High-Speed Manipulation of Microobjects Using an Automated Two-Fingered Microhand for 3D Microassembly
Abstract
1. Introduction
2. Materials and Methods
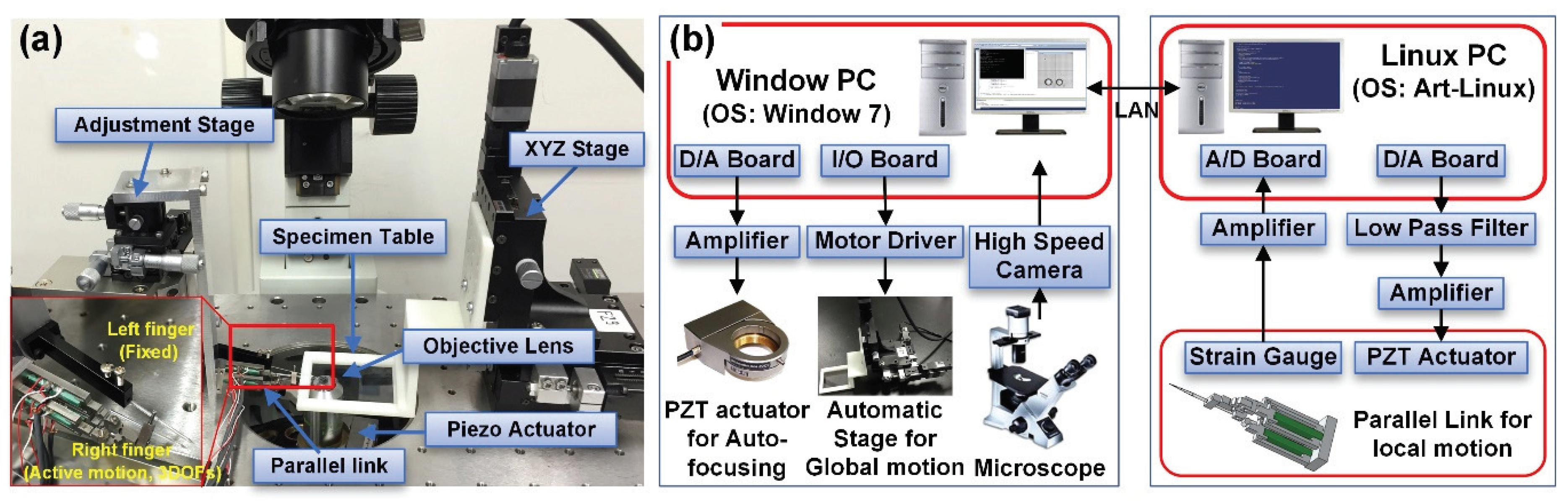
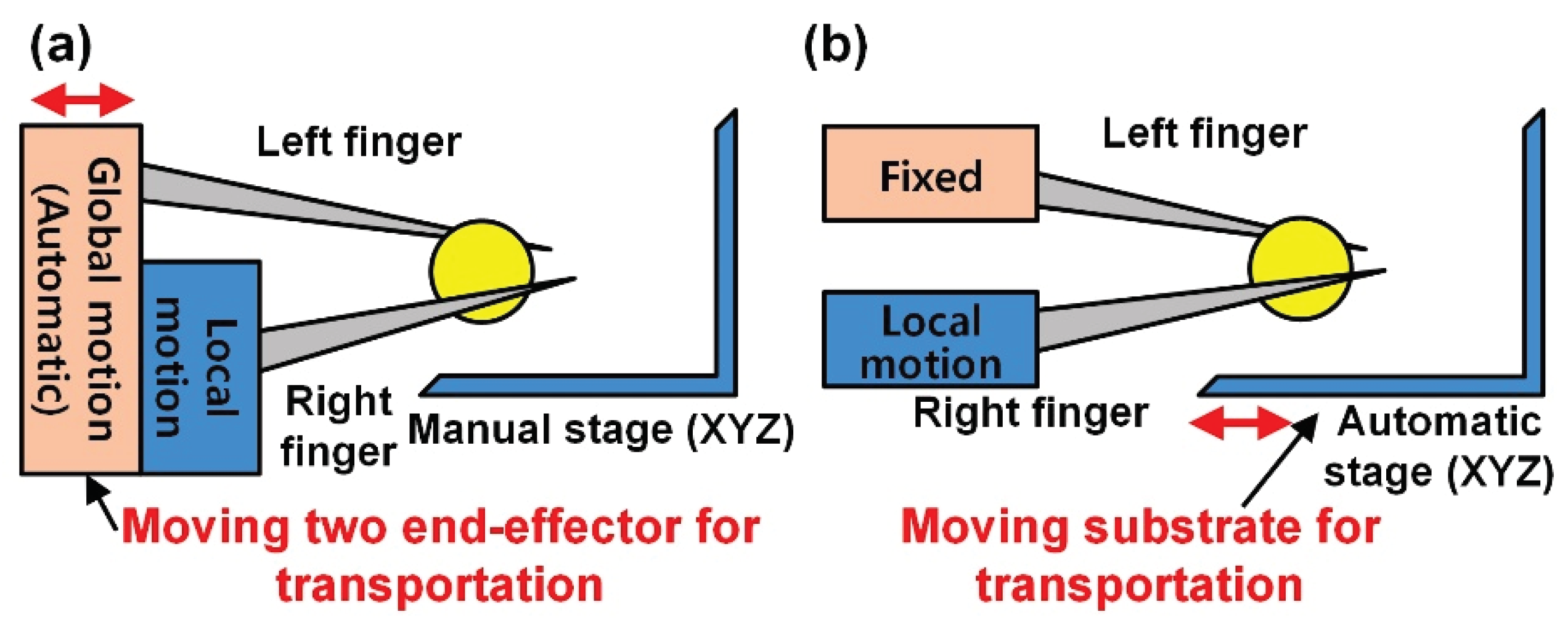
2.1. System for Micromanipulation
2.2. Accurate and High-Speed Manipulation
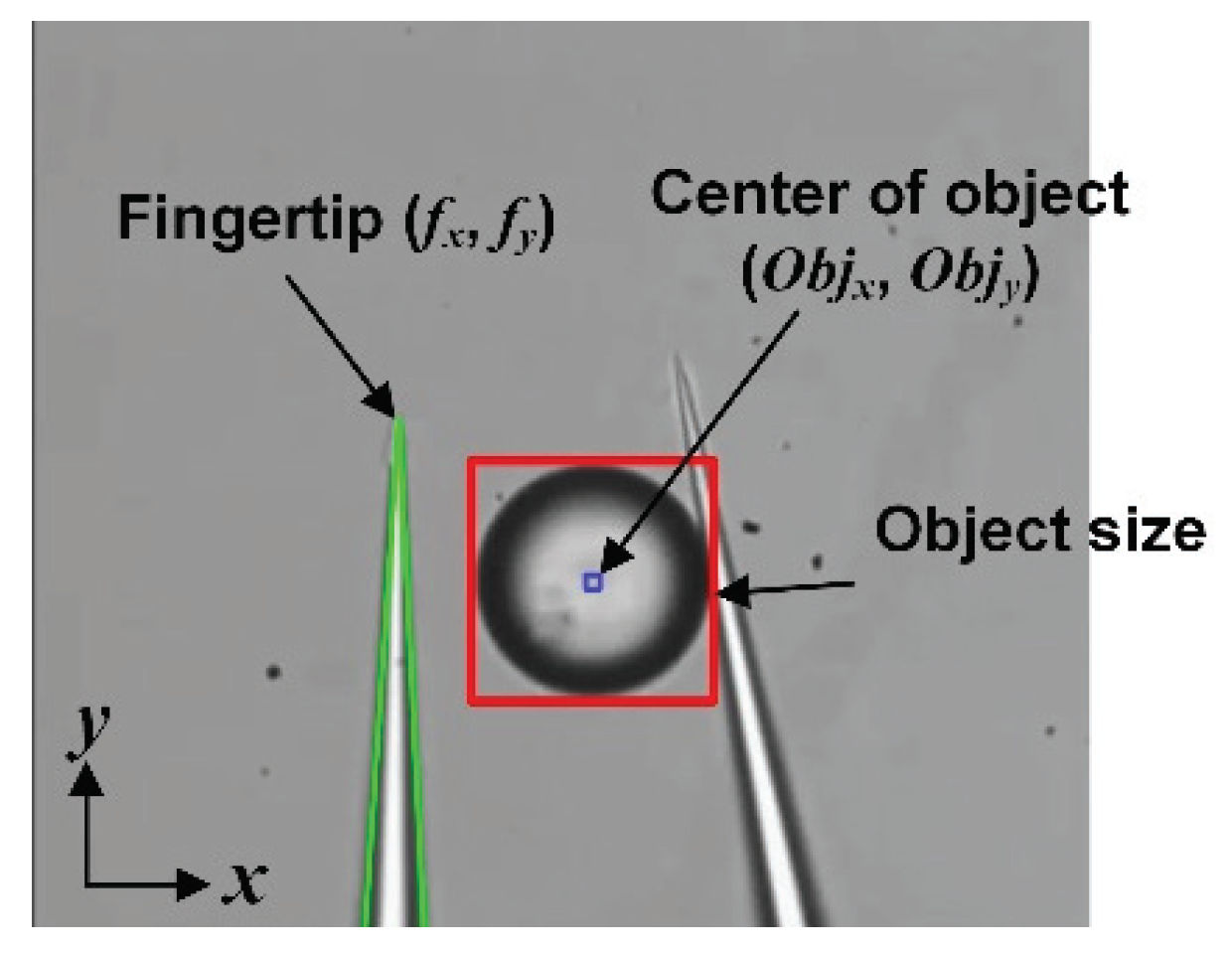
2.2.1. Object and Fingertip Detection for Stable Grasping
2.2.2. High-Speed Transportation in Liquid
2.2.3. Compensating Error during Grasping and Transporting
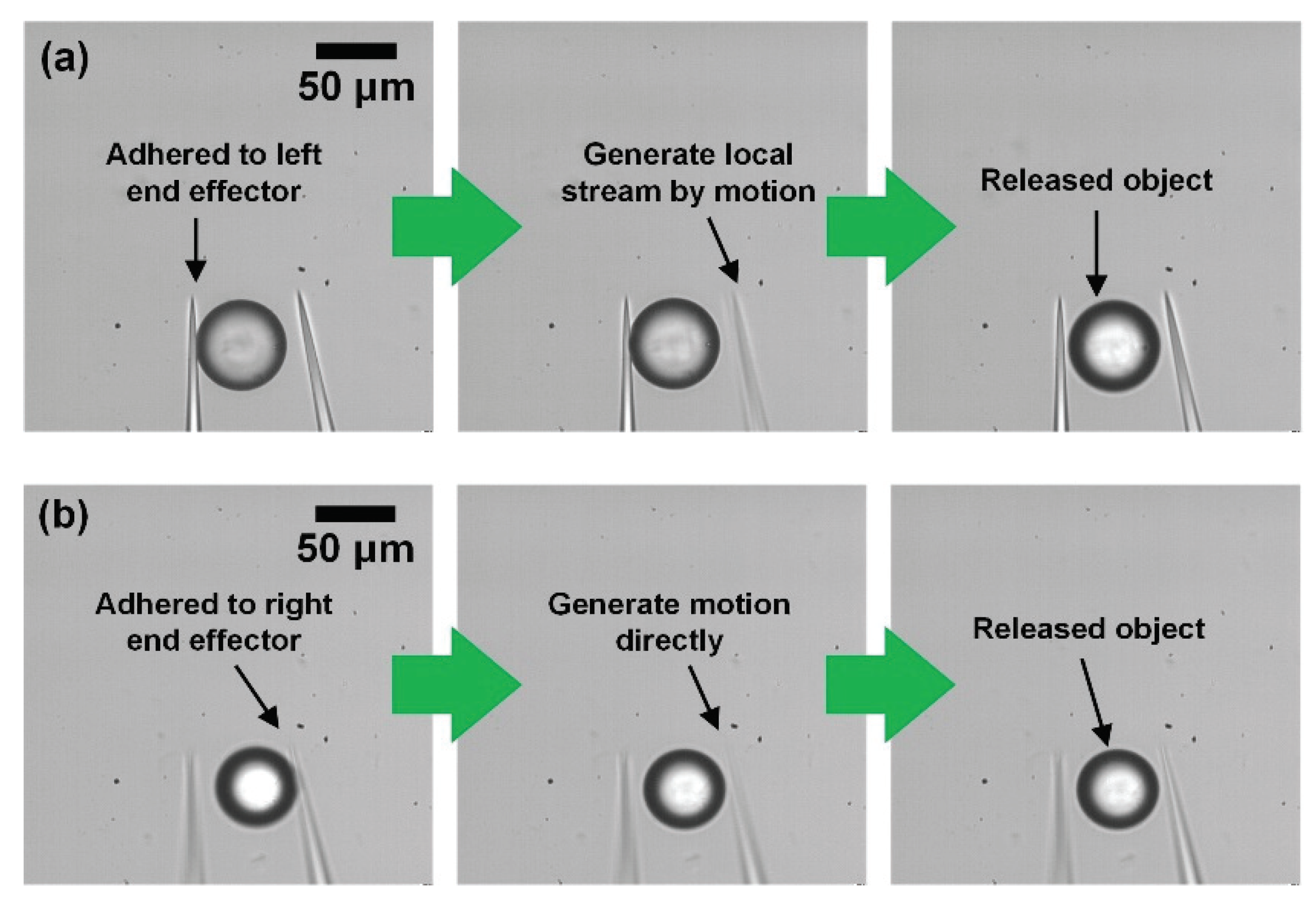
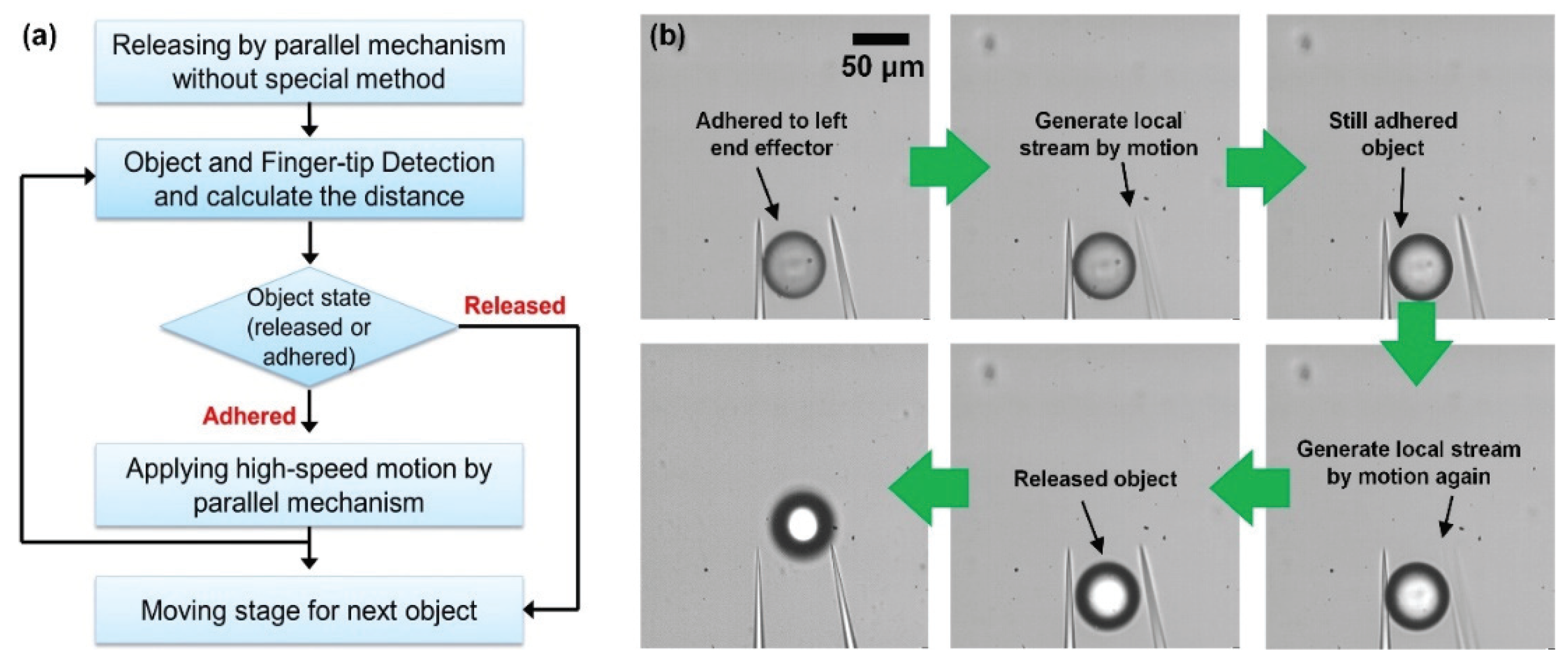
2.2.4. Release and Precise Positioning of Objects
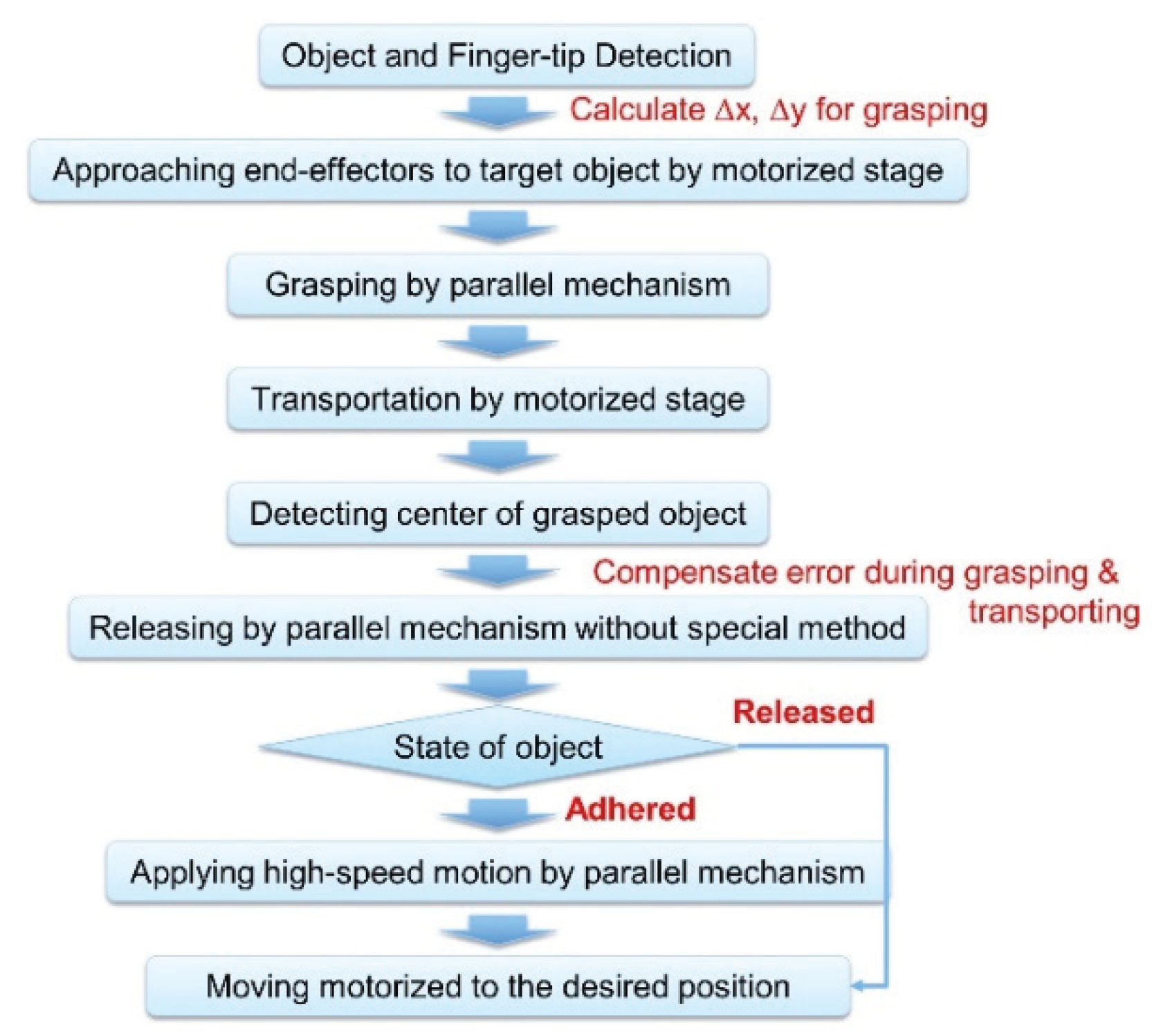
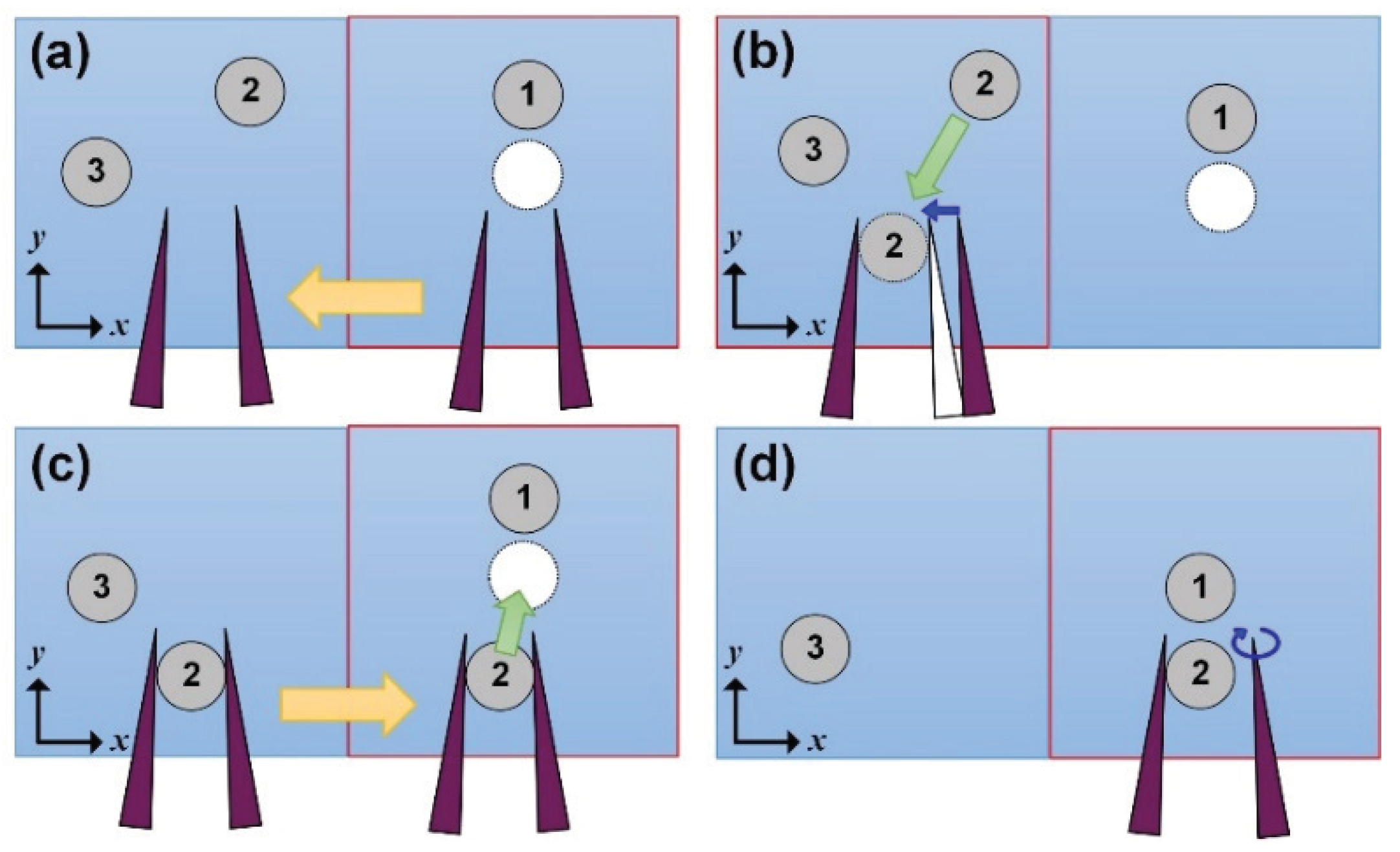
2.2.5. Manipulation Process for Assembling Objects
3. Results and Discussion
3.1. Automated Manipulation of an Object
3.2. Microassembly of 2D and 3D Objects
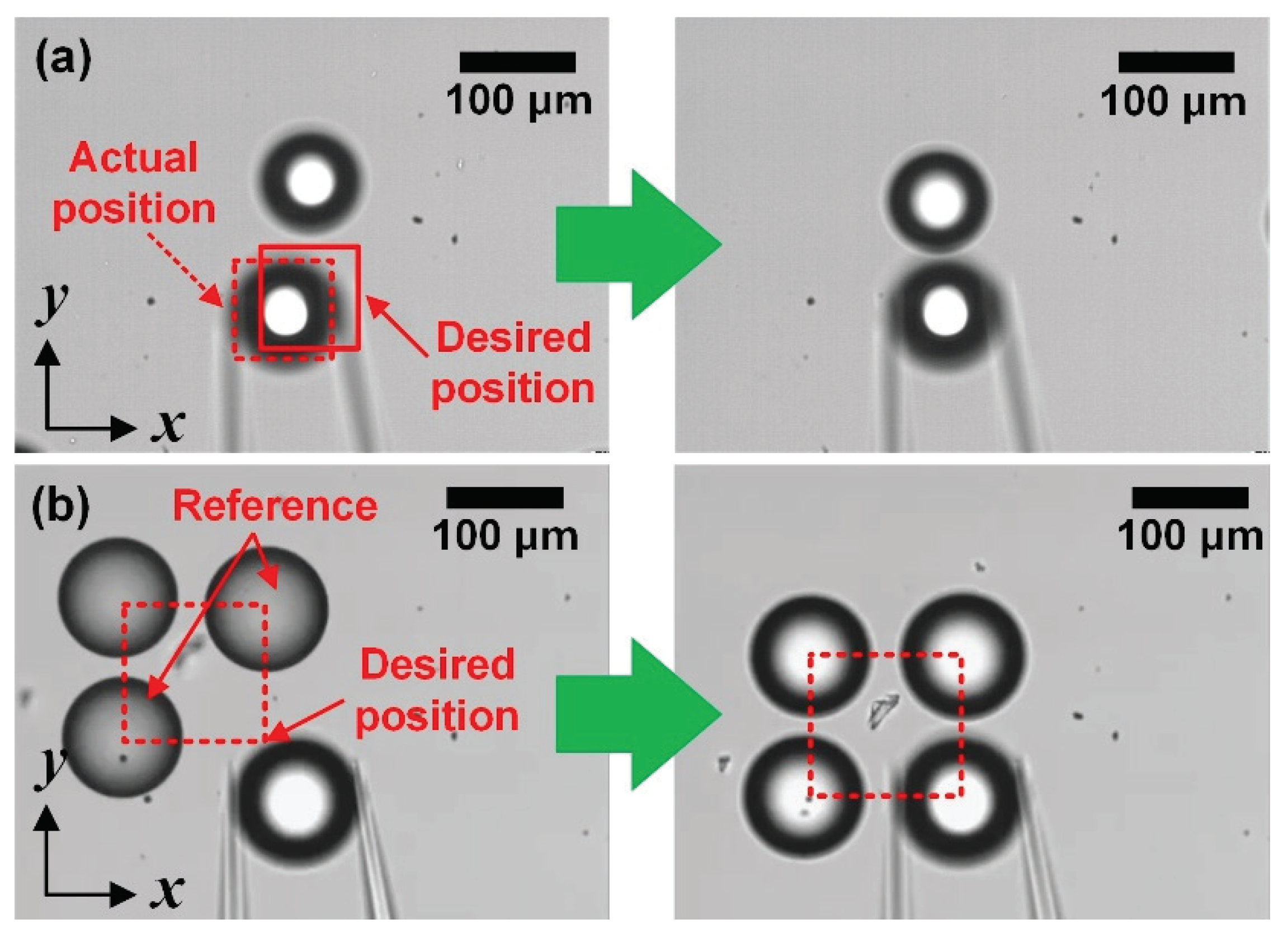
3.2.1. Error Recovery for Assembling Objects
3.2.2. Automated Assembly of 2D and 3D Structure
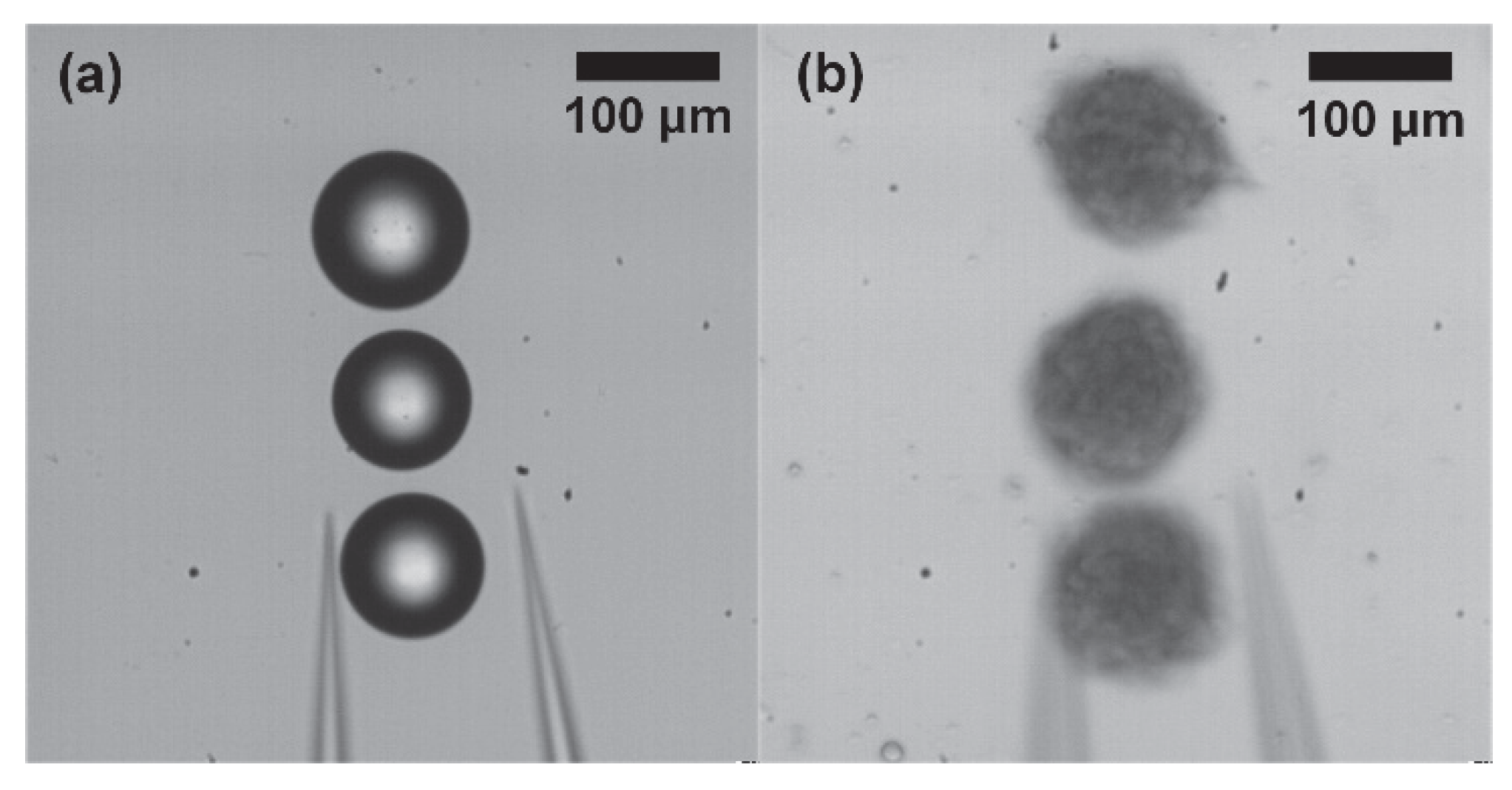
3.2.3. Automated Manipulation Using Spheroids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D bioprinting for vascularized tissue fabrication. Ann. Biomed. Eng. 2017, 45, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Nat. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850. [Google Scholar] [CrossRef]
