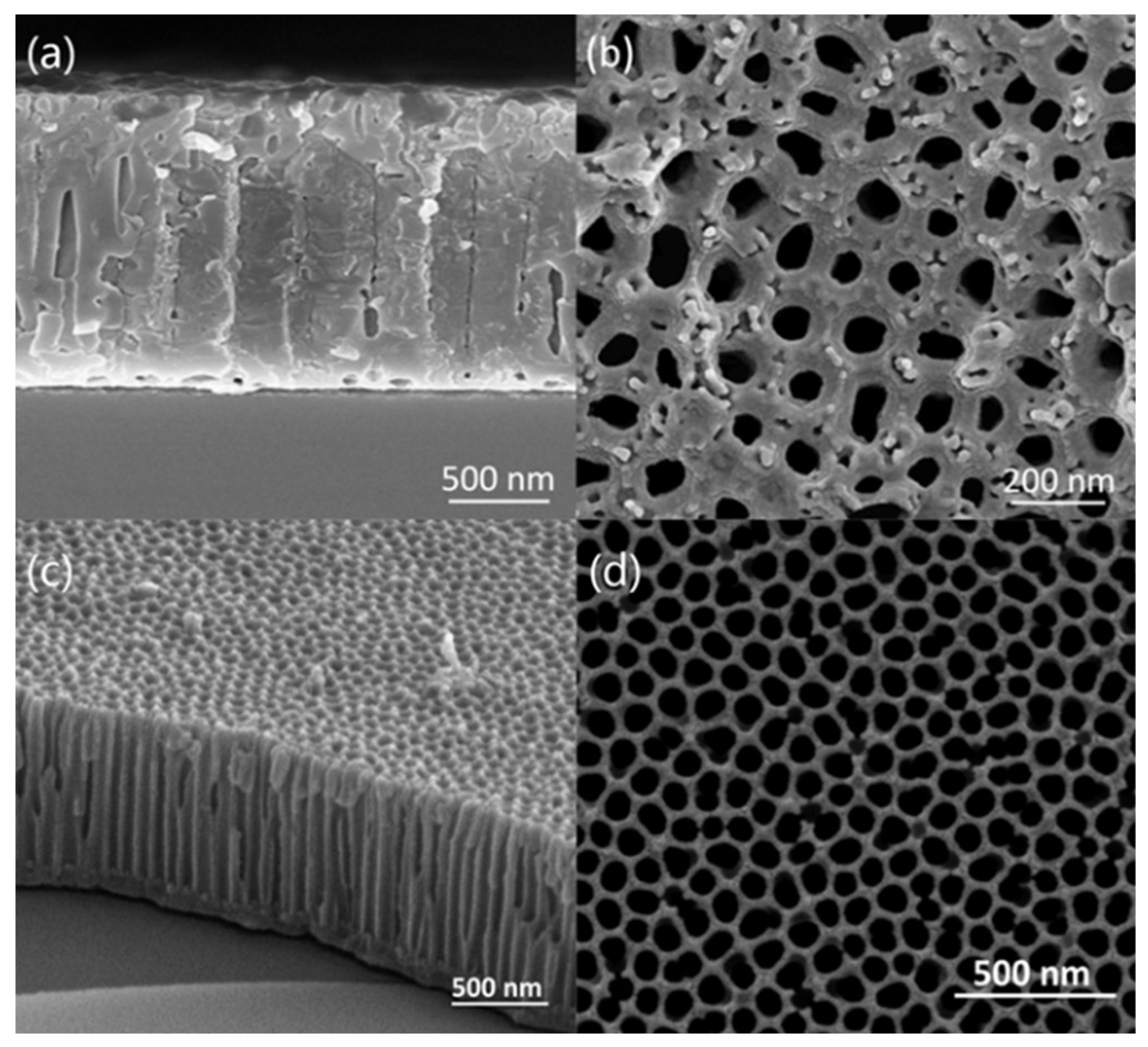
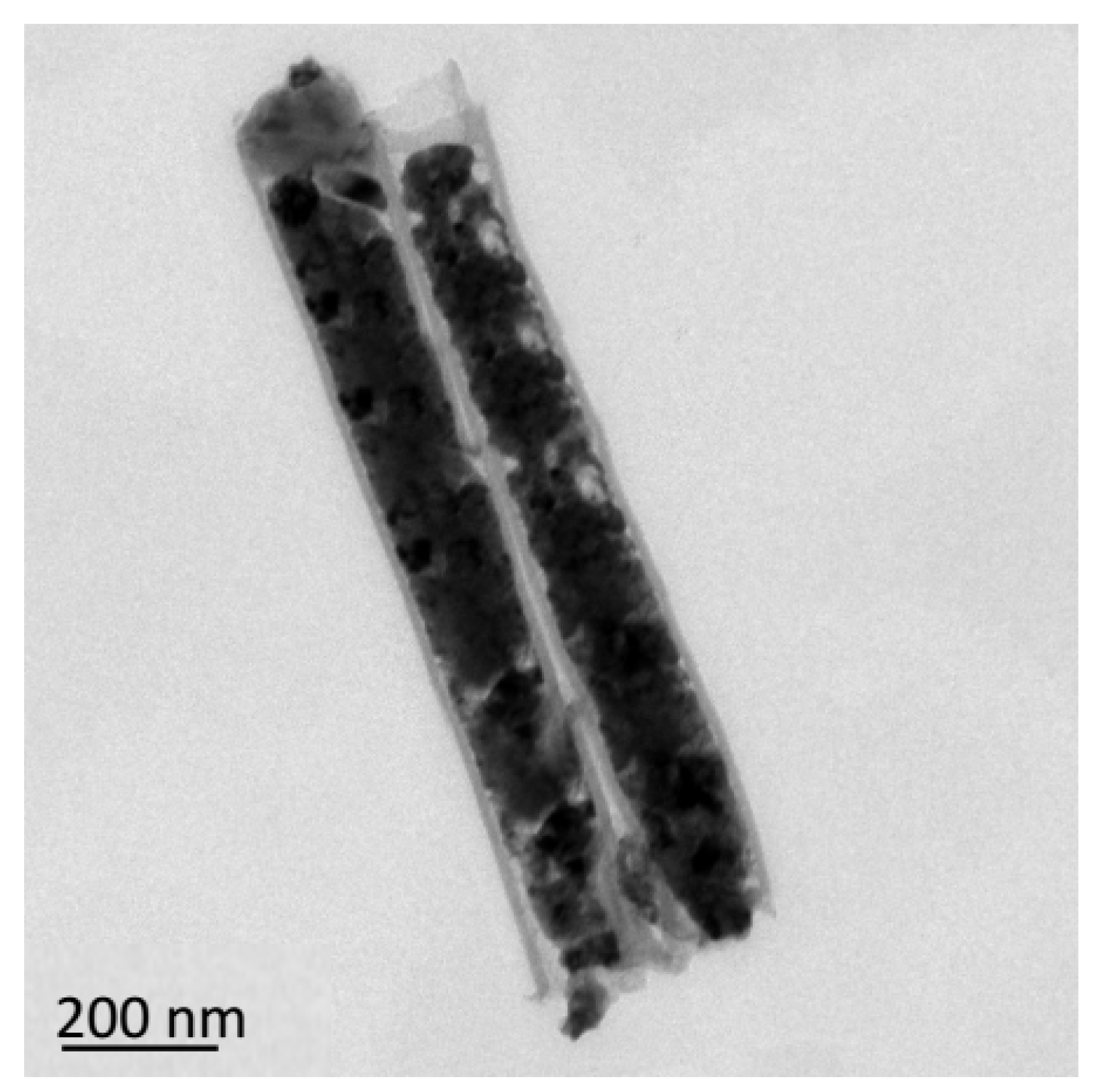
Figure 19 shows the SEM images of the cross-section of CNTs grown on silicon-based porous alumina films, silicon-based Cu@CNTs film and silicon-based Cu(N
3)
2@CNTs film. It can be seen from
Figure 19a that the carbon nanotubes were tightly attached to the inner walls of the pores of the porous alumina template, and the shape of the pores was well reproduced. Its upper end was open, providing an entrance for the deposition of the copper nanoparticles.
Figure 19b shows the cross-section of the film after the copper nanoparticles were filled. The copper nanoparticles were relatively evenly distributed in the carbon nanotubes, without obvious agglomeration. After the azide reaction, the copper nanoparticles were converted to energetic copper azide (
Figure 19c). It can be seen that its particles’ size become slightly larger, and the ordered directional carbon nanotube arrays have not been destroyed during the azide process.
Carbon nanotubes have excellent electrical conductivity. When the copper azide is filled into the hollow cavity of the oriented carbon nanotube, the electrostatic charge will be dispersed on the surface of the carbon nanotube instead of accumulating on the surface of the copper azide. Therefore, the explosion of copper azide caused by the electrostatic charge can be avoided, and the electrostatic sensitivity of the composite energetic film can be effectively reduced. Compared with the E
50% of pure nano-copper azide (0.05 mJ) reported before [
33], the electrostatic sensitivity of the composite film is significantly reduced.
In future research, it will be an interesting direction to combine carbon materials with different structures and copper azide to obtain energetic materials with good performance. Copper azide can take advantage of its high energy density in composite materials, and carbon materials with different structures can isolate copper azide particles in various ways, thereby reducing the mechanical sensitivity of composite energetic materials. Meanwhile, the excellent conductivity of carbon materials can also reduce the electrostatic sensitivity of composite energetic materials. On the one hand, the advantages of copper azide are used, and on the other hand, carbon materials are used to make up for the shortcomings of copper azide as a primary explosive. The composite energetic films designed and prepared in this paper will also be further optimized to be applied to MEMS pyrotechnic devices. Furthermore, an in-depth study of the mechanism of azide reaction will also be a valuable work.