Concentration Gradient Constructions Using Inertial Microfluidics for Studying Tumor Cell–Drug Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Concentration Gradient Stability of the Microfluidic Devices
2.3. The Coefficient of Variation
2.4. Experimental Setup
2.5. Microscopy and Image Analysis
2.6. Cell Perfusion
3. Results and Discussion
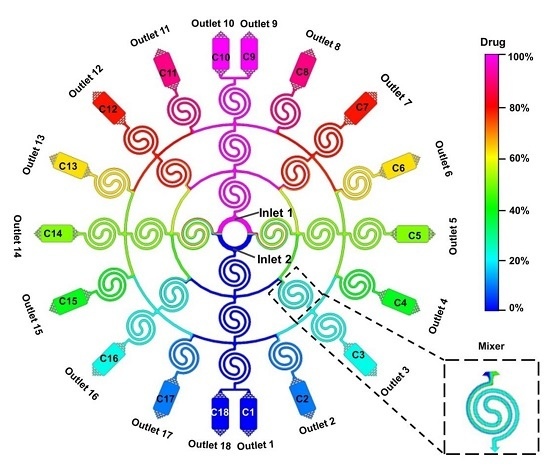
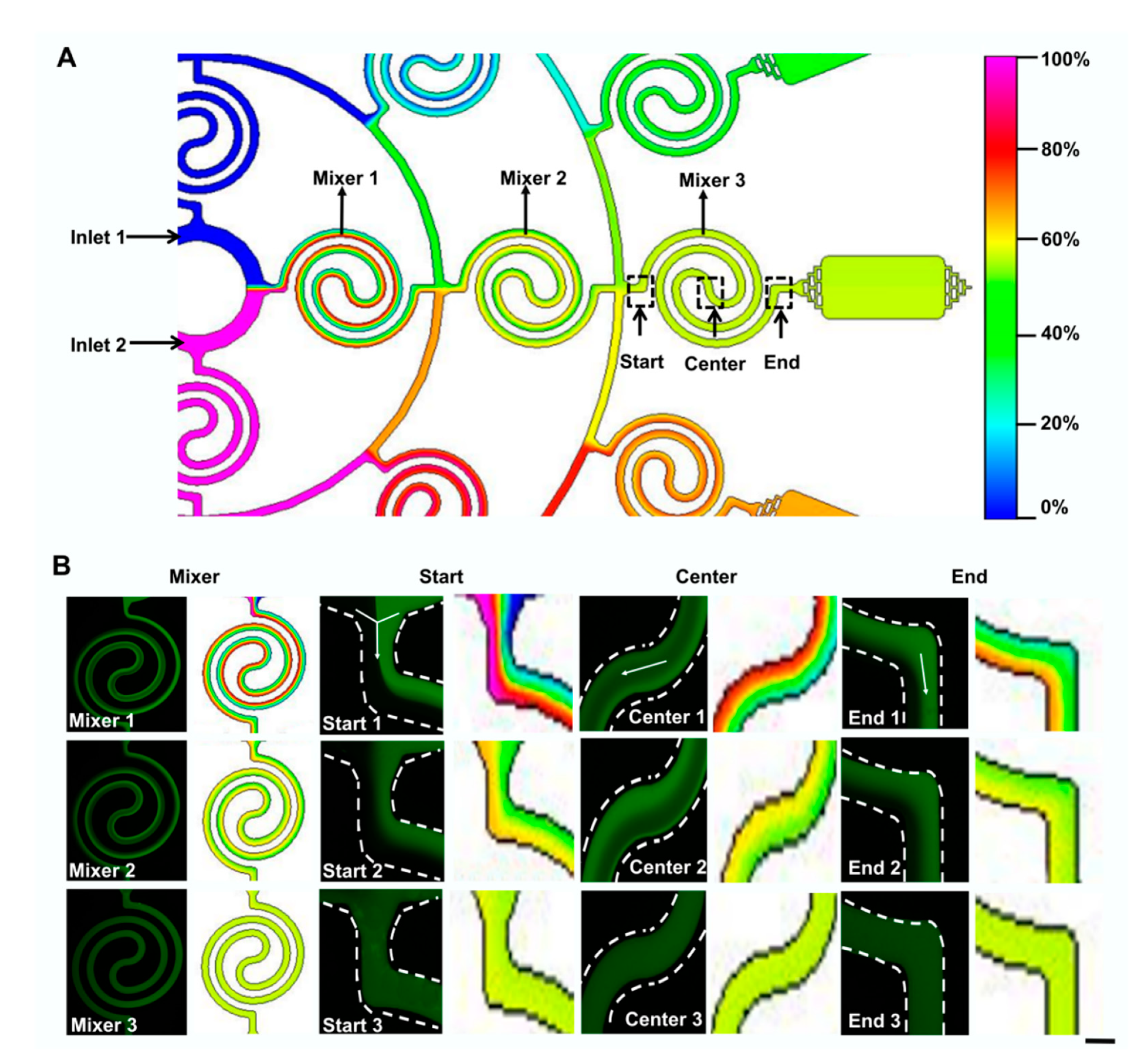
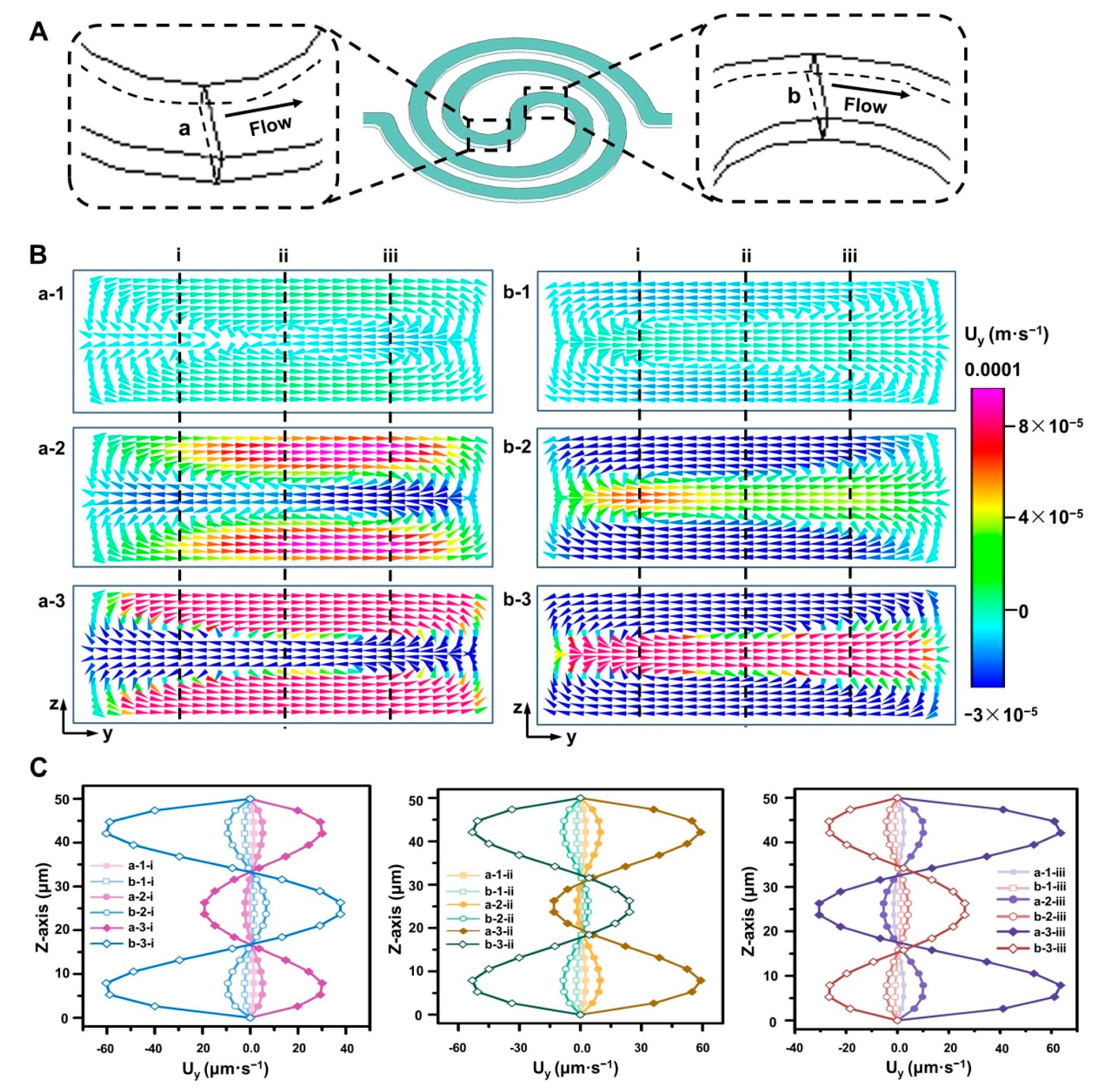
3.1. Device Design
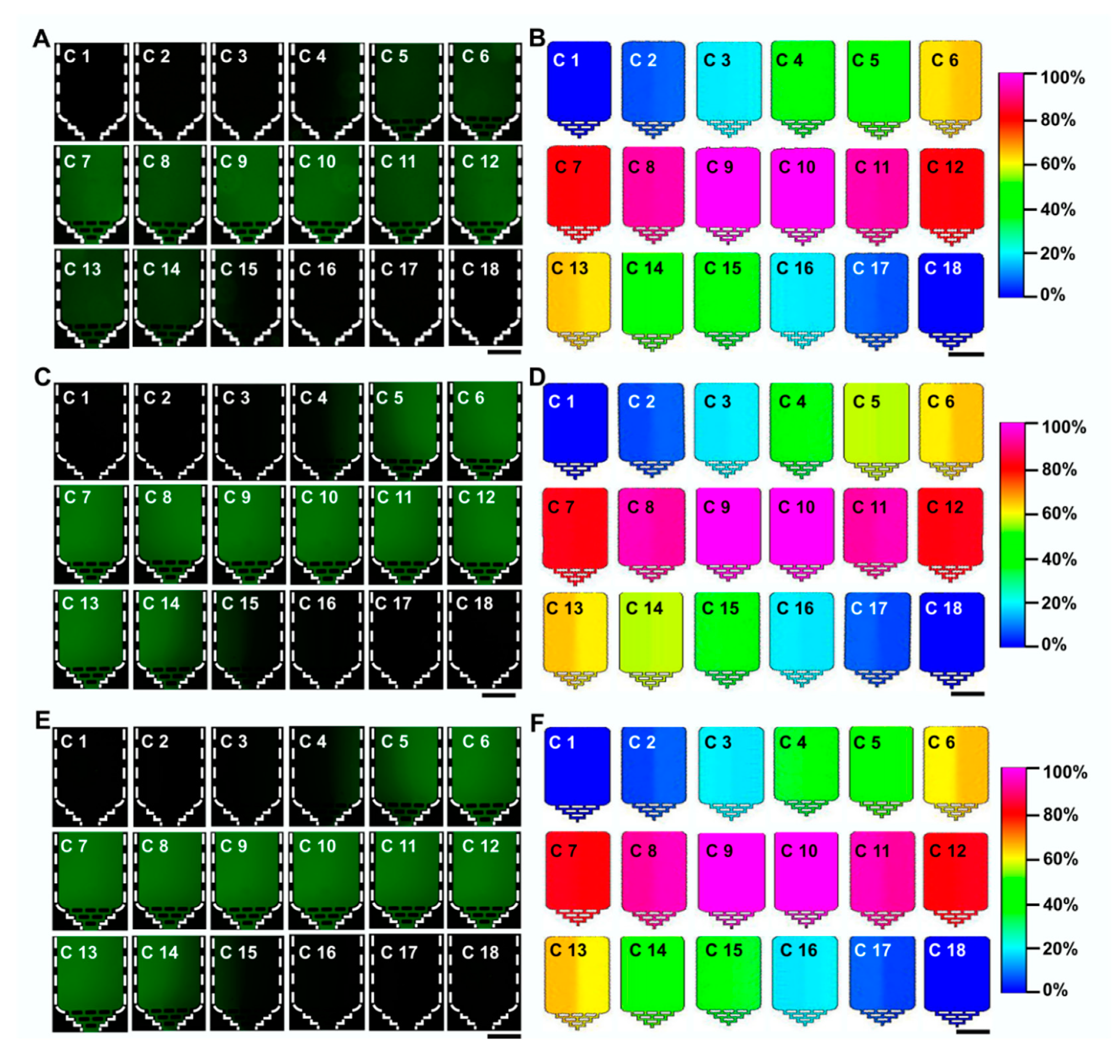
3.2. Dual Concentration-Gradients Formation with a Wide Range of Flow Rates
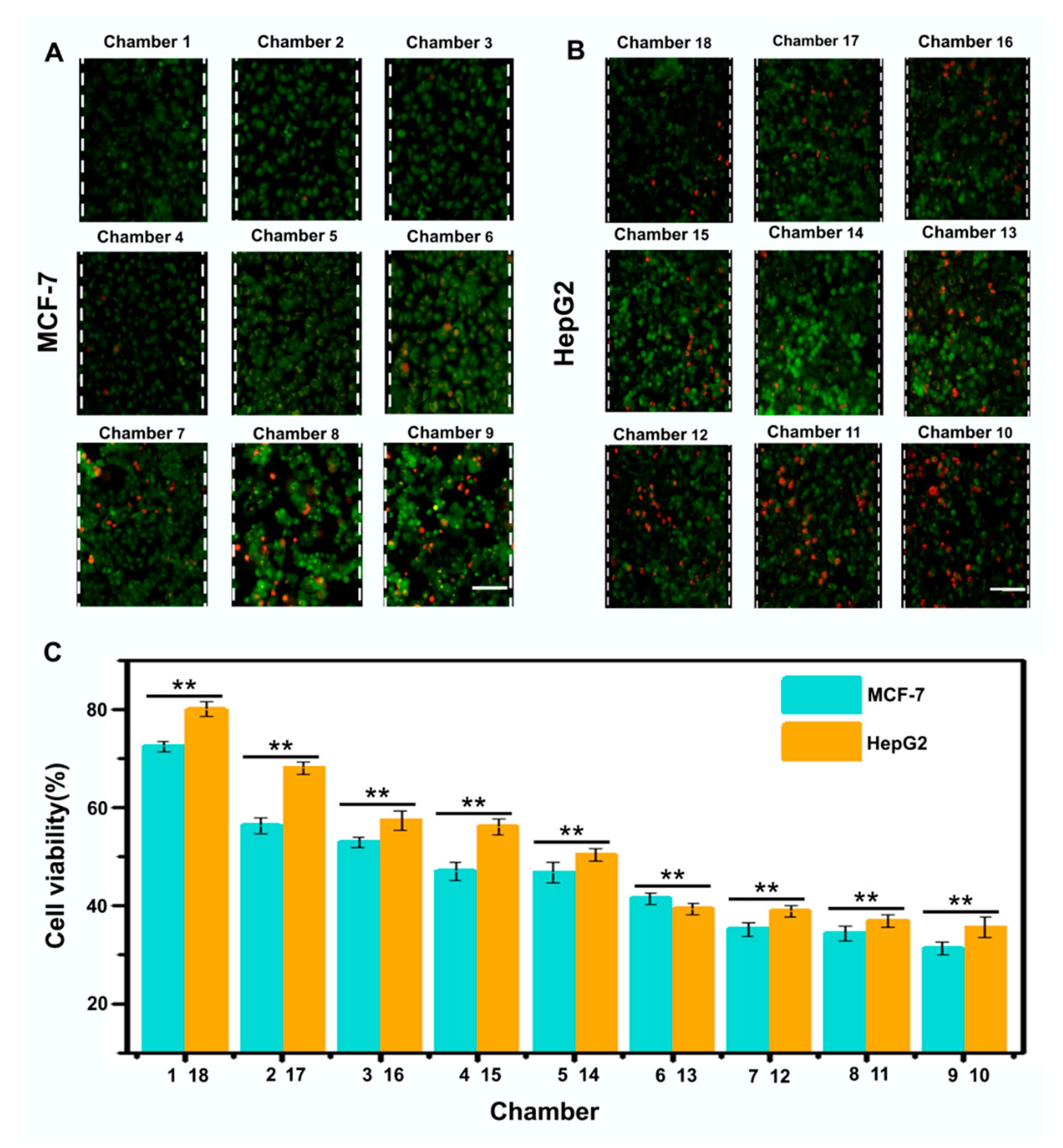
3.3. Evaluation of Drugs on Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.H.; Jung, H.S.; Wang, T.; Shin, M.H.; Kim, Y.E.; Kim, K.H.; Ahn, G.-O.; Hahn, S.K. Microneedle biosensor for real-time electrical detection of nitric oxide for in situ cancer diagnosis during endomicroscopy. Adv. Healthc. Mater. 2015, 4, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Das, V.; Bruzzese, F.; Konecny, P.; Iannelli, F.; Budillon, A.; Hajdúch, M. Pathophysiologically relevant in vitro tumor models for drug screening. Drug Discov. Today 2015, 20, 848–855. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Varadaraj, S.; Dash, S.K.; Sharma, A.; Verma, R.S. Organotypic cancer tissue models for drug screening: 3D constructs, bioprinting and microfluidic chips. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed cell death, from a cancer perspective: An overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef]