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Chen, T.; Chen, L.; Sun, L.; Li, X. Design and fabrication of a four-arm-structure MEMS gripper. IEEE Trans. Ind. Electron. 2008, 56, 996–1004. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shimizu, T.; Wada, M.; Yamato, M.; Okano, T. Automatic fabrication of 3-dimensional tissues using cell sheet manipulator technique. Biomaterials 2014, 35, 2428–2435. [Google Scholar] [CrossRef]
- Nagai, M.; Kato, K.; Oohara, K.; Shibata, T. Pick-and-Place Operation of Single Cell Using Optical and Electrical Measurements for Robust Manipulation. Micromachines 2017, 8, 350. [Google Scholar] [CrossRef]
- Penny, H.; Hayman, D.T.; Avci, E. Micromanipulation System for Isolating a Single Cryptosporidium Oocyst. Micromachines 2020, 11, 3. [Google Scholar] [CrossRef]
- Xu, F.; Finley, T.D.; Turkaydin, M.; Sung, Y.; Gurkan, U.A.; Yavuz, A.S.; Guldiken, R.O.; Demirci, U. The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials 2011, 32, 7847–7855. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Mao, Z.; Chen, Y.; Xie, Z.; Lata, J.P.; Li, P.; Ren, L.; Liu, J.; Yang, J.; Dao, M. Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Nat. Acad. Sci. USA 2016, 113, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, S.-C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.-K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Nat. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Grilli, S.; Ferraro, P. Dielectrophoretic trapping of suspended particles by selective pyroelectric effect in lithium niobate crystals. Appl. Phys. Lett. 2008, 92, 232902. [Google Scholar] [CrossRef]
- Thakur, A.; Chowdhury, S.; Švec, P.; Wang, C.; Losert, W.; Gupta, S.K. Indirect pushing based automated micromanipulation of biological cells using optical tweezers. Int. J. Robot. Res. 2014, 33, 1098–1111. [Google Scholar] [CrossRef]
- Chung, S.K.; Cho, S.K. 3-D manipulation of millimeter-and micro-sized objects using an acoustically excited oscillating bubble. Microfluid. Nanofluid. 2009, 6, 261–265. [Google Scholar] [CrossRef]
- Hayakawa, T.; Sakuma, S.; Fukuhara, T.; Yokoyama, Y.; Arai, F. A single cell extraction chip using vibration-induced whirling flow and a thermo-responsive gel pattern. Micromachines 2014, 5, 681–696. [Google Scholar] [CrossRef]
- Rong, W.; Fan, Z.; Wang, L.; Xie, H.; Sun, L. A vacuum microgripping tool with integrated vibration releasing capability. Rev. Sci. Instrum. 2014, 85, 085002. [Google Scholar] [CrossRef]