- Ocana, S.; Casas, M.L.; Buhigas, I.; Lledo, J.L. Diagnostic strategy for occult hepatitis B virus infection. World J. Gastroenterol. 2011, 17, 1553–1557. [Google Scholar] [CrossRef]
- Fernandes, T.G.; Diogo, M.M.; Clark, U.S.; Dordick, J.S.; Cabral, J.M.S. High-throughput cellular microarray platforms: Applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 2009, 27, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Wang, S. Application of microfluidic chip technology in pharmaceutical analysis: A review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Wu, M.; Segall, J.E.; Wu, M. Microfluidic modeling of the biophysical microenvironment in tumor cell invasion. Lab Chip 2017, 17, 3221–3233. [Google Scholar] [CrossRef]
- Dhiman, N.; Kingshott, P.; Sumer, H.; Sharma, C.S.; Rath, S.N. On-chip anticancer drug screening—Recent progress in microfluidic platforms to address challenges in chemotherapy. Biosens. Bioelectron. 2019, 137, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Soon, R.H.; Lim, C.T.; Khoo, B.L.; Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, X.; Wang, Y.; Du, W.; Chen, P.; Liu, C.; Liu, B.-F. Assembly of multiple cell gradients directed by three-dimensional microfluidic channels. Lab Chip 2015, 15, 3203–3210. [Google Scholar] [CrossRef]
- Ebadi, M.; Moshksayan, K.; Kashaninejad, N.; Saidi, M.; Nguyen, N.-T. A tool for designing tree-like concentration gradient generators for lab-on-a-chip applications. Chem. Eng. Sci. 2019, 212, 115339. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, C.; Liu, W.; Wang, K.; Wei, Y.; Wang, H.; Wang, J.; Liu, S. Determination of benzopyrene-induced lung inflammatory and cytotoxic injury in a chemical gradient-integrated microfluidic bronchial epithelium system. ACS Sens. 2018, 3, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ferreira, M.M.; Heilshorn, S.C. Small-molecule axon-polarization studies enabled by a shear-free microfluidic gradient generator. Lab Chip 2014, 14, 2047–2056. [Google Scholar] [CrossRef] [Green Version]
- Toh, A.G.G.; Wang, Z.P.; Yang, C.; Nguyen, A.V. Engineering microfluidic concentration gradient generators for biological applications. Microfluid. Nanofluid. 2013, 16, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y.; Li, Z.-Y.; Ueno, K.; Misawa, H.; Ren, N.; Sun, K. On-chip MIC by combining concentration gradient generator and flanged chamber arrays. Micromachines 2020, 11, 207. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Meng, J.; Li, S.; Yu, C.; Li, J.; Wei, C.; Dai, S. A microfluidic concentration gradient maker with tunable concentration profiles by changing feed flow rate ratios. Micromachines 2020, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Y.-C.; Urs, S.; Chen, L.; Simeone, D.M.; Yoon, E. Scalable multiplexed drug-combination screening platforms using 3D microtumor model for precision medicine. Small 2018, 14, 1703617. [Google Scholar] [CrossRef] [PubMed]
- Griner, L.A.M.; Guha, R.; Shinn, P.; Young, R.M.; Keller, J.M.; Liu, D.; Goldlust, I.S.; Yasgar, A.; McKnight, C.; Boxer, M.B.; et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell–like diffuse large B-cell lymphoma cells. Proc. Natl. Acad. Sci. USA 2014, 111, 2349–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, S.; Auld, U.; Klumpp, C.; Jadhav, A.; Zheng, W.; Thorne, N.; Austin, C.P.; Inglese, J.; Simeonov, A. A robotic platform for quantitative high-throughput screening. Assay Drug Dev. Technol. 