- Takahashi, K.; Kajihara, H.; Urago, M.; Saito, S.; Mochimaru, Y.; Onzawa, T. Voltage required to detach an adhered particle by Coulomb interaction for micromanipulation. J. Appl. Phys. 2001, 90, 432–437. [Google Scholar] [CrossRef]
- Avci, E.; Ohara, K.; Nguyen, C.-N.; Theeravithayangkura, C.; Kojima, M.; Tanikawa, T.; Mae, Y.; Arai, T. High-speed automated manipulation of microobjects using a two-fingered microhand. IEEE Trans. Ind. Electron. 2014, 62, 1070–1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.K.; Liu, X.; Sun, Y. Autonomous robotic pick-and-place of microobjects. IEEE Trans. Robot. 2009, 26, 200–207. [Google Scholar] [CrossRef]
- Tamadazte, B.; Piat, N.L.-F.; Dembélé, S. Robotic micromanipulation and microassembly using monoview and multiscale visual servoing. IEEE/ASME Trans. Mechatron. 2010, 16, 277–287. [Google Scholar] [CrossRef]
- Gauthier, M.; Régnier, S.; Rougeot, P.; Chaillet, N. Analysis of forces for micromanipulations in dry and liquid media. J. MicroMechatron. 2006. [Google Scholar] [CrossRef]
- Gauthier, M.; Régnier, S.; Lopez-Walle, B.; Gibeau, E.; Rougeot, P.; Hériban, D.; Chaillet, N. Micro-assembly and modeling of the liquid microworld: The PRONOMIA project. In IEEE/RSJ International Conference Intelligent Robots Systems; IEEE: Piscataway, NJ, USA, 2007; pp. 50–62. [Google Scholar]