2008, 6, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Mitxelena-Iribarren, O.; Zabalo, J.; Arana, S.; Mujika, M. Improved microfluidic platform for simultaneous multiple drug screening towards personalized treatment. Biosens. Bioelectron. 2019, 123, 237–243. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J.; Jeon, N.L. Biological applications of microfluidic gradient devices. Integr. Biol. 2010, 2, 584–603. [Google Scholar] [CrossRef]
- Shen, S.; Kou, L.; Zhang, X.; Wang, D.; Niu, Y.B.; Wang, J. Regulating secondary flow in ultra-low aspect ratio microchannels by dimensional confinement. Adv. Theory Simul. 2018, 1, 1700034. [Google Scholar] [CrossRef]
- Yuan, D.; Tan, S.H.; Sluyter, R.; Zhao, Q.; Yan, S.; Nguyen, A.V.; Guo, J.; Zhang, J.; Li, W. On-chip microparticle and cell washing using coflow of viscoelastic fluid and newtonian fluid. Anal. Chem. 2017, 89, 9574–9582. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yuan, D.; Zhao, Q.; Yan, S.; Tang, S.-Y.; Tan, S.H.; Guo, J.; Xia, H.; Nguyen, A.V.; Li, W. Tunable particle separation in a hybrid dielectrophoresis (DEP)-inertial microfluidic device. Sens. Actuators B Chem. 2018, 267, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yuan, D.; Zhao, Q.; Teo, A.J.T.; Yan, S.; Ooi, C.H.; Li, W.; Nguyen, N.-T. Fundamentals of differential particle inertial focusing in symmetric sinusoidal microchannels. Anal. Chem. 2019, 91, 4077–4084. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Tian, C.; Li, T.; Xu, J.; Tu, Q.; Liu, W.; Wang, J.; Chen, S.; Yuan, M. Spiral microchannel with ordered micro-obstacles for continuous and highly-efficient particle separation. Lab Chip 2017, 17, 3578–3591. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, F.; Wang, S.; Wang, J.; Long, D.; Wang, D.; Niu, Y.B. Ultra-low aspect ratio spiral microchannel with ordered micro-bars for flow-rate insensitive blood plasma extraction. Sens. Actuators B Chem. 2019, 287, 320–328. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, X.; Zhang, F.; Wang, D.; Long, D.; Niu, Y.B. Three-gradient constructions in a flow-rate insensitive microfluidic system for drug screening towards personalized treatment. Talanta 2020, 208, 120477. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Nguyen, D.T.; Akay, Y.; Xu, F.; Akay, M. Engineering a brain cancer chip for high-throughput drug screening. Sci. Rep. 2016, 6, 25062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Liu, W.; Wang, Y.; Wang, J.-C.; Tu, Q.; Liu, R.; Wang, J. Construction of oxygen and chemical concentration gradients in a single microfluidic device for studying tumor cell–drug interactions in a dynamic hypoxia microenvironment. Lab Chip 2013, 13, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Gard, D.L.; Kirschner, M.W.; Lin, J.J.; Helfman, D.M.; Hughes, S.H.; Chou, C.S. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J. Cell Biol. 1985, 100, 764–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parness, J.; Horwitz, S. Taxol binds to polymerized tubulin in vitro. J. Cell Biol. 1981, 91, 479–487. [Google Scholar] [CrossRef]