- Dejeu, J.; Bechelany, M.; Rougeot, P.; Philippe, L.; Gauthier, M. Adhesion control for micro-and nanomanipulation. ACS Nano 2011, 5, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Lopez-Walle, B.; Clévy, C. Comparison between micro-objects manipulations in dry and liquid mediums. In Proceedings of the 2005 International Symposium on Computational Intelligence in Robotics and Automation, Espoo, Finland, 27–30 June 2005; pp. 707–712. [Google Scholar]
- Xie, H.; Régnier, S. Three-dimensional automated micromanipulation using a nanotip gripper with multi-feedback. J. Micromech. Microeng. 2009, 19, 075009. [Google Scholar] [CrossRef]
- Lu, Z.; Moraes, C.; Zhao, Y.; You, L.; Simmons, C.A.; Sun, Y. A micromanipulation system for single cell deposition. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 494–499. [Google Scholar]
- Ramadan, A.A.; Takubo, T.; Mae, Y.; Oohara, K.; Arai, T. Developmental process of a chopstick-like hybrid-structure two-fingered micromanipulator hand for 3-D manipulation of microscopic objects. IEEE Trans. Ind. Electron. 2009, 56, 1121–1135. [Google Scholar] [CrossRef]
- Kim, E.; Kojima, M.; Kamiyama, K.; Horade, M.; Mae, Y.; Arai, T. High-speed active release end-effector motions for precise positioning of adhered micro-objects. World J. Eng. Technol. 2017, 6, 81–103. [Google Scholar] [CrossRef][Green Version]
- Kim, E.; Kojima, M.; Kamiyama, K.; Horade, M.; Mae, Y.; Arai, T. Releasing of micro-objects based on local stream generation by high speed motion of end-effector. Int. J. Mechatron. Autom. 2016, 5, 171–179. [Google Scholar] [CrossRef]
- Kim, E.; Kojima, M.; Kamiyama, K.; Horade, M.; Mae, Y.; Arai, T. Accurate releasing of biological cells using two release methods generated by high speed motion of an end effector. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Korea, 9–14 October 2016; pp. 2572–2577. [Google Scholar]
- Jasper, D.; Diederichs, C.; Stolle, C.; Fatikow, S. Automated robot-based separation and palletizing of microcomponents. In Proceedings of the 2011 IEEE International Symposium on Assembly and Manufacturing (ISAM), Tampere, Finland, 25–27 May 2011; pp. 1–6. [Google Scholar]
- Chowdhury, S.; Thakur, A.; Švec, P.; Wang, C.; Losert, W.; Gupta, S.K. Automated manipulation of biological cells using gripper formations controlled by optical tweezers. IEEE Trans. Automatisci. Eng. 2013, 11, 338–347. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, D.; Huang, W.; Xi, N. Dynamics analysis and motion planning for automated cell transportation with optical tweezers. IEEE/ASME Trans. Mechatron. 2012, 18, 706–713. [Google Scholar] [CrossRef]
- Lu, Z.; Moraes, C.; Ye, G.; Simmons, C.A.; Sun, Y. Single cell deposition and patterning with a robotic system. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kojima, M.; Xiaoming, L.; Hattori, T.; Kamiyama, K.; Mae, Y.; Arai, T. Analysis of rotational flow generated by circular motion of an end effector for 3D micromanipulation. ROBOMECH J. 2017, 4, 5. [Google Scholar] [CrossRef]
- Horade, M.; Kojima, M.; Kamiyama, K.; Kurata, T.; Mae, Y.; Arai, T. Development of an optimum end-effector with a nano-scale uneven surface for non-adhesion cell manipulation using a micro-manipulator. J. Micromech. Microeng. 2015, 25, 115002. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, H.-W. Core–shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015, 21, 2–19. [Google Scholar] [CrossRef]




| Parameter | Air | Liquid | ||
|---|---|---|---|---|
| Xie et al. 2009 | Zhang et al. 2010 | Lu et al. 2010 | Avci et al. 2015 | |
| Transfer speed | NA | 82 µm/s | 0.2 mm/s | 2.3 mm/s |
| Transfer distance | 9 µm/sphere | 77 µm/sphere | 1 mm/sphere | 60 µm/sphere |
| Total speed | 48 s/sphere | 6 s/sphere | 15 s/sphere | 1 s/sphere |