- Murphy, D.B.; Johnson, K.A.; Borisy, G.G. Role of tubulin-associated proteins in microtubule nucleation and elongation. J. Mol. Biol. 1977, 117, 33–52. [Google Scholar] [CrossRef]
- Schiff, P.B.; Horwitz, S.B. Taxol assembles tubulin in the absence of exogenous guanosine 5′-triphosphate or microtubule-associated proteins. Biochemistry 1981, 20, 3247–3252. [Google Scholar] [CrossRef]
- Patt, D.; Gauthier, M.; Giordano, S.H. Paclitaxel in breast cancer. Women’s Health 2006, 2, 11–21. [Google Scholar] [CrossRef]
- Ajabnoor, G.M.; Crook, T.; Coley, H.M. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012, 3, e260. [Google Scholar] [CrossRef] [Green Version]
- Young, E.W.K.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Zhang, F.; Gao, M.; Niu, Y. Concentration Gradient Constructions Using Inertial Microfluidics for Studying Tumor Cell–Drug Interactions. Micromachines 2020, 11, 493. https://doi.org/10.3390/mi11050493
Shen S, Zhang F, Gao M, Niu Y. Concentration Gradient Constructions Using Inertial Microfluidics for Studying Tumor Cell–Drug Interactions. Micromachines. 2020; 11(5):493. https://doi.org/10.3390/mi11050493
Chicago/Turabian StyleShen, Shaofei, Fangjuan Zhang, Mengqi Gao, and Yanbing Niu. 2020. "Concentration Gradient Constructions Using Inertial Microfluidics for Studying Tumor Cell–Drug Interactions" Micromachines 11, no. 5: 493. https://doi.org/10.3390/mi11050493
APA StyleShen, S., Zhang, F., Gao, M., & Niu, Y. (2020). Concentration Gradient Constructions Using Inertial Microfluidics for Studying Tumor Cell–Drug Interactions. Micromachines, 11(5), 493. https://doi.org/10.3390/mi11050493