| Success rate | 100% | 100% | 95.13% | 84% |
| Structure | 3D pyramid | 2D patterns | 2D (microwell) | 1D string |
| Manipulation Process | 55 µm Microbeads | 100 µm Microbeads |
|---|---|---|
| Detecting and approaching | 125 ms | 170 ms |
| Grasping | 100 ms | 100 ms |
| Transporting | 105 ms | 105 ms |
| Releasing | 382.5 ms | 330 ms |
| Total | 712.5 ms | 705 ms |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Kojima, M.; Mae, Y.; Arai, T. High-Speed Manipulation of Microobjects Using an Automated Two-Fingered Microhand for 3D Microassembly. Micromachines 2020, 11, 534. https://doi.org/10.3390/mi11050534
Kim E, Kojima M, Mae Y, Arai T. High-Speed Manipulation of Microobjects Using an Automated Two-Fingered Microhand for 3D Microassembly. Micromachines. 2020; 11(5):534. https://doi.org/10.3390/mi11050534
Chicago/Turabian StyleKim, Eunhye, Masaru Kojima, Yasushi Mae, and Tatsuo Arai. 2020. "High-Speed Manipulation of Microobjects Using an Automated Two-Fingered Microhand for 3D Microassembly" Micromachines 11, no. 5: 534. https://doi.org/10.3390/mi11050534
APA StyleKim, E., Kojima, M., Mae, Y., & Arai, T. (2020). High-Speed Manipulation of Microobjects Using an Automated Two-Fingered Microhand for 3D Microassembly. Micromachines, 11(5), 534. https://doi.org/10.3390/mi11050